Abstract
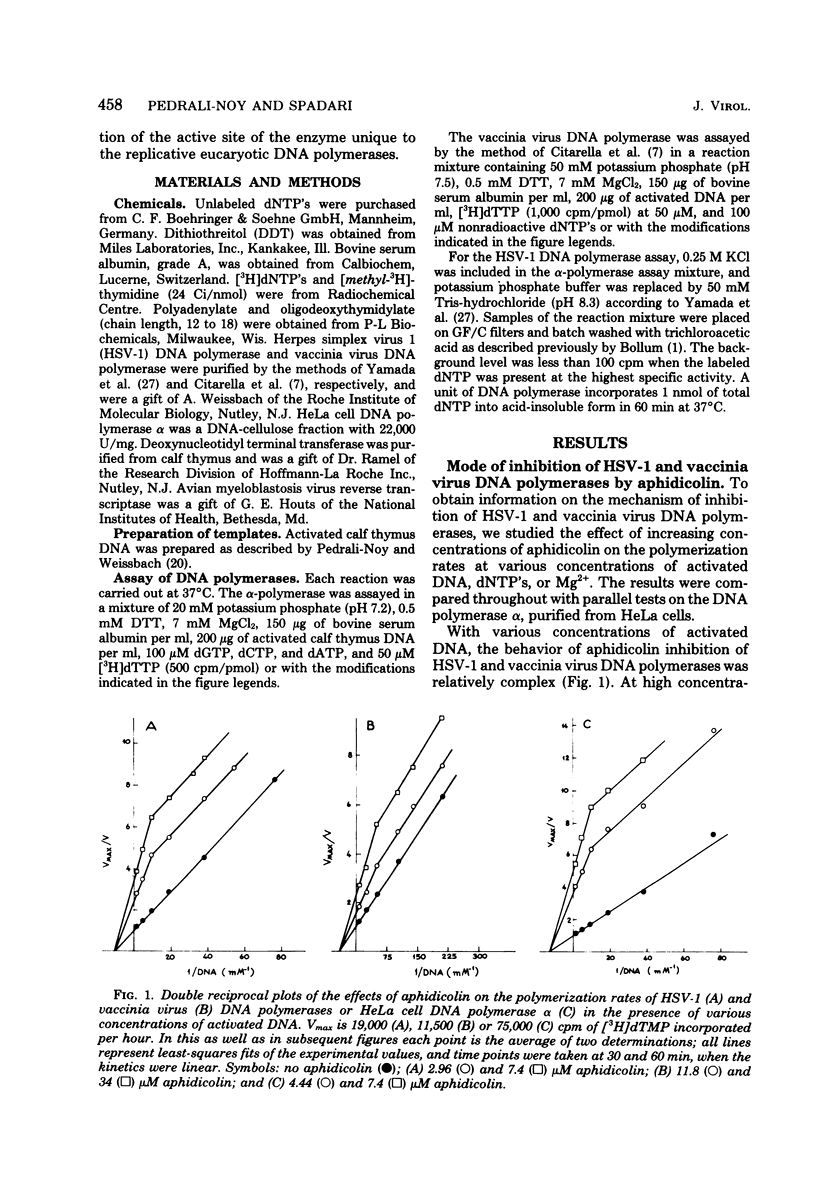
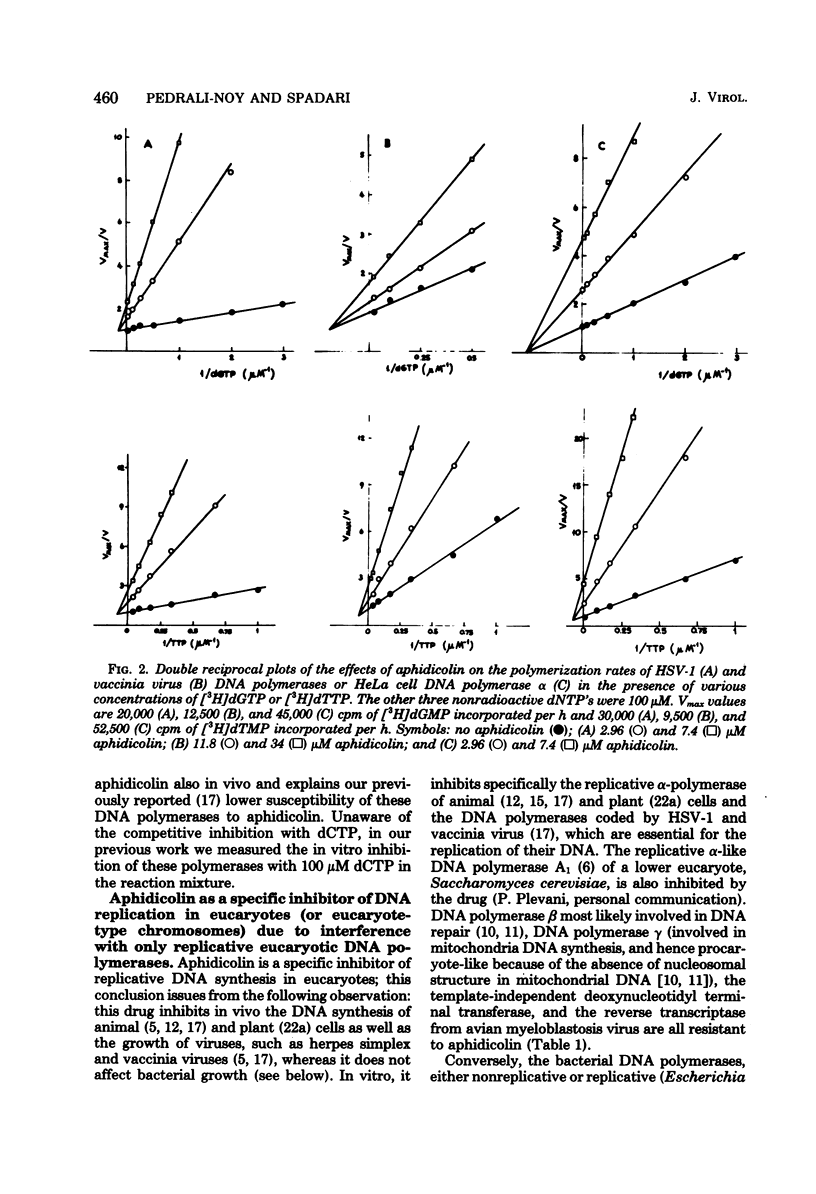
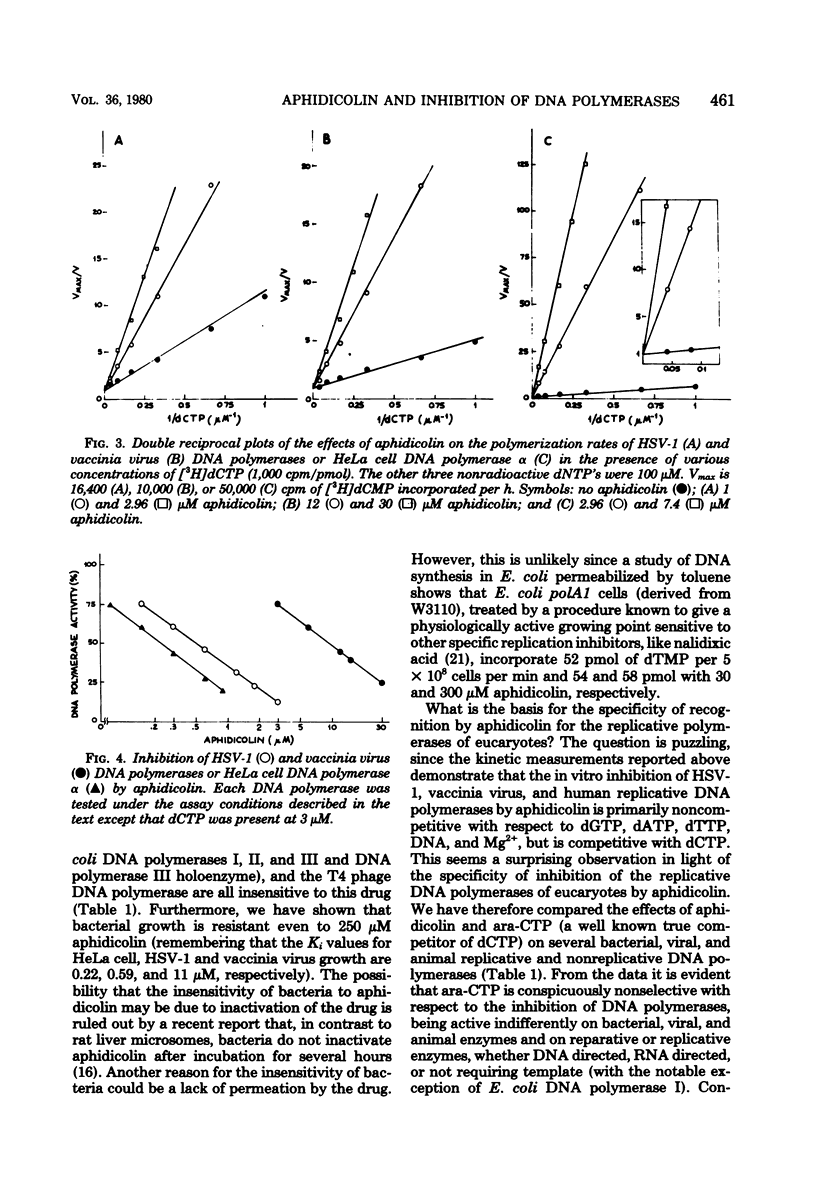
The inhibition in vitro of herpes simplex virus 1 and vaccinia virus DNA polymerases by aphidicolin is primarily noncompetitive with dGTP, dATP, dTTP, DNA, and Mg2+ and competitive with dCTP in analogy with the mode of inhibition of cellular alpha-polymerase. The degree of inhibition of viral or cellular growth in vivo can be quantitatively predicted by the degree of inhibition of the isolated replicative DNA polymerases at the same concentration of aphidicolin in suitable conditions (limiting dCTP concentration). Thus, the only in vivo target for aphidicolin is probably the replicative DNA polymerase, and aphidicolin is a highly specific inhibitor of replicative nuclear DNA synthesis in eucaryotes. This, coupled with the lack of mutagenic effect, represents a valuable property for an anticancer drug. The specificity of inhibition (contrary to the aspecific effect on almost all DNA polymerases by a true competitive inhibitor, such as 1-beta-D-arabinofuranosylcytidine 5'-triphosphate) and the structure of the drug, which does not resemble that of the triphosphates, suggest that aphidicolin must recognize a site common only to the replicative DNA polymerases of eucaryotes and different from the binding site for deoxyribonucleic triphosphates and DNA, which should be similar in reparative and procaryote-type DNA polymerase; the aphidicolin binding site is probably very near to, or even overlaping with, the binding site for dCTP so that the drug mimics a competitive effect with this nucleotide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Bollum F. J. Mammalian DNA polymerases. Prog Nucleic Acid Res Mol Biol. 1975;15(0):109–144. doi: 10.1016/s0079-6603(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Bray G., Brent T. P. Deoxyribonucleoside 5'-triphosphate pool fluctuations during the mammalian cell cycle. Biochim Biophys Acta. 1972 May 10;269(2):184–191. doi: 10.1016/0005-2787(72)90425-x. [DOI] [PubMed] [Google Scholar]
- Bucknall R. A., Moores H., Simms R., Hesp B. Antiviral effects of aphidicolin, a new antibiotic produced by Cephalosporium aphidicola. Antimicrob Agents Chemother. 1973 Sep;4(3):294–298. doi: 10.1128/aac.4.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Citarella R. V., Muller R., Schlabach A., Weissbach A. Studies on vaccinia virus-directed deoxyribonucleic acid polymerase. J Virol. 1972 Oct;10(4):721–729. doi: 10.1128/jvi.10.4.721-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicioccio R. A., Srivastava B. I. Kinetics of inhibition of deoxynucleotide-polymerizing enzyme activities from normal and leukemic human cells by 9-beta-D-arabinofuranosyladenine 5'-triphosphate and 1-beta-D-arabinofuranosylcytosine 5'-triphosphate. Eur J Biochem. 1977 Oct 3;79(2):411–418. doi: 10.1111/j.1432-1033.1977.tb11823.x. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Limacher W., Scherrer P., Spadari S. Functions of DNA polymerases alpha, beta, and gamma in neurons during development. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):625–629. doi: 10.1101/sqb.1979.043.01.069. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Functional roles of DNA polymerases beta and gamma. Proc Natl Acad Sci U S A. 1979 May;76(5):2316–2320. doi: 10.1073/pnas.76.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Noy G. P., Weissbach A. HeLa cell DNA polymerases: the effect of cycloheximide in vivo and detection of a new form of DNA polymerase alpha. Biochim Biophys Acta. 1977 Jul 5;477(1):70–83. doi: 10.1016/0005-2787(77)90161-7. [DOI] [PubMed] [Google Scholar]
- Oguro M., Shioda M., Nagano H., Mano Y., Hanaoka F., Yamada M. The mode of action of aphidicolin on DNA synthesis in isolated nuclei. Biochem Biophys Res Commun. 1980 Jan 15;92(1):13–19. doi: 10.1016/0006-291x(80)91512-0. [DOI] [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Ohashi M., Taguchi T., Ikegami S. Aphidicolin: a specific inhibitor of DNA polymerases in the cytosol of rat liver. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1084–1090. doi: 10.1016/0006-291x(78)90298-x. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Aphidicolin allows a rapid and simple evaluation of DNA-repair synthesis in damaged human cells. Mutat Res. 1980 May;70(3):389–394. doi: 10.1016/0027-5107(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Effect of aphidicolin on viral and human DNA polymerases. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1194–1202. doi: 10.1016/0006-291x(79)91106-9. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S., Miller-Faurès A., Miller A. O., Kruppa J., Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980 Jan 25;8(2):377–387. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini A. M., Geroldi D., Siccardi A., Falaschi A. Studies on the mode of action of nalidixic acid. Eur J Biochem. 1972 Feb 15;25(2):359–365. doi: 10.1111/j.1432-1033.1972.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Rama Reddy G. V., Goulian M., Hendler S. S. Inhibition of E. coli DNA polymerase II by ara-CTP. Nat New Biol. 1971 Dec 29;234(52):286–288. doi: 10.1038/newbio234286a0. [DOI] [PubMed] [Google Scholar]
- Sala F., Parisi B., Burroni D., Amileni A. R., Pedrali-Noy G., Spadari S. Specific and reversible inhibition by aphidicolin in the alpha-like DNA polymerase of plant cells. FEBS Lett. 1980 Aug 11;117(1):93–98. doi: 10.1016/0014-5793(80)80920-3. [DOI] [PubMed] [Google Scholar]
- Schrecker A. W., Smith R. G., Gallo R. C. Comparative inhibition of purified DNA polymerases from murine leukemia virus and human lymphocytes by 1-beta-D-arabinofuranosylcytosine 5'-triphosphate. Cancer Res. 1974 Feb;34(2):286–292. [PubMed] [Google Scholar]
- Waqar M. A., Evans M. J., Huberman J. A. Effect of 2',3'-dideoxythymidine-5'-triphosphate on HeLa cell in vitro DNA synthesis: evidence that DNA polymerase alpha is the only polymerase required for cellular DNA replication. Nucleic Acids Res. 1978 Jun;5(6):1933–1946. doi: 10.1093/nar/5.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]
- Yamada M., Brun G., Weissbach A. Synthesis of viral and host DNA in isolated chromatin from herpes simplex virus-infected HeLa cells. J Virol. 1978 May;26(2):281–290. doi: 10.1128/jvi.26.2.281-290.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Yamada M., Masaki S. Inhibition of DNA polymerase-alpha and -beta of calf thymus by 1-beta-D-arabinofuranosylcytosine-5'-triphosphate. Biochim Biophys Acta. 1977 Jul 15;477(2):144–150. doi: 10.1016/0005-2787(77)90230-1. [DOI] [PubMed] [Google Scholar]



