Abstract
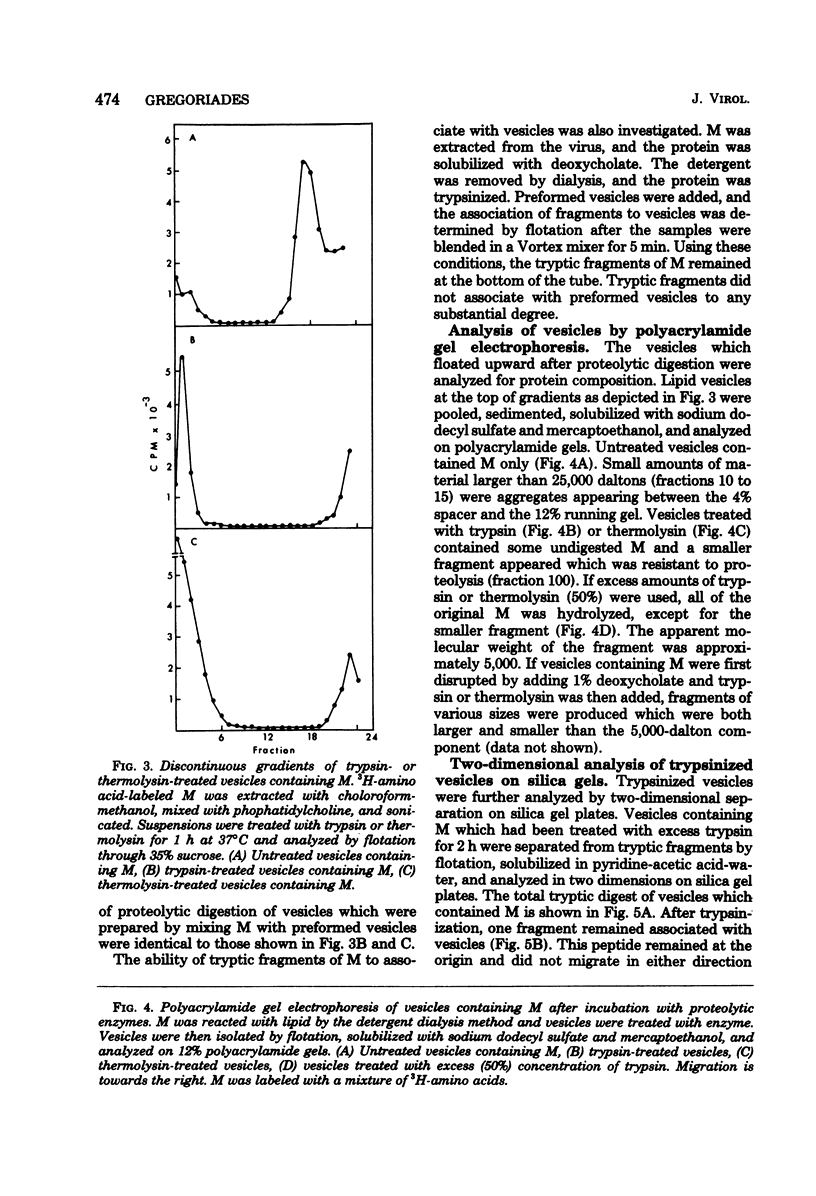
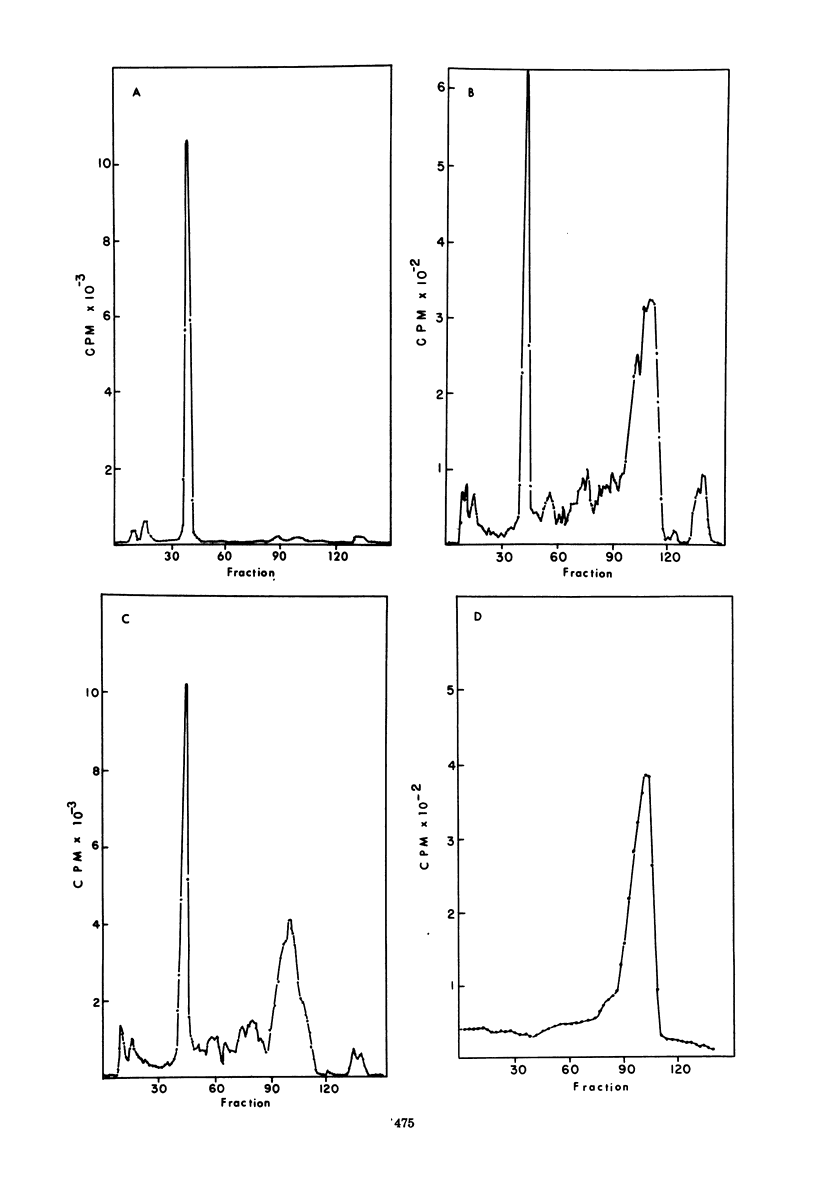
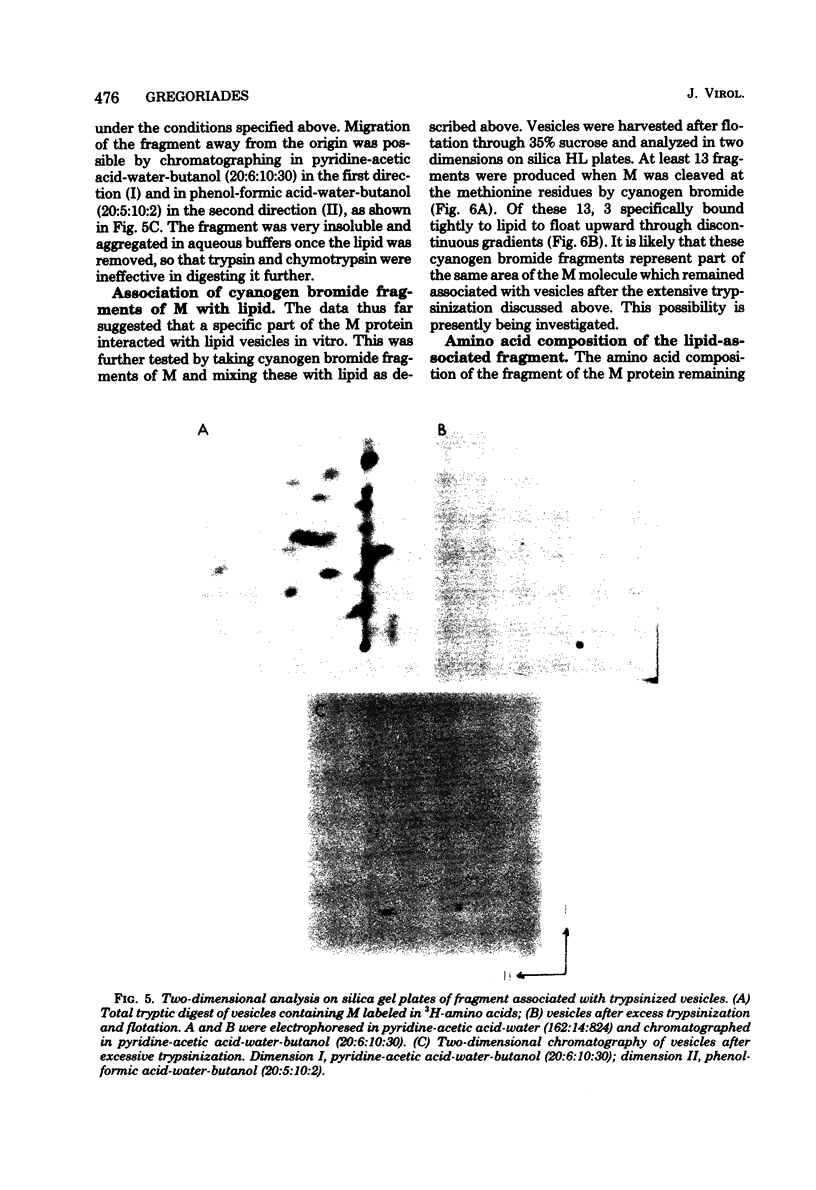
The M protein of influenza is the predominant structural component of the virus. The interactions of this protein with the viral lipid or with other proteins are not known. The ability of M to interact with viral or other lipids was investigated. Purified M was mixed with viral lipid or egg phosphatidylcholine and was incorporated into vesicles (i) by addition of sodium deoxycholate followed by dialysis or (ii) by sonication. Between 90 and 100% of the M became firmly associated with the lipid by either of these two methods, whereas nucleoprotein failed to associate with the vesicles. From association also occurred if M was mixed with performed vesicles. Most of the M attached to the vesicles could be hydrolyzed with proteolytic enzymes such as trypsin or thermolysin, except for a small fragment of about 5,000 daltons which remained associated with the lipid vesicles. The ability of fragments of M to interact with lipids was also investigated. Of 13 fragments produced by cleavage with cyanogen bromide, 3 specifically associated with lipid vesicles. The data indicate that a specific portion of the M molecule has a high affinity for lipid bilayers of various origins.
Full text
PDF

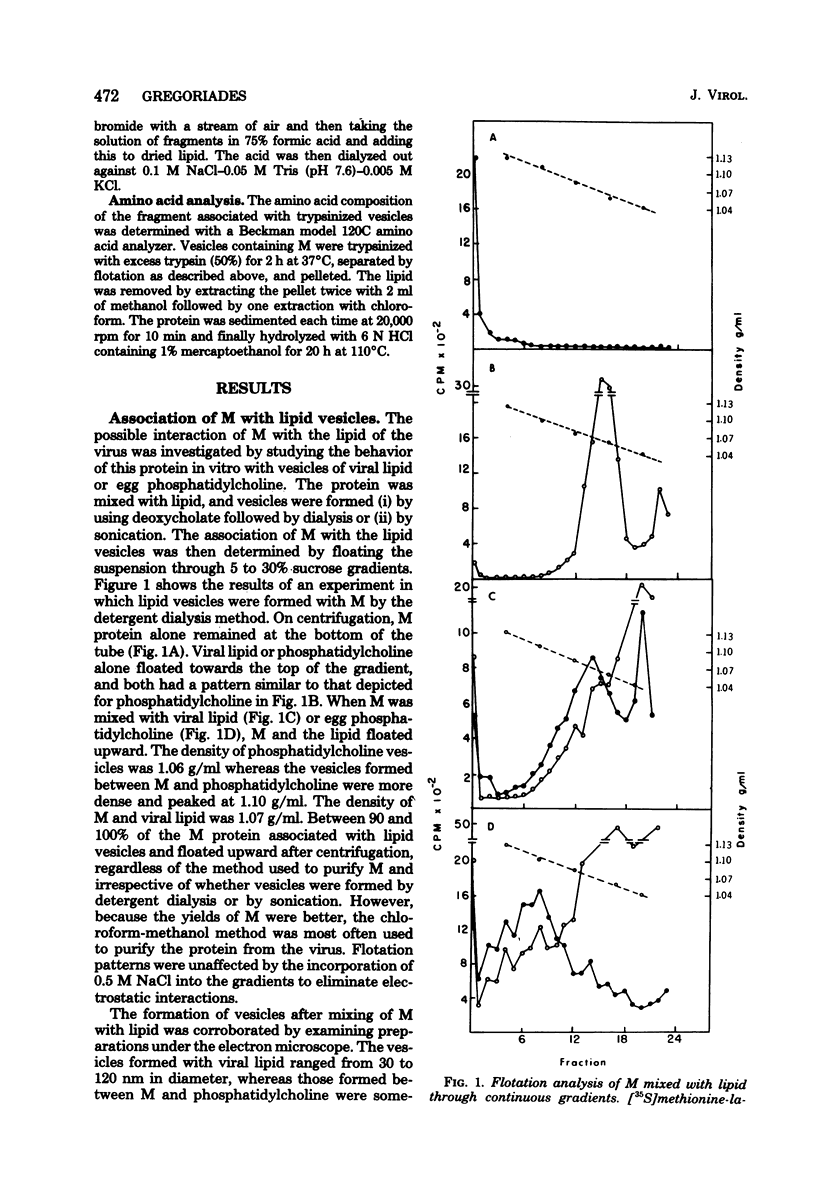
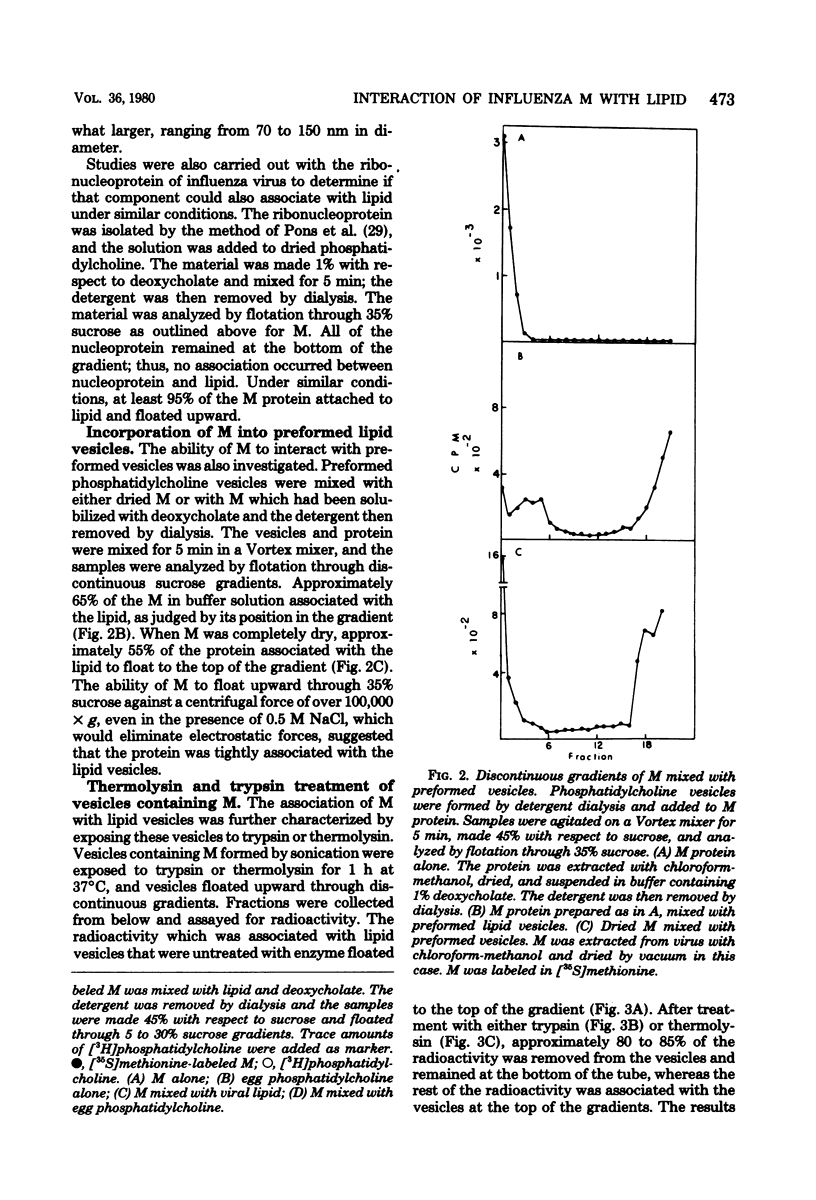



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Edwards D. C., Brand C. M., Heath T. D. Formation of virosomes from influenza subunits and liposomes. Lancet. 1975 Nov 8;2(7941):899–901. doi: 10.1016/s0140-6736(75)92130-3. [DOI] [PubMed] [Google Scholar]
- Apostolov K., Flewett T. H. Further observations on the structure of influenza viruses A and C. J Gen Virol. 1969 Apr;4(3):365–370. doi: 10.1099/0022-1317-4-3-365. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Doherty P. C., Webster R. G. Antibody to influenza virus matrix protein detects a common antigen on the surface of cells infected with type A influenza viruses. J Exp Med. 1977 Sep 1;146(3):690–697. doi: 10.1084/jem.146.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Air G. M. Nucleotide sequence coding for the N-terminal region of the matrix protein influenza virus. Eur J Biochem. 1979 May 15;96(2):363–372. doi: 10.1111/j.1432-1033.1979.tb13048.x. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Bucher D. J., Li S. S., Kehoe J. M., Kilbourne E. D. Chromatographic isolation of the hemagglutinin polypeptides from influenza virus vaccine and determination of their amino-terminal sequences. Proc Natl Acad Sci U S A. 1976 Jan;73(1):238–242. doi: 10.1073/pnas.73.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol. 1969 Nov;4(5):769–776. doi: 10.1128/jvi.4.5.769-776.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Engelhard V. H., Guild B. C., Helenius A., Terhorst C., Strominger J. L. Reconstitution of purified detergent-soluble HLA-A and HLA-B antigens into phospholipid vesicles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3230–3234. doi: 10.1073/pnas.75.7.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A., Hirst G. K. Mechanism of influenza recombination. III. Biochemical studies of temperature-sensitive mutants belonging to different recombination groups. Virology. 1976 Jan;69(1):81–92. doi: 10.1016/0042-6822(76)90196-3. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. Influenza virus-induced proteins in nuclei and cytoplasm of infected cells. Virology. 1977 Jun 15;79(2):449–454. doi: 10.1016/0042-6822(77)90372-5. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. The membrane protein of influenza virus: extraction from virus and infected cell with acidic chloroform-methanol. Virology. 1973 Aug;54(2):369–383. doi: 10.1016/0042-6822(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). I. Assembly of hemolytic and fusion factors from envelopes solubilized by Nonidet P40. Virology. 1972 Sep;49(3):627–639. doi: 10.1016/0042-6822(72)90519-3. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Association of the envelope glycoproteins of influenza virus with liposomes--a model study on viral envelope assembly. Virology. 1979 Aug;97(1):212–217. doi: 10.1016/0042-6822(79)90390-8. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. X. Correlation of morphology and function in submitochondrial particles. J Biol Chem. 1966 May 25;241(10):2475–2482. [PubMed] [Google Scholar]
- Laver W. G., Baker N. Amino acid composition of polypeptides from influenza virus particles. J Gen Virol. 1972 Oct;17(1):61–67. doi: 10.1099/0022-1317-17-1-61. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Haslam E. A., White D. O. The polypeptides of influenza virus. VI. Composition of the neuraminidase. Virology. 1972 Sep;49(3):758–765. doi: 10.1016/0042-6822(72)90532-6. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Cullen S. E., Schwartz B. D. Insertion of Ia and H-2 alloantigens into model membranes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):902–906. doi: 10.1073/pnas.76.2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno K., Yoshii S., Yoshida T., Iinuma M., Kawamoto Y. Intracellular development of membrane protein of influenza virus. Microbiol Immunol. 1977;21(8):427–438. doi: 10.1111/j.1348-0421.1977.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. Further investigation on the fine structure of influenza virus. J Gen Virol. 1972 Dec;17(3):317–331. doi: 10.1099/0022-1317-17-3-317. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., Schild G. C. Immunological and physicochemical studies of influenza matrix (M) polypeptides. Virology. 1976 Oct 15;74(2):394–402. doi: 10.1016/0042-6822(76)90345-7. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979 Jun 10;254(11):4313–4316. [PubMed] [Google Scholar]
- Pons M. W., Schulze I. T., Hirst G. K., Hauser R. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969 Oct;39(2):250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Reginster M., Rentier B., Dierickx L. Impairment of the M-protein and unmasking of a superficial type-specific antigen by proteolytic treatment of influenza A virions with preservation of host-specific antigenicity. Intervirology. 1975;6(4-5):239–248. doi: 10.1159/000149478. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Compans R. W., Reich E. A specific labeling procedure for proteins on the outer surface of membranes. J Biol Chem. 1972 Oct 25;247(20):6432–6437. [PubMed] [Google Scholar]
- Robertson B. H., Bhown A. S., Compans R. W., Bennett J. C. Structure of the membrane protein of influenza virus. I. Isolation and characterization of cyanogen bromide cleavage products. J Virol. 1979 Jun;30(3):759–766. doi: 10.1128/jvi.30.3.759-766.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Schwarz H., Hunsmann G. Rosettes from Friend leukemia virus envelope: preparation and physicochemical and partial biological characterization. J Virol. 1979 Feb;29(2):624–632. doi: 10.1128/jvi.29.2.624-632.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972 Jan;47(1):181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Haslam E. A. The polypeptides of influenza virus. V. Localization of polypeptides in the virion by iodination techniques. Virology. 1971 Dec;46(3):764–773. doi: 10.1016/0042-6822(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: relationship of the envelope to virus assembly. Virology. 1976 Nov;75(1):242–255. doi: 10.1016/0042-6822(76)90023-4. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G., Skehel J. J., Charlwood P. A., Brand C. M. The size and shape of influenza virus neuraminidase. Virology. 1973 Feb;51(2):525–529. doi: 10.1016/0042-6822(73)90457-1. [DOI] [PubMed] [Google Scholar]