Abstract
Objectives.
To prospectively examine the relationship between body weight, frailty, and the disablement process.
Method.
Longitudinal data from the Health and Retirement Study (1998–2006) were used to examine the relationship between being underweight, overweight, or obese (compared with normal weight) and the onset and progression of functional limitations and disabilities in instrumental activities of daily living (IADL) and activities of daily living (ADL) among a nationally representative sample of community-dwelling older adults (aged 50 and older) with characteristics of frailty (n = 11,491). Nonlinear multilevel models additionally adjusted for demographic characteristics and intra-individual changes in body weight, socioeconomic status, health behaviors, and health conditions over the course of 8 years.
Results.
Compared with their nonfrail normal weight counterparts, prefrail obese respondents have a 16% (p ≤ 0.001) reduction in the expected functional limitations rate and frail overweight and obese respondents have a 10% (p ≤ 0.01) and 36% (p ≤ 0.001) reduction in the expected functional limitations rate, respectively. In addition, frail obese respondents have a 27% (p ≤ 0.05) reduction in the expected ADL disability rate.
Discussion.
This study’s findings suggest that underweight, overweight, and obese status differentially affect the risk for functional limitations and disabilities in IADL and ADL. Among prefrail and frail adults, some excess body weight in later life may be beneficial, reducing the rate of functional limitations and disability.
Key Words: Sarcopenia, Obesity, Disease
About 32% of community-dwelling older adults report functional limitations and more than 10% report disabilities in activities of daily living (ADL), including bathing, eating, and dressing (Fuller-Thomson, Yu, Nuru-Jeter, Guralnik, & Minkler, 2009). Consistent with the disablement process, the level of disability experienced varies largely by the type and severity of disease (Verbrugge & Jette, 1994) with each progressive loss in functioning increasing the risk for dependence, institutionalization, and mortality (Fried & Guralnik, 1997). Disease progression is also associated with muscle, strength (Baumgartner, Waters, Gallagher, Morley, & Garry, 1999), and weight loss (Ferraro, Su, Gretebeck, Black, & Badylak, 2002), which may lead to an increased risk for functional limitations and disability in old age.
Frailty is similarly associated with muscle, strength, and weight loss (Fried et al., 2001) and has been used interchangeably with disability in some studies (Rockwood, Hogan, & MacKnight, 2000). Despite this overlap in symptoms, frailty may be an independent stage in the disablement process (Ensrud et al., 2008). For example, cohort studies report an association between frailty and an increased risk for disabilities in ADL (Boyd, Xue, Simpson, Guralnik, & Fried, 2005). Even though frailty is a “wasting disorder” and underweight older adults are vulnerable (Abate et al., 2007; Fried et al., 2001; Morley, 2008; Rolland et al., 2008), overweight and obese older adults also experience age-related muscle loss and may also be frail (Barzilay et al., 2007; Blaum, Xue, Michelon, Semba, & Fried, 2005).
The relationship between body weight, frailty, and the disablement process in later life is unclear. Although excess body weight increases the risk for disease, disability, and other adverse outcomes (Larrieu et al., 2004), it also reduces the risk for osteoporosis and injurious falls (Rosen & Klibanski, 2009). Thus, for frail older adults, some excess body weight in later life may be protective. This study will examine the relationship between body weight and the disablement process over time using a nationally representative sample of community-dwelling older adults.
Frailty and Body Weight
Frailty, as a syndrome of weakness, impaired mobility, balance, and minimal energy reserve (Buchner & Wagner, 1992), has been associated with falls, hospitalization, and death (Ensrud et al., 2009; Fried et al., 2001). Frailty is most often defined as having at least three of the following risk factors: unintentional weight loss, fatigue or exercise intolerance, weakness, slowed motor performance, and low physical activity (Fried et al., 2001). An older adult with only one or two of these risk factors is considered prefrail. However, frailty definitions vary, and frailty is largely conceptualized as increased vulnerability across multiple systems (Rockwood & Mitnitski, 2007). This study conceptualizes frailty as a fall (gait and balance deficits), difficulty getting up from a chair (weakness), fatigue (minimal energy reserve), and little/no physical activity.
It is well documented that underweight older adults are vulnerable with minimal reserve capacity (Fried et al., 2001) and an increased risk for malnutrition, institutionalization, and death (Payette, Coulombe, Boutier, & Gray-Donald, 2000). Underweight older adults also have an increased risk for gait and balance deficits and osteoporosis, increasing the risk for falls, fractures, and other fall-related injuries (Waters, Hale, Grant, Herbison, & Goulding, 2010). For these reasons, frailty researchers have largely focused on exercise and other weight-bearing interventions to increase strength and muscle among underweight older adults (Fried et al., 2009). However, overweight and obese older adults are also at risk for adverse outcomes; excess body weight in later life is associated with disease, functional limitations, and disability (Himes, 2000). Other studies have shown that overweight and obese older adults may be frail, with lower muscle quality, strength, endurance, and balance than their underweight or normal weight counterparts (Morley, Kim, Haren, Kevorkian, & Banks, 2005; Roubenoff, 2000, 2004). This is due, in part, to age-related changes in body composition and skeletal muscle mass that lead to concurrent weight gain and muscle loss over time (Roubenoff, 2000, 2004). However, overweight and obese older adults have more energy reserve and are at a reduced risk for osteoporosis and injurious falls, which decreases the risk for functional limitations and disability (Ensrud et al., 2009; Rosen & Klibanski, 2009). Thus, some excess body weight in later life may be beneficial among prefrail and frail older adults.
This study will prospectively examine how bodyweight may vary the relationship between frailty, functional limitations, and disabilities in instrumental activities of daily living (IADL) and ADL over the course of 8 years using a nationally representative sample of community-dwelling older adults. Frailty is conceptualized as increasing vulnerability—where three or four risk factors (including a fall, difficulty getting up from a chair, fatigue, and low physical activity) indicate frailty and one or two of these risk factors indicate prefrailty. In this study, prefrailty and frailty are expected to independently increase the risk for functional limitations and disabilities in IADL and ADL, accounting for demographic characteristics, socioeconomic status (SES), health behaviors, and health conditions. However, bodyweight is expected to vary the relationship between prefrailty, frailty, and the disablement process. Although excess bodyweight will be associated with a higher rate of functional limitations and IADL and ADL disability—among prefrail and frail overweight and obese older adults—excess body weight is expected to be associated with lower rates of functional limitations and IADL and ADL disability.
Method
Data
The Health and Retirement Study (HRS), a prospective multistage probability cohort sample of U.S. households, was conducted by the University of Michigan with support from the National Institute of Aging. The first wave of the HRS occurred in 1992 with a 51- to 61-year-old cohort and was merged with the older Asset and Health Dynamics of the Oldest Old Study (AHEAD; born 1890–1923) in 1998. Two additional cohorts, Children of the Depression (CODA; 1924–1930) and War Babies (WB; born 1942–1947), were added in 1998 to fill in the gaps between these two groups, resulting in a sample design nationally representative of the U.S. population aged 50 and older in 1998. Further details on the HRS design and methods have been previously published (Heeringa & Connor, 1995).
To account for the effects of cognitive impairment on frailty and disability (Boyle, Buchman, Wilson, Leurgans, & Bennett, 2010; Jones, Song, & Rockwood, 2004), respondents unable to answer survey questions at baseline (year 1998) and those scoring below the 10th percentile (n = 2,346) on immediate (fewer than three words; range 0–10) and delayed (fewer than two words; range 0–10) wordlist recall tests were excluded. Data were weighted using respondent-level sampling weights to account for the sample design in the HRS and to generalize findings to the community-dwelling older adult population (Heeringa & Connor, 1995). This study, using five waves of data from the HRS (1998–2006), is nationally representative sample of non-institutionalized adults aged 50 and older (n = 11,491). Approximately 74.3% of respondents participated in all five waves of the HRS (1998–2006). By survey year 2006, approximately 17% of respondents were deceased, and 8.7% were lost to follow-up. Nonresponse rates increased with age and were higher for non-whites, men, and respondents with low SES.
Variable Measurement
The variables age, marital status, SES, health behaviors, health conditions, prefrailty/frailty, body weight, functional limitations, IADL, and ADL were measured at baseline (1998) and subsequently every 2 years over the course of the study, allowing for an examination of intra-individual changes in these over the 8-year course of the study. Other variables (e.g., sex, race/ethnicity, and education) did not change over the course of the study and were treated as fixed.
Dependent Variables
Consistent with the disablement process, disablement is conceptualized as a progressive loss of functioning and disabilities in IADL and ADL (Verbrugge & Jette, 1994). To measure functional limitations, respondents were asked if they had difficulties (yes/no) walking one block, walking several blocks, walking across a room, climbing one flight of stairs, and climbing several flights of stairs; for IADL, respondents were asked if they had difficulties (yes/no) using the phone, managing money, taking medications, shopping for groceries, and preparing hot meals; for ADL, respondents were asked if they had difficulties (yes/no) walking across a room, bathing, eating, dressing, and getting in and out of bed. Each scale ranges from 0 to 5. These scales were used because they predict disablement (Bowen & Gonzalez, 2010; Clarke & George, 2005) and have good reliability (functional limitations, α = 0.79; IADL, α = 0.65; and ADL, α = 0.72).
Independent Variables
The independent variable of interest in this study is body weight, measured by body mass index (BMI; based on self-reports of height and weight) with normal weight (BMI = 18.6–24.9; reference), underweight (BMI ≤ 18.5), overweight (BMI = 25–29.9), and obese (BMI ≥ 30) categories.
Frailty is a syndrome of increased vulnerability, impaired mobility, balance, and minimal reserve (Buchner & Wagner, 1992). To measure frailty, this study used the following: a fall (yes/no), fatigue (report of whether everything is an effort; yes/no), difficulty getting up from a chair (yes/no), and low physical activity (defined as participation in a job involving physical labor, heavy housework, aerobics, bicycling, running/jogging, or swimming less than 3 times per week). Three or four of these risk factors is considered frail; one or two of these is considered prefrail (Fried et al., 2001). The latter measure of physical activity was used because it is the only physical activity measure asked consistently across all survey waves of the HRS.
This study also included demographic conditions [age (≥51 in 1998), sex (male/female), race/ethnicity (white/non-white), and marital status (married)], SES, health behaviors, and health conditions in multivariate models as these may affect the relationship between body weight, functional limitations, and disability (Adler et al., 1994; Link & Phelan, 1995; Verbrugge & Jette, 1994). SES included education (0–17+ years), income (the log transformed total household income including earnings, pensions, and social security), and wealth (the log-transformed value of assets minus the sum of all debt, including mortgages). Health behaviors included smoking (never smoked, current smoker, and former smoker) and drinking alcohol (drinks per week over the past 3 months). Health conditions (scale ranging from 0 to 5) included whether (yes/no) a doctor had ever told the respondent that they had high blood pressure (or hypertension), diabetes (or high blood sugar), stroke (or a transient ischemic attack), heart problems (including coronary heart disease, heart attack, congestive heart failure, and the occurrence of heart surgery), and arthritis (or rheumatism). Self-reported health conditions have shown substantial agreement with both survey and medical record reports (Bush, Miller, Golden, & Hale, 1989).
Data Analysis
To examine how body weight may vary the relationship between prefrailty/frailty, functional limitations, and disabilities in IADL and ADL, multilevel statistical modeling techniques were used. Hierarchical Linear Modeling software, version 6.08 (HLM; Scientific Software International, Lincolnwood, IL), was used to examine individual and aggregate levels of data over time and to account for the complex HRS sampling design and the subset analyzed (Raudenbush & Bryk, 2002). Model diagnostics (e.g., fitted residuals) were assessed to check for assumptions of normality; functional loss and IADL and ADL disability distributions were non-normal, reflecting the higher frequency of intact functioning among the general older adult population. Alternative distributions were examined, and nonlinear models that modeled Poisson distributions of the functional limitations, IADL, and ADL were a better fit. The results of the nonlinear analyses are reported subsequently.
At the first level, each individual respondent’s trajectory of change in functional limitations and IADL and ADL disability is represented as a function of person-time-specific parameters (e.g., prefrailty/frailty, income, wealth, health behaviors, and health conditions) plus random error. These variables are time-varying, measured at baseline (year 1998) and subsequently every 2 years over the 8-year course of the survey. The second level statistically models individual variations in growth parameters across a population of persons (e.g., sex, race/ethnicity, and education). Multilevel models account for between-subject heterogeneity and within-individual correlations and model cluster-induced errors in the intercepts and coefficients to increase the efficiency of the estimates. Effect estimates are presented in terms of event rate ratios (ERRs), which are the beta coefficient exponentiated in a Poisson model. An ERR is interpreted as the percentage change in a dependent variable associated with a 1-U increase in an independent variable.
In a sequential model-building process, the first multivariate models for each outcome (shown in Table 2; Models 1, 3, and 5) include demographic characteristics, SES, health behaviors, frailty, and body weight. Models 2, 4, and 6 additionally include prefrailty/frailty interactions with body weight. Attrition variables (death and lost to follow-up) were added to multivariate models to examine the ways in which these may affect study results (see bottom of Table 2). For each outcome, goodness of fit is determined by comparing the baseline x 2 and Level-2 variance model with the prefrailty/frailty model.
Table 2.
Hierarchical Pois\son Models Examining the Relationship Between Frailty and the Disablement Process in the HRS (1998–2006; n = 11,491)a
| Functional limitations | IADL | ADL | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| ERR (CI) | ERR (CI) | ERR (CI) | ERR (CI) | ERR (CI) | ERR (CI) | |
| Demographic characteristics | ||||||
| Age | 1.02*** (1.02–1.03) | 1.01*** (1.01–1.02) | 1.04*** (1.04–1.05) | 1.03*** (1.03–1.04) | 1.03*** (1.02–1.03) | 1.01*** (1.01–1.02) |
| Female | 1.52*** (1.46–1.58) | 1.43*** (1.38–1.48) | 1.19*** (1.10–1.28) | 1.06 (0.99–1.14) | 1.20*** (1.13–1.29) | 1.08* (1.01–1.14) |
| White | 0.97 (0.93–1.01) | 1.02 (0.99–1.06) | 0.82*** (0.76–0.89) | 0.88*** (0.83–0.95) | 0.77*** (0.71–0.82) | 0.83*** (0.79–0.89) |
| Married | 1.01 (0.98–1.04) | 1.00 (0.98–1.03) | 0.92* (0.87–0.98) | 0.93* (0.87–0.99) | 0.95 (0.90–1.00) | 0.94* (0.89–0.99) |
| Socioeconomic status | ||||||
| Education (years) | 0.96*** (0.96–0.97) | 0.97*** (0.97–0.98) | 0.94*** (0.95–0.96) | 0.96*** (0.95–0.97) | 0.96*** (0.95–0.97) | 0.97*** (0.97–0.98) |
| Income (logged value) | 0.87*** (0.87–0.90) | 0.89*** (0.87–0.90) | 0.79*** (0.76–0.83) | 0.80*** (0.78–0.83) | 0.81*** (0.80–0.84) | 0.83*** (0.80–0.86) |
| Wealth (logged value) | 0.97*** (0.97–0.98) | 0.98*** (0.97–0.98) | 0.92*** (0.91–0.93) | 0.93*** (0.93–0.94) | 0.94*** (0.93–0.95) | 0.95*** (0.95–0.96) |
| Health behaviors | ||||||
| Current smoker (ref. = never smoked) | 1.34*** (1.29–1.41) | 1.31*** (1.26–1.36) | 1.19*** (1.09–1.30) | 1.10* (1.01–1.20) | 1.24*** (1.13–1.35) | 1.15*** (1.06–1.24) |
| Former smoker | 1.19*** (1.15–1.23) | 1.16*** (1.13–1.20) | 1.07 (0.99–1.14) | 1.04 (0.97–1.11) | 1.13*** (1.07–1.21) | 1.10** (1.04–1.17) |
| Drink alcohol (number of drinks per week) | 0.99** (0.98–0.99) | 0.99*** (0.98–0.99) | 0.98*** (0.97–0.98) | 0.98*** (0.97–0.98) | 0.99*** (0.98–0.99) | 0.99*** (0.98–0.99) |
| Health conditions (range 0–5) | 1.36*** (1.34–1.37) | 1.29*** (1.28–1.30) | 1.52*** (1.49–1.55) | 1.36*** (1.34–1.39) | 1.49*** (1.46–1.52) | 1.32*** (1.30–1.35) |
| Body weight | ||||||
| Underweight (BMI ≤ 18.5) | 1.22*** (1.15–1.29) | 1.25 (0.94–1.64) | 1.51*** (1.38–1.65) | 1.59 (0.91–2.80) | 1.52*** (1.38–1.68) | 2.12 (0.80–5.64) |
| Overweight (BMI = 25–29.9) | 1.12*** (1.09–1.15) | 1.18*** (1.09–1.28) | 0.77*** (0.73–0.82) | 0.82 (0.66–1.03) | 0.99 (0.94–1.05) | 1.06 (0.81–1.38) |
| Obese (BMI ≥ 30) | 1.51*** (1.46–1.56) | 1.90*** (1.73–2.08) | 0.94* (0.88–0.99) | 0.85 (0.65–1.12) | 1.34*** (1.26–1.43) | 1.47* (1.06–2.02) |
| Frailty risk factors (ref. = nonfrail, normal weight) | ||||||
| Prefrail (1 or 2 indicators) | 2.36*** (2.28–2.45) | 2.49*** (2.34–2.65) | 3.04*** (2.74–3.36) | 3.22*** (2.76–3.74) | 7.58*** (6.70–8.60) | 7.83*** (2.08–2.21) |
| × underweight | — | 0.93 (0.71–1.23) | — | 0.89 (0.50–1.56) | — | 0.61 (0.22–1.63) |
| × overweight | — | 0.95 (0.88–1.03) | — | 0.85 (1.03–1.11) | — | 0.91 (0.70–1.20) |
| × obese | — | 0.84*** (0.77–0.92) | — | 0.85 (0.65–1.12) | — | 0.98 (0.71–1.36) |
| Frail (three or four indicators) | 3.59*** (3.45–3.74) | 4.29*** (4.01–4.60) | 7.47*** (6.68–8.34) | 7.62*** (6.48–8.96) | 20.78*** (18.21–23.72) | 24.04*** (19.52–29.52) |
| × underweight | — | 0.92 (0.70–1.23) | — | 0.90 (0.51–1.59) | — | 0.68 (0.25–1.84) |
| × overweight | — | 0.90** (0.82–0.98) | — | 0.97 (0.77–1.23) | — | 0.90 (0.67–1.19) |
| × obese | — | 0.64*** (0.58–0.71) | — | 1.00 (0.76–1.33) | — | 0.73* (0.52–0.99) |
| Intercept | 0.32*** (0.29–0.36) | 0.38*** (0.34–0.42) | 0.12*** (0.09–0.15) | 0.14*** (0.12–0.18) | 0.04*** (0.03–0.05) | 0.08*** (0.07–0.10) |
| Attrition | ||||||
| Deceased/Lost to follow-up | 1.35*** (1.31–1.40) | 1.36*** (1.32–1.40) | 1.78*** (1.68–1.88) | 1.73*** (1.64–1.83) | 1.79*** (1.70–1.90) | 1.77*** (1.69–1.87) |
| Model statistics | ||||||
| x 2 | 71,698.91 | 56,025.81*** | 43,854.74 | 32,997.35*** | 60,005.63 | 37,073.12*** |
| Level-2 variance | 0.74 | 0.55 | 1.58 | 1.27 | 1.56 | 1.05 |
Note. ADL = activities of daily living; BMI = body mass index; CI = confidence intervals; ERR = event rate ratio; IADL = instrumental activities of daily living.
aEstimates are weighted to using respondent level sampling weights to account for the sample design in the HRS and to generalize findings to the community-dwelling older adult population.
*p < .05. **p < .01. ***p ≤ .001.
Results
Bivariate Results
As shown in Table 1, respondents were more likely to report functional limitations than IADL and ADL disabilities. Compared with their counterparts, frail respondents reported more functional limitations and disabilities in IADL and ADL. Respondents were most likely to report little/no physical activity (54%) followed by difficulty getting up from a chair (34%). Respondents were also more likely to be overweight or normal weight compared with underweight or obese. Of all health conditions, respondents were most likely to report arthritis (46%) and high blood pressure (40%). Frail respondents were more likely than their counterparts to report a health condition.
Table 1.
Means (M) at Baseline (Year 1998) for Model Predictors and Outcomes Including Functional Limitations, Disabilities in IADL, and ADL in the HRSa
| Total sample, M b | Nonfrail, M | Prefrail,c M | Frail,d M | |
|---|---|---|---|---|
| (n = 11,491) | (n = 3,218) | (n = 6,894) | (n = 1,379) | |
| Demographic characteristics | ||||
| Age (years) | 64.25 | 62.16 | 64.14* | 69.54* |
| Female | 0.57 | 0.50 | 0.58* | 0.69* |
| White | 0.84 | 0.89 | 0.84* | 0.76* |
| Married | 0.66 | 0.73 | 0.67* | 0.52* |
| Functional limitations and disabilities | ||||
| Functional limitations (range = 0–5) | 0.82 | 0.18 | 0.78* | 2.42* |
| IADL (range = 0–5) | 0.14 | 0.01 | 0.10* | 0.62* |
| ADL (range = 0–5) | 0.22 | 0.01 | 0.16* | 1.00* |
| Frailty risk factors | ||||
| Fall (yes/no) | 0.12 | 0.14 | 0.10* | 0.45* |
| Difficulty getting up from chair (yes/no) | 0.34 | 0.29 | 0.37* | 0.94* |
| Fatigue (yes/no) | 0.23 | 0.25 | 0.21 | 0.81* |
| Little/no physical activity (yes/no) | 0.54 | 0.30 | 0.69* | 0.96* |
| Body weight | ||||
| Underweight (BMI ≤ 18.5) | 0.02 | 0.01 | 0.02* | 0.03* |
| Normal weight (BMI = 18.6–24.9) | 0.34 | 0.41 | 0.33* | 0.28* |
| Overweight (BMI = 25–29.9) | 0.39 | 0.43 | 0.39* | 0.35* |
| Obese (BMI ≥ 30) | 0.24 | 0.15 | 0.26* | 0.33* |
| Socioeconomic status | ||||
| Education (years) | 12.61 | 13.29 | 12.59* | 11.21* |
| Income (logged value) | 4.52 | 4.65 | 4.51* | 4.25* |
| Wealth (logged value) | 4.72 | 5.03 | 4.73* | 4.00* |
| Health behaviors | ||||
| Never smoked | 0.40 | 0.42 | 0.39* | 0.39* |
| Current smoker | 0.18 | 0.16 | 0.18* | 0.19* |
| Former smoker | 0.42 | 0.42 | 0.42 | 0.42 |
| Drinking alcohol (drinks per week) | 1.83 | 2.32 | 1.78* | 1.02* |
| Health conditions | ||||
| High blood pressure (yes/no) | 0.40 | 0.39 | 0.42* | 0.57* |
| Diabetes (yes/no) | 0.12 | 0.09 | 0.11* | 0.22* |
| Stroke (yes/no) | 0.05 | 0.03 | 0.05* | 0.14* |
| Heart condition (yes/no) | 0.18 | 0.21 | 0.17* | 0.36* |
| Arthritis (yes/no) | 0.46 | 0.40 | 0.47* | 0.76* |
Note. ADL = activities of daily living; BMI = body mass index; IADL = instrumental activities of daily living.
aEstimates are weighted to using respondent level sampling weights to account for the sample design in the HRS and to generalize findings to the community-dwelling older adult population.
bM is mean; standard errors = 0.00.
cPrefrail is 1 or 2 frailty risk factors.
dFrail is 2 or 3 frailty risk factors.
*p < .001.
Multivariate Results
As shown in Table 2, Model 1, each year increase in age is associated with a 22% (p ≤ .001) increase in functional limitations accounting for demographic conditions, SES, health behaviors, and health conditions. Women are 52% (p ≤ .001) more likely than men to experience functional limitations. Increased education, income, and wealth are associated with a reduced functional limitations rate. Compared to respondents who have never smoked, respondents who currently smoke are 34% (p ≤ .001) more likely to have functional limitations and respondents who formerly smoked are 19% (p ≤ .001) more likely to have functional limitations. Each unit increase in the number of drinks per week was associated with a reduced risk of functional limitations, and each unit increase in the number of health conditions reported was associated with a 36% (p ≤ .001) increase in functional limitations. Also in Model 1, being underweight, compared with normal weight, was associated with a 22% (p ≤ .001) increase in functional limitations; being overweight was associated with a 12% (p ≤ .001) increase in functional limitations; and being obese was associated with a 51% (p ≤ .001) increase in functional limitations, accounting for the other factors considered in the model.
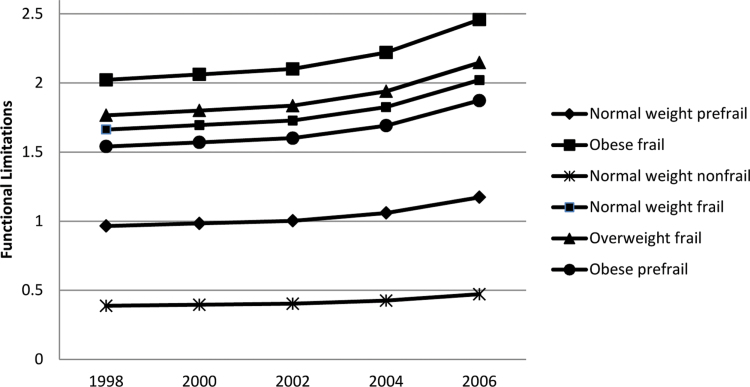
In Model 2, prefrailty and frailty are added to the functional limitations model and interactions between prefrailty, frailty, and bodyweight are examined. As shown in Model 2, prefrail respondents were more than twice as likely than nonfrail respondents to have functional limitations; frail respondents were more than four times as likely to have functional limitations. However, the relationship between prefrailty, frailty, and functional limitations varies by bodyweight. Compared with nonfrail normal weight respondents, prefrail obese respondents have a 16% reduction in the expected functional limitations rate, accounting for the other factors considered in the model. Similarly, among frail overweight and frail obese respondents, there is a significant 10% and 36% reduction in the expected functional limitations rate, respectively. Whereas obese and prefrail respondents remain at an increased risk for functional limitations, compared with their nonfrail normal weight counterparts, these respondents have a lower risk than would be expected given their obese and frail status (Figure 1). As shown at the bottom of Table 2 (Models 1 and 2), the addition of prefrailty and frailty to the model improved model fit, significantly reducing chi-square and explaining an additional 19% of the variance in functional limitations.
Figure 1.
The effects of bodyweight and frailty status on functional limitations by year (1998–2006).
In the IADL disability model, Model 3 (Table 2), increased age and being female were associated with a higher IADL disability rate, whereas increased SES was associated with a lower IADL disability rate, accounting for demographic conditions, SES, health behaviors, and health conditions. However, unlike the functional limitations model, in the IADL model being white and married were associated with a lower IADL disability rate. In Model 3, being underweight, compared with normal weight, was associated with a 51% (p ≤ .001) increase in IADL disabilities. However, being overweight or obese were associated with a reduced IADL disability rate, accounting for the other factors considered in the model. In Model 4, the addition of prefrailty and frailty statistically explained the relationship between body weight and IADL disability. In this model, prefrail respondents, compared to nonfrail respondents, were more than three times as likely to have IADL disability; frail respondents were more than seven times as likely to have IADL disability, accounting for demographic conditions, SES, health behaviors, and health conditions. As shown at the bottom of Table 2 (Models 3 and 4), the addition of prefrailty and frailty to the model improved model fit, significantly reducing chi-square and explaining an additional 31% of the variance in IADL disability.
In the ADL disability model, Model 5 (Table 2), increased age and being female were associated with a higher ADL disability rate, whereas being white (compared with non-white) and increased SES were associated with a lower ADL disability rate. Each unit increase in health conditions was associated with a 49% (p ≤ .001) increase in ADL disability. In this model, underweight or obese respondents were associated with a 52% (p ≤ .001) increase and a 32% (p ≤ .001) increase in ADL disability, respectively. In Model 6, when prefrailty and frailty were added to the ADL disability model, prefrail respondents were over seven times more likely to have ADL disability, compared to nonfrail respondents; frail respondents were over 24 times more likely to have ADL disability. The relationship between frailty and ADL disability varies by bodyweight. Compared with their nonfrail normal weight counterparts, frail obese respondents have a 27% (p ≤ .05) reduction in the expected ADL disability rate accounting for the other factors considered in the model. As with the functional limitations model, whereas obese frail respondents remain at an increased risk for ADL disability, these respondents have a lower risk than would be expected given their obese and frail status. As shown at the bottom of Table 2 (Models 5 and 6), the addition of prefrailty and frailty to the model improved model fit, significantly reducing chi-square and explaining an additional 51% of the variance in ADL disability.
There is a lack of evidence in the literature on differential weighting. The models presented assume each frailty item contributes similar to disability outcomes. To examine this assumption, supplementary analyses were conducted in HLM to examine the contributions of each frailty item. ERRs for each of the frailty items were comparable across disability models except for a fall, which had a somewhat weaker relationship, for example, in the IADL model, difficulty getting up from a chair (ERR = 2.4), fatigue (ERR = 2.3), little/no physical activity (ERR = 1.9), and a fall (ERR = 1.4).
Discussion
In this nationally representative prospective study of community-dwelling older adults, there was a gradient association between prefrailty, frailty, functional limitations, and disabilities in IADL and ADL independent of the other factors considered here. Most importantly for this study, the effect of prefrailty and frailty on the disablement process varied by bodyweight; when compared with nonfrail normal weight respondents, prefrail obese respondents were associated with a reduced rate of functional limitations as were frail overweight and obese respondents. Frail obese respondents were also associated with a reduced rate of ADL disability. This study’s findings are particularly strong given that this study accounted for intra-individual changes in body weight over the course of the study and the role of other disablement risk factors, including health behaviors and health conditions.
This study’s findings are consistent with previous work in this area, suggesting that frailty may be an independent stage in the disablement process, increasing the risk for functional limitations, IADL and ADL disabilities (Ensrud et al., 2008). Also consistent with previous work, this study’s findings support the idea that the overall risk for disablement increases with the number of frailty symptoms (Boyd et al., 2005). As such, strength, balance, endurance training, and exercise (Rubenstein et al., 2000) may be effective interventions, reducing the risk for disablement among the older adult population. In addition, this study’s findings confirm that being underweight, overweight (functional limitations only), or obese (functional limitations, ADL disability only) in later life increases the risk of disablement (Ferraro et al., 2002; Larrieu et al., 2004).
However, there may be some benefits to excess body weight, particularly among frail older adults. This may be because frail older adults who can maintain or gain weight—rather than lose weight (e.g., due to health conditions) benefit from the additional nutrients and energy, reducing their overall vulnerability to stressors (Villareal, Banks, Siener, Sinacore, & Klein, 2004). In addition, frail older adults with excess body weight have higher bone mineral density than their counterparts, reducing their risk for osteoporosis (Barrera et al., 2004), injurious falls, hip fractures (Vellas, Wayne, Garry, & Baumgartner, 1997), and other adverse events that may lead to functional limitations and disability. With respect to these findings, future work is needed to examine the exact “tipping” point at which weight gain or at least maintenance may be more important than weight loss for good health outcomes such as functional limitations and disability.
This study’s findings also suggest that being underweight is not a defining characteristic of frailty (Abate et al., 2007; Fried et al., 2001; Morley, 2008; Rolland et al., 2008). The relationship between being underweight and disability was statistically explained by the inclusion of frailty risk factors. Thus, this study’s findings support emergent work on sarcopenic obesity; overweight and obese older adults are also at risk for frailty with characteristics such as low muscle quality, strength, and fatigue (Launer, Harris, Rumpel, & Madans, 1994; Morley et al., 2005; Roubenoff, 2000, 2004). There may be another explanation for this study’s findings: the conceptualization and measurement of frailty used here and in other studies may not fully capture the increasing vulnerability of overweight and obese frail older adults. Future work is needed to examine the characteristics of the frail overweight and obese and the mechanisms through which frailty indicators may vary by body weight.
There are several limitations to consider when interpreting results. First, this study used an accumulation of deficits perspective (Rockwood & Mitnitski, 2007) to capture deficits across multiple systems (Lipsitz, 2002). Although this is a commonly used measure of frailty, it is notable that frailty definitions and measures vary across studies. Also, though the frailty index used in this study consisted of unweighted frailty items, frailty items may contribute differently to disability outcomes. There was little evidence for this in this study except for falls. This may be because information on falls in the HRS is limited to fall reports and does not capture fall-related injuries, which may be more strongly associated with disability outcomes. These results should be confirmed with additional data and future work addressing weighting issues may improve model predictability. Second, attrition is a concern in longitudinal health studies (Cao & Hill, 2005; Groves & Couper, 1998). In an attempt to account for this, proxy respondents and respondents scoring below the 10th percentile on learning and memory tests at baseline were excluded from this study. In addition, deceased respondents and respondents lost to follow-up were included in multilevel models to determine how attrition may affect results. Finally, this study used a representative sample of community-dwelling older adults who are at less risk for mortality than their institutionalized counterparts. Nevertheless, it remains that the healthiest adults are most likely to enter into and remain in the study over time, and this study’s findings may underestimate the relationship between frailty, body weight, and the disablement process.
Despite these limitations, the findings from this study are consistent with previous work indicating that interventions focused on strength, balance, endurance training, and exercise (Rubenstein et al., 2000) may reduce the risk for functional limitations and ADL disability in later life among prefrail/frail older adults (Boyd et al., 2005). Similarly, interventions to maintain or promote weight among frail older adults may be protective against further decline. Among older adults with no prefrailty/frailty risk factors, the maintenance of a healthy weight may help prevent functional limitations and disability.
F unding
Dr. M. E. Bowen is supported by a Rehabilitation Research and Development Career (RR&D) Development Award (CDA) from the Department of Veterans Affairs (VA). The Health and Retirement Study is sponsored by the National Institute on Aging (NIA U01AG009740). The data used in this article were made available by the Inter-University Consortium for Political and Social Research at the University of Michigan.
The author’s views are her own and do not necessarily represent the views of the VA.
Acknowledgments
The author would like to acknowledge Dr. Melville Bradley and Dr. Meredeth Rowe for their helpful comments on this article. The authors would also like to acknowledge the editor and anonymous reviewers at The Journals of Gerontology: Social Sciences for their valuable comments and suggestions.
References
- Abate M.,, Di Iorio A.,, Di Renzo D.,, Paganelli R.,, Saggini R.,, Abate G. (2007). Frailty in the elderly: The physical dimension. Europa Medicophysica 43 407–415 [PubMed] [Google Scholar]
- Adler N. E.,, Boyce T.,, Chesney M. A.,, Cohen S.,, Folkman S.,, Kahn R. L.,, Syme S. L. (1994). Socioeconomic status and health. The challenge of the gradient. The American Psychologist 49 15–24 [DOI] [PubMed] [Google Scholar]
- Barrera G.,, Bunout D.,, Gattás V.,, de la Maza M. P.,, Leiva L.,, Hirsch S. (2004). A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects. Nutrition 20 769–771 [DOI] [PubMed] [Google Scholar]
- Barzilay J. I.,, Blaum C.,, Moore T.,, Xue Q. L.,, Hirsch C. H.,, Walston J. D.,, Fried L. P. (2007). Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Archives of Internal Medicine 167 635–641 [DOI] [PubMed] [Google Scholar]
- Baumgartner R. N.,, Waters D. L.,, Gallagher D.,, Morley J. E.,, Garry P. J. (1999). Predictors of skeletal muscle mass in elderly men and women. Mechanisms of Ageing and Development 107 123–136 [DOI] [PubMed] [Google Scholar]
- Blaum C. S.,, Xue Q. L.,, Michelon E.,, Semba R. D.,, Fried L. P. (2005). The association between obesity and the frailty syndrome in older women: The Women’s Health and Aging Studies. Journal of the American Geriatrics Society 53 927–934 [DOI] [PubMed] [Google Scholar]
- Bowen M. E., Gonzalez H. M. (2010). Childhood socioeconomic position and disability in later life: Results of the health and retirement study American Journal of Public Health 100(Suppl. )S197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. M.,, Xue Q. L.,, Simpson C. F.,, Guralnik J. M.,, Fried L. P. (2005). Frailty, hospitalization, and progression of disability in a cohort of disabled older women. The American Journal of Medicine 118 1225–1231 [DOI] [PubMed] [Google Scholar]
- Boyle P. A.,, Buchman A. S.,, Wilson R. S.,, Leurgans S. E.,, Bennett D. A. (2010). Physical frailty is associated with incident mild cognitive impairment in community-based older persons. Journal of the American Geriatrics Society 58 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner D. M.,, Wagner E. H. (1992). Preventing frail health. Clinics in Geriatric Medicine 8 1–17 [PubMed] [Google Scholar]
- Bush T. L.,, Miller S. R.,, Golden A. L.,, Hale W. E. (1989). Self-report and medical record report agreement of selected medical conditions in the elderly. American Journal of Public Health 79 1554–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Hill D. H. Active versus passive sample attrition: The Health and Retirement Study Econometrics 0505006, EconWPA. Ann Arbor, MI: University of Michigan.; (2005). [Google Scholar]
- Clarke P.,, George L. K. (2005). The role of the built environment in the disablement process. American journal of public health 95 1933–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud K. E.,, Ewing S. K.,, Cawthon P. M.,, Fink H. A., Taylor B. C., Cauley J. A., Cummings S. R. (2009). A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatrics Society 57 492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud K. E.,, Ewing S. K.,, Taylor B. C.,, Fink H. A., Cawthon P. M., Stone K. L., Cummings S. R. (2008). Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Archives of Internal Medicine 168 382–389 [DOI] [PubMed] [Google Scholar]
- Ferraro K. F.,, Su Y. P.,, Gretebeck R. J.,, Black D. R.,, Badylak S. F. (2002). Body mass index and disability in adulthood: A 20-year panel study. American Journal of Public Health 92 834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried L. P.,, Guralnik J. M. (1997). Disability in older adults: Evidence regarding significance, etiology, and risk. Journal of the American Geriatrics Society 45 92–100 [DOI] [PubMed] [Google Scholar]
- Fried L. P.,, Tangen C. M.,, Walston J.,, Newman A. B., Hirsch C., Gottdiener J., McBurnie M. A. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 56 M146–M156 [DOI] [PubMed] [Google Scholar]
- Fried L. P.,, Xue Q. L.,, Cappola A. R.,, Ferrucci L., Chaves P., Varadhan R., Bandeen-Roche K. (2009). Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 64 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Thomson E.,, Yu B.,, Nuru-Jeter A.,, Guralnik J. M.,, Minkler M. (2009). Basic ADL disability and functional limitation rates among older AMERICANS from 2000-2005: The end of the decline? The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 64 1333–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R., Couper M. Nonresponse in household surveys. New York: John Wiley and Sons; (1998). [Google Scholar]
- Heeringa S. G., Connor J. (1995). Technical description of the Health and Retirement Study sample design: HRS/AHEAD documentation report DR-002
- Himes C. L. (2000). Obesity, disease, and functional limitation in later life. Demography 37 73–82 [PubMed] [Google Scholar]
- Jones D. M.,, Song X.,, Rockwood K. (2004). Operationalizing a frailty index from a standardized comprehensive geriatric assessment. Journal of the American Geriatrics Society 52 1929–1933 [DOI] [PubMed] [Google Scholar]
- Larrieu S.,, Péres K.,, Letenneur L.,, Berr C., Dartigues J. F., Ritchie K., Barberger-Gateau P. (2004). Relationship between body mass index and different domains of disability in older persons: The study. International Journal of Obesity 28 1555–1560 [DOI] [PubMed] [Google Scholar]
- Launer L. J.,, Harris T.,, Rumpel C.,, Madans J. (1994). Body mass index, weight change, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. Journal of the American Medical Association 271 1093–1098 [PubMed] [Google Scholar]
- Link B. G.,, Phelan J. (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior Spec No 80–94 [PubMed] [Google Scholar]
- Lipsitz L. A. (2002). Dynamics of stability: The physiologic basis of functional health and frailty. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 57 B115–B125 [DOI] [PubMed] [Google Scholar]
- Morley J. E. (2008). Diabetes, sarcopenia, and frailty. Clinics in Geriatric Medicine 24 455–69, vi [DOI] [PubMed] [Google Scholar]
- Morley J. E.,, Kim M. J.,, Haren M. T.,, Kevorkian R.,, Banks W. A. (2005). Frailty and the aging male. The Aging Male 8 135–140 [DOI] [PubMed] [Google Scholar]
- Payette H.,, Coulombe C.,, Boutier V.,, Gray-Donald K. (2000). Nutrition risk factors for institutionalization in a free-living functionally dependent elderly population. Journal of Clinical Epidemiology 53 579–587 [DOI] [PubMed] [Google Scholar]
- Raudenbush S. W., Bryk A. S. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage Publications; (2002). [Google Scholar]
- Rockwood K.,, Hogan D. B.,, MacKnight C. (2000). Conceptualisation and measurement of frailty in elderly people. Drugs & Aging 17 295–302 [DOI] [PubMed] [Google Scholar]
- Rockwood K.,, Mitnitski A. (2007). Frailty in relation to the accumulation of deficits. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 62 722–727 [DOI] [PubMed] [Google Scholar]
- Rolland Y.,, Czerwinski S.,, Abellan Van Kan G.,, Morley J. E., Cesari M., Onder G., Vellas B. (2008). Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. The Journal of Nutrition, Health & Aging 12 433–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. J.,, Klibanski A. (2009). Bone, fat, and body composition: Evolving concepts in the pathogenesis of osteoporosis. The American Journal of Medicine 122 409–414 [DOI] [PubMed] [Google Scholar]
- Roubenoff R. (2000). Sarcopenic obesity: Does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Annals of the New York Academy of Sciences 904 553–557 [DOI] [PubMed] [Google Scholar]
- Roubenoff R. (2004). Sarcopenic obesity: The confluence of two epidemics. Obesity Research 12 887–888 [DOI] [PubMed] [Google Scholar]
- Rubenstein L. Z.,, Josephson K. R.,, Trueblood P. R.,, Loy S.,, Harker J. O.,, Pietruszka F. M.,, Robbins A. S. (2000). Effects of a group exercise program on strength, mobility, and falls among fall-prone elderly men. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 55 M317–M321 [DOI] [PubMed] [Google Scholar]
- Vellas B. J.,, Wayne S. J.,, Garry P. J.,, Baumgartner R. N. (1997). A two-year longitudinal study of falls in 482 community-dwelling elderly adults. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 53 M264–M274 [DOI] [PubMed] [Google Scholar]
- Verbrugge L. M.,, Jette A. M. (1994). The disablement process. Social Science & Medicine 38 1–14 [DOI] [PubMed] [Google Scholar]
- Villareal D. T.,, Banks M.,, Siener C.,, Sinacore D. R.,, Klein S. (2004). Physical frailty and body composition in obese elderly men and women. Obesity Research 12 913–920 [DOI] [PubMed] [Google Scholar]
- Waters D. L.,, Hale L.,, Grant A. M.,, Herbison P.,, Goulding A. (2010). Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporosis International 21 351–357 [DOI] [PubMed] [Google Scholar]