Abstract
Background
Proton radiotherapy (PRT) is an emerging treatment for prostate cancer despite limited knowledge of clinical benefit or potential harms compared with other types of radiotherapy. We therefore compared patterns of PRT use, cost, and early toxicity among Medicare beneficiaries with prostate cancer with those of intensity-modulated radiotherapy (IMRT).
Methods
We performed a retrospective study of all Medicare beneficiaries aged greater than or equal to 66 years who received PRT or IMRT for prostate cancer during 2008 and/or 2009. We used multivariable logistic regression to identify factors associated with receipt of PRT. To assess toxicity, each PRT patient was matched with two IMRT patients with similar clinical and sociodemographic characteristics. The main outcome measures were receipt of PRT or IMRT, Medicare reimbursement for each treatment, and early genitourinary, gastrointestinal, and other toxicity. All statistical tests were two-sided.
Results
We identified 27,647 men; 553 (2%) received PRT and 27,094 (98%) received IMRT. Patients receiving PRT were younger, healthier, and from more affluent areas than patients receiving IMRT. Median Medicare reimbursement was $32,428 for PRT and $18,575 for IMRT. Although PRT was associated with a statistically significant reduction in genitourinary toxicity at 6 months compared with IMRT (5.9% vs 9.5%; odds ratio [OR] = 0.60, 95% confidence interval [CI] = 0.38 to 0.96, P = .03), at 12 months post-treatment there was no difference in genitourinary toxicity (18.8% vs 17.5%; OR = 1.08, 95% CI = 0.76 to 1.54, P = .66). There was no statistically significant difference in gastrointestinal or other toxicity at 6 months or 12 months post-treatment.
Conclusions
Although PRT is substantially more costly than IMRT, there was no difference in toxicity in a comprehensive cohort of Medicare beneficiaries with prostate cancer at 12 months post-treatment.
Over the past decade, intensity modulated radiotherapy (IMRT) has become the standard form of radiotherapy for the treatment of prostate cancer, accounting for more than 80% of all radiotherapy (1). Even as IMRT has been widely adopted, other radiotherapy modalities have come to market, most notably proton radiotherapy (PRT). Although PRT predates IMRT, dissemination of PRT has been increasing rapidly in recent years. In part because of its high capital cost, Medicare is reported to reimburse PRT at a rate 1.4 to 2.5 times that of IMRT (2–4), despite many unexplored questions.
First, there is a lack of data regarding national patterns of use and the true cost of PRT among Medicare beneficiaries. Currently, there are only nine PRT centers in operation in the United States (5), and this relatively low treatment capacity limits costs. However, eight other centers are in development (5), along with smaller and more affordable proton machines (6), conceivably opening the door to more widespread adoption of PRT across the country.
Second, the Institute for Clinical and Economic Review concluded unanimously that the state of current knowledge of comparative clinical effectiveness was “insufficient” (7,8). Because differences in cancer cure rates and survival from prostate cancer treatment often take many years to become evident, it has been suggested that initial study of prostate cancer treatments should focus on treatment-related toxicity (8). Proponents of PRT argue that the physical properties of protons may decrease the most common side effects associated with prostate radiotherapy—gastrointestinal and genitourinary toxicity (9). Early outcomes from single-arm, prospective trials investigating PRT are forthcoming, indicating low levels of radiation-induced toxicity with early follow-up (10,11). However, IMRT itself has a robust literature describing excellent efficacy and low toxicity in the treatment of prostate cancer (12). Therefore, it is unclear that PRT offers a statistically significant benefit beyond IMRT. Prior studies investigating PRT in Medicare beneficiaries using the Surveillance, Epidemiology, and End Results–Medicare database have been single-institution studies (13,14) and, therefore, are not of the whole country. These studies (13,14) noted a statistically significant reduction of gastrointestinal toxicity for patients undergoing IMRT compared with PRT. A comprehensive comparison of PRT with IMRT requires examination of the entire country for the most recent years available.
As more PRT centers become operational, it will be crucial for patients, providers, and policy makers to understand the cost and national pattern of adoption of PRT and the incidence of treatment-related toxicity compared with IMRT. Therefore, we used a national sample of Medicare beneficiaries with prostate cancer to investigate the patterns and cost of PRT delivery, as well as the early treatment-related toxicity associated with PRT compared with IMRT.
Methods
Data Source and Study Sample
Our data source was the Chronic Condition Warehouse, a comprehensive national database of 100% of Medicare fee-for-service claims for patients with specific chronic conditions (5). Health system characteristics at the level of hospital referral region (HRR) were obtained from the Dartmouth Atlas of Healthcare (15,16). HRRs are geographical units representing regional healthcare markets. The Yale Human Investigation Committee determined that this study did not constitute human subjects research.
Using Medicare claims from 2008 and 2009, we identified a sample of early-stage, treated prostate cancer patients aged 66 to 94 years using a multistep algorithm (Supplementary Figure 1, available online). Only patients who received IMRT or PRT as primary treatment were included. Treatment date was assigned as the date of first radiation treatment. We excluded patients who did not have Medicare Parts A and B fee-for-service coverage from 9 months prior through 3 months after treatment date to ensure completeness of claims.
For the analysis of treatment selection, we included all patients. For the analysis of 6-month toxicity and costs, we included only patients who initiated radiotherapy prior to July 1, 2009, and had Medicare Parts A and B fee-for-service coverage for 6 months post-treatment. For the analysis of 12-month toxicity, we included only patients who initiated radiotherapy prior to January 1, 2009, and had coverage for 12 months post-treatment.
Construction of Variables
We used Healthcare Common Procedure Coding System codes to identify the type of radiotherapy received. Patients were assigned to the PRT group if there were any codes for PRT delivery; patients were assigned to the IMRT group if there were four or more codes for IMRT treatment delivery or if they had the IMRT treatment planning code (77301) in addition to four or more generic external beam treatment delivery codes.
The cost of IMRT or PRT treatment was calculated for each patient using the sum of Medicare reimbursements for all outpatient and physician claims with Healthcare Common Procedure Coding System codes indicative of radiotherapy, including treatment planning, management, and delivery in the 3 months following initiation of radiotherapy.
Using insights from prior studies investigating Medicare claims for prostate cancer treatment-related toxicity (17–22), we rigorously compiled a list of potential treatment-related toxicity (Supplementary Table 1, available online), specifically excluding codes that were thought to be due to surgical complications. We searched claims for Healthcare Common Procedure Coding System or International Classification of Diseases, 9th revision diagnosis or procedure codes associated with the following categories of toxicity, which were constructed a priori: genitourinary (infection, upper urinary tract dysfunction, urethral stricture/obstruction, incontinence, erectile dysfunction); gastrointestinal (fistula, rectal repair, stenosis, bowel resection, other); and other toxicity (local musculoskeletal damage, red blood cell transfusion, systemic derangements, infection, nerve injury, and fractures). Some codes may be indicative of preexisting conditions and be unrelated to treatment; if a patient had one of these codes after treatment but also had evidence of the code in the 9 months prior to treatment, we did not count it as a complication. Our outcome was whether a patient had a complication between 0 and 6 months or 0 and 12 months after start of treatment.
Patient characteristics included age, race, year of treatment, residence in a metropolitan county, median household income at the zip code level, and distance to the nearest proton center. Because access to primary care may be an important factor in the development and reporting of toxicity, receipt of an influenza vaccination or visit to a primary care provider in the 9 months prior to treatment was recorded. The use of androgen deprivation therapy (ADT) was assessed by adapting algorithms used in prior studies (Supplementary Table 2, available online) (23). We identified comorbidities by searching inpatient, outpatient, and physician claims billed between 9 months and 1 month prior to treatment date for specific International Classification of Diseases, 9th revision diagnosis codes that appeared on at least one inpatient claim or two or more outpatient/physician claims billed more than 30 days apart. Using the comorbidity categories outlined by Elixhauser et al. (24), we looked for conditions that we had previously found were statistically significantly associated with survival in a sample of noncancer patients.
Each patient was assigned to a HRR based on their zip code. HRR-level variables included the number of discharges for ambulatory care sensitive conditions, number of available acute care beds, and density of primary care providers and radiation oncologists. We created a variable indicating whether each patient resided in a state that required a certificate of need prior to expansion or creation of new radiation facilities, used by some states to restrict unnecessary or redundant increases in healthcare services and facilities.
Statistical Analysis
We used χ2 tests to assess bivariate associations between the independent variables and receipt of PRT. We used random effects logistic regression with clustering by HRR to investigate the unadjusted and adjusted associations between covariates and use of PRT versus IMRT. To illustrate the travel patterns of patients receiving PRT, we created a map with lines going from the centroid of patients’ home states to the PRT center at which treatment was administered. We only included lines representing two or more patients.
Usual techniques to account for treatment selection bias, such as instrumental variables and propensity score matching, were computationally problematic because of the small number of PRT patients. To adjust for treatment selection bias from known confounders in our analyses of cost and toxicity outcomes, we used Mahalanobis matching (25). Matching was based on the Mahalanobis distance calculated using age, race, residence in a metropolitan county, comorbidity, receipt of ADT, prior influenza vaccination or prior visit to a primary care physician (both as proxies for access to care), and income. Matches were assigned by choosing the two best IMRT patient matches for each PRT patient; when two or more PRT patients matched the same control (that is, had Mahalanobis distance minimized by the same control), one was randomly selected as a match, with this process reiterated until all PRT patients had two matched controls. Matching was done separately for patients with 6-month and 12-month follow-up. We assessed the validity of the matching by comparing risk factors between the PRT and IMRT groups using χ2 tests.
To compare the relative cost of PRT and IMRT, we calculated the median and interquartile range (IQR) of Medicare reimbursement for patients in the matched 6-month complication sample. To estimate the effect of PRT on outcomes, we estimated a conditional logit model for the 6- and 12-month complication samples, including an indicator for whether the patient was treated with PRT or IMRT. Because Mahalonobis matching takes into account all known variables simultaneously to match appropriate IMRT patients with PRT patients, there were slight imbalances between individual variables after the matching. Therefore each model was conditioned on the matched grouping and adjusted for factors that were not perfectly balanced between groups (age, race, comorbidity, and use of ADT).
All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC), Stata version 12.1 (StataCorp, College Station, TX), and ArcMap version 10 (ESRI, Redlands, CA).
Results
Patterns of Care and Costs
We identified 27,647 patients who received either IMRT (n = 27,094; 98.0%) or PRT (n = 553; 2.0%) during the study period (Table 1). Patients who were aged 66 to 69 years were three times as likely to receive PRT than those aged 85 to 94 years (3.3% vs 1.0%; P < .001). White patients were more likely to receive PRT than black patients (2.2% vs 0.5%; P < .001). Patients with no comorbidity were statistically significantly more likely to receive PRT than those with three or more comorbidities (2.6% vs 0.8%; P < .001). Patients who received ADT were less likely to have received PRT than those who did not receive ADT (1.0% vs 2.9%; P < .001).
Table 1.
Distribution of overall sample and percent who received proton radiotherapy (PRT) and distribution of matching variables in the 12-month toxicity sample*
| Characteristics | Overall sample | 12-month toxicity sample | |||||
|---|---|---|---|---|---|---|---|
| Received PRT | PRT | IMRT | |||||
| No. | % | % | P† | No. (%) | No. (%) | P† | |
| Overall | 27,647 | 100 | 2.0 | 314 (33.3) | 628 (66.7) | ||
| Patient characteristics | |||||||
| Age, y | <.001 | 1.00 | |||||
| 66–69 | 6232 | 22.5 | 3.3 | 114 (36.3) | 228 (36.3) | ||
| 70–74 | 9735 | 35.2 | 2.1 | 115 (36.6) | 230 (36.6) | ||
| 75–79 | 7886 | 28.5 | 1.4 | 64 (20.4) | 128 (20.4) | ||
| 80–84 | 3112 | 11.3 | 1.0 | >10 (>3.2)‡ | >31 (>4.9)‡ | ||
| 85–94 | 682 | 2.5 | <1.6‡ | <11 (<3.5)‡ | <11 (<1.8)‡ | ||
| Race | <.001 | 1.00 | |||||
| White | 23,696 | 85.7 | 2.2 | 292 (93.0) | 584 (93.0) | ||
| Black | 2801 | 10.1 | 0.5 | <11 (<3.5)‡ | 18 (2.9) | ||
| Other | 1150 | 4.2 | 1.8 | >11 (>3.5)‡ | 26 (4.1) | ||
| Year of treatment | .20 | — | |||||
| 2008 | 16,628 | 60.1 | 1.9 | — | — | ||
| 2009 | 11,019 | 39.9 | 2.1 | — | — | ||
| Residence in metro county | <.001 | .86 | |||||
| Yes | 21,665 | 78.4 | 2.1 | 249 (79.3) | 503 (80.1) | ||
| No§ | 5982 | 21.6 | 1.6 | 65 (20.7) | 125 (19.9) | ||
| Median household income | <.001 | 1.00 | |||||
| Q1 (≤$31,848) | 5300 | 19.2 | 1.2 | 35 (11.1) | 70 (11.1) | ||
| Q2 ($31,849–$38,040) | 5305 | 19.2 | 2.1 | 65 (20.7) | 130 (20.7) | ||
| Q3 ($38,044–$45,494) | 5303 | 19.2 | 2.0 | 65 (20.7) | 130 (20.7) | ||
| Q4 ($45,495–$57,284) | 5302 | 19.2 | 1.9 | 51 (16.2) | 102 (16.2) | ||
| Q5 (≥$57,294) | 5302 | 19.2 | 2.3 | 68 (21.7) | 135 (21.5) | ||
| Unknown | 1135 | 4.1 | 4.3 | 30 (9.6) | 61 (9.7) | ||
| Distance to nearest proton center, miles | <.001 | — | |||||
| <75 | 2737 | 9.9 | 4.9 | — | — | ||
| 75–500 | 23,116 | 83.6 | 1.5 | — | — | ||
| >500 | 1751 | 6.3 | 4.2 | — | — | ||
| Unknown | 43 | 0.2 | <25.6‡ | — | — | ||
| Overall sample | 12-month complications sample | ||||||
| Received PRT | PRT | IMRT | |||||
| No. | % | % | P† | No. (%) | No. (%) | P† | |
| Clinical characteristics | |||||||
| Comorbidity | <.001 | .99 | |||||
| 0 conditions | 15,660 | 56.6 | 2.6 | 231 (73.6) | 461 (73.4) | ||
| 1–2 conditions | 9984 | 36.1 | 1.3 | >72 (>22.9)‡ | 146 (23.2) | ||
| ≥3 conditions | 2003 | 7.2 | 0.8 | <11 (<3.5)‡ | 21 (3.3) | ||
| Receipt of androgen-deprivation therapy | <.001 | .91 | |||||
| No | 15,213 | 55.0 | 2.9 | 249 (79.3) | 496 (79.0) | ||
| Yes | 12,434 | 45.0 | 1.0 | 65 (20.7) | 132 (21.0) | ||
| Receipt of influenza vaccination (9 months prior to start of radiation) | <.001 | 1.00 | |||||
| No | 18,557 | 67.1 | 2.3 | 249 (79.3) | 498 (79.3) | ||
| Yes | 9090 | 32.9 | 1.4 | 65 (20.7) | 130 (20.7) | ||
| Visit to primary care physician (9 months prior to start of radiation) | .32 | 1.00 | |||||
| No | 539 | 2.0 | 2.6 | <11 (<3.5)‡ | 14 (2.2) | ||
| Yes | 27,108 | 98.1 | 2.0 | >303 (>96.5)‡ | 614 (97.8) | ||
| Health system characteristics | |||||||
| State certificate of need for radiation facility | <.001 | — | |||||
| No | 16,099 | 58.2 | 2.7 | — | — | ||
| Yes | 11,548 | 41.8 | 1.0 | — | — | ||
* Results were similar for the 6-month toxicity sample. IMRT = intensity-modulated radiotherapy; — = variables were not used in the matching.
† P value calculated using χ2 test. All P values are two-sided.
‡ Actual value is not provided because of the Centers for Medicare and Medicaid Services prohibition of publication of groups of less than 11 patients.
§ Category also includes less than 11 patients with unknown metropolitan residence because of the Centers for Medicare and Medicaid Services privacy regulation.
In the adjusted analysis, patients who were younger, white, and had less comorbidity were more likely to receive PRT (Table 2) than others. Geographically, patients from more affluent areas and from states not requiring a certificate of need were more likely to receive PRT. No HRR-level characteristics were associated with receipt of PRT. Patients who received an influenza vaccination in the 9 months prior to treatment were also less likely to receive PRT than those who did not receive an influenza vaccination (odds ratio [OR] = 0.63, 95% confidence interval [CI] = 0.52 to 0.78).
Table 2.
Odds ratios (ORs) for receipt of proton radiotherapy*
| Characterisitics | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P† | OR | 95% CI | P† | |
| Patient characteristics | ||||||
| Age, y | <.001 | <.001 | ||||
| 66–69 | 1.00 (referent) | — | 1.00 (referent) | — | ||
| 70–74 | 0.63 | 0.51 to 0.77 | 0.66 | 0.53 to 0.80 | ||
| 75–79 | 0.43 | 0.34 to 0.54 | 0.45 | 0.35 to 0.57 | ||
| 80–84 | 0.28 | 0.19 to 0.42 | 0.33 | 0.22 to 0.48 | ||
| 85–94 | 0.12 | 0.04 to 0.39 | 0.15 | 0.05 to 0.46 | ||
| Race | <.001 | <.001 | ||||
| White | 1.00 (referent) | — | 1.00 (referent) | — | ||
| Black | 0.22 | 0.12 to 0.38 | 0.22 | 0.13 to 0.39 | ||
| Other | 0.61 | 0.38 to 0.97 | 0.64 | 0.40 to 1.10 | ||
| Year of treatment | .43 | |||||
| 2008 | 1.00 (referent) | — | ||||
| 2009 | 1.07 | 0.90 to 1.28 | ||||
| Residence in metro county | .048 | .42 | ||||
| Yes | 1.00 (referent) | — | 1.00 (referent) | — | ||
| No | 0.78 | 0.61 to 0.998 | 0.90 | 0.68 to 1.17 | ||
| Median household income | <.001 | <.001 | ||||
| Q1 (≤$31,848) | 1.00 (referent) | — | 1.00 (referent) | — | ||
| Q2 ($31,849–$38,040) | 1.66 | 1.20 to 2.29 | 1.38 | 0.995 to 1.91 | ||
| Q3 ($38,044–$45,494) | 1.65 | 1.19 to 2.28 | 1.32 | 0.94 to 1.86 | ||
| Q4 ($45,495–$57,284) | 1.62 | 1.16 to 2.26 | 1.18 | 0.83 to 1.69 | ||
| Q5 (≥$57,294) | 2.20 | 1.59 to 3.07 | 1.55 | 1.09 to 2.22 | ||
| Unknown | 2.85 | 1.92 to 4.22 | 2.42 | 1.60 to 3.66 | ||
| Distance to nearest proton center, miles | <.001 | .002 | ||||
| <75 | 1.00 (referent) | — | 1.00 (referent) | — | ||
| 75–500 | 0.54 | 0.36 to 0.82 | 0.61 | 0.40 to 0.92 | ||
| >500 | 1.59 | 0.92 to 2.74 | 1.14 | 0.64 to 2.05 | ||
| Unknown | 0.48 | 0.06 to 3.73 | 0.21 | 0.03 to 1.77 | ||
| Clinical characteristics | ||||||
| Comorbidity | <.001 | <.001 | ||||
| 0 conditions | 1.00 (referent) | — | 1.00 (referent) | — | ||
| 1–2 conditions | 0.47 | 0.38 to 0.58 | 0.52 | 0.43 to 0.64 | ||
| ≥3 conditions | 0.28 | 0.16 to 0.47 | 0.34 | 0.20 to 0.57 | ||
| Receipt of androgen deprivation therapy | <.001 | <.001 | ||||
| No | 1.00 (referent) | — | 1.00 (referent) | — | ||
| Yes | 0.33 | 0.27 to 0.41 | 0.38 | 0.31 to 0.47 | ||
| Flu shot (9 months prior to start of radiation) | <.001 | <.001 | ||||
| No | 1.00 (referent) | — | 1.00 (referent) | — | ||
| Yes | 0.63 | 0.51 to 0.77 | 0.63 | 0.52 to 0.78 | ||
| Visit to primary care physician (9 months prior to start of radiation) | .43 | |||||
| No | 1.00 (referent) | — | ||||
| Yes | 0.80 | 0.46 to 1.39 | ||||
| Health system characteristics | ||||||
| State certificate of need for radiation facility | <.001 | <.001 | ||||
| No | 1.00 (referent) | — | 1.00 (referent) | — | ||
| Yes | 0.41 | 0.31 to 0.56 | 0.53 | 0.39 to 0.71 | ||
| Discharges for ambulatory care sensitive conditions per 1000 Medicare enrollees | 0.98 | 0.97 to 0.99 | <.001 | 0.99 | 0.98 to 1.01 | .41 |
| Acute care hospital beds per 1000 residents | 0.52 | 0.39 to 0.69 | <.001 | 0.79 | 0.53 to 1.17 | .23 |
| Primary care providers per 100,000 residents | 0.99 | 0.98 to 1.004 | .17 | |||
| Radiation oncologists per 100,000 residents | 0.84 | 0.50 to 1.42 | .52 | |||
* All odds ratios restricted to patients with known hospital referral region and metro status. CI = confidence interval; — = no 95% CI for referent values.
† Wald P value. All P values are two-sided.
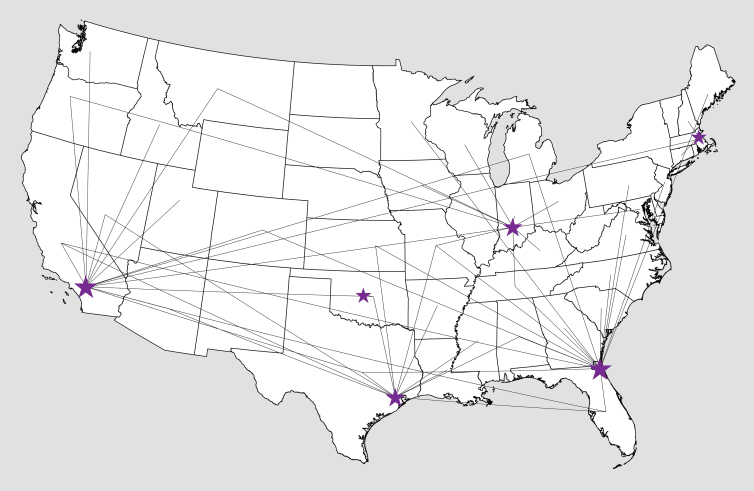
Patients living closest to (<75 miles) and furthest from (>500 miles) a PRT center were more likely to receive PRT than those living 75 to 500 miles from a center (4.9% and 4.2%, respectively, compared with 1.5% for 75–500 miles; P < .001). Patients traveled substantial distances to undergo PRT treatment (Figure 1). Approximately 25% of patients undergoing PRT traveled less than 75 miles and 15% traveled more than 500 miles for treatment.
Figure 1.
Travel patterns among patients receiving proton therapy. The lines go from centroid of each patient’s home state to the proton therapy center at which treatment was administered. Only lines representing two or more patients are shown. Larger stars indicate more patients treated.
The median amount reimbursed by Medicare was $32,428 (IQR = $31,265–$34,189) for PRT patients and $18,575 (IQR = $14,911–$23,022) for the matched group of IMRT patients.
Comparison of Toxicity
There were 421 PRT patients matched with 842 IMRT controls for the analysis of 6-month toxicity and 314 PRT patients matched with 628 IMRT controls for the analysis of 12-month toxicity. The samples were well matched for sociodemographic characteristics, comorbidities, and ADT use, with the bivariate P value for independence for each variable ranging from .86 to 1.00 (Table 1; results shown for the 12-month toxicity sample only, results were consistent for the 6-month sample). The rate of cumulative genitourinary toxicity at 6 months was 9.5% for IMRT vs 5.9% for PRT, a statistically significant difference (Table 3) (OR = 0.60, 95% CI = 0.38 to 0.96; P = .03). However, at 12 months post-treatment, cumulative genitourinary toxicity was no longer statistically significantly different between treatment groups (IMRT = 17.5% vs PRT = 18.8%; OR = 1.08, 95% CI = 0.76 to 1.54; P = .66). Gastrointestinal toxicity at 6 months was 3.6% vs 2.9% for IMRT vs PRT (OR = 0.84, 95% CI = 0.42 to 1.66; P = .61) and 10.2% vs 9.9% at 12 months (OR = 0.97, 95% CI = 0.61 to 1.53; P = .89). Other toxicity was 2.5% vs less than 2.6% for IMRT vs PRT at 6 months (OR = 0.69, 95% CI = 0.29 to 1.66; P = .41) and 5.6% vs 4.5% at 12 months (OR = 0.78, 95% CI = 0.41 to 1.50; P = .46). A more-precise estimate of the rate of 6-month other toxicity for PRT is not reportable because the Centers for Medicare and Medicare Services prohibits the description of groups of less than 11 patients. This prohibition also prevents us from describing complication rates for individual complication codes.
Table 3.
Numbers of patients with 6-month and 12-month toxicity and odds ratios (ORs) for patients receiving proton radiotherapy (PRT) and a matched intensity-modulated radiotherapy (IMRT) cohort*
| 6-month toxicity | 12-month toxicity | |||||||
|---|---|---|---|---|---|---|---|---|
| Complications category | IMRT, n = 842, No. (%) | PRT, n = 421, No. (%) | OR† (95% CI) | P‡ | IMRT, n = 628, No. (%) | PRT, n = 314, No. (%) | OR (95% CI) | P‡ |
| Genitourinary | 80 (9.5) | 25 (5.9) | 0.60 (0.38 to 0.96) | .03 | 110 (17.5) | 59 (18.8) | 1.08 (0.76 to 1.54) | .66 |
| Gastrointestinal | 30 (3.6) | 12 (2.9) | 0.84 (0.42 to 1.66) | .61 | 64 (10.2) | 31 (9.9) | 0.97 (0.61 to 1.53) | .89 |
| Other | 21 (2.5) | <11 (<2.6)§ | 0.69 (0.29 to 1.66) | .41 | 35 (5.6) | 14 (4.5) | 0.78 (0.41 to 1.50) | .46 |
* CI = confidence interval.
† Odds ratio is likelihood of complication for PRT, with IMRT as the reference group.
‡ P value is calculated using a conditional logit model and is two-sided.
§ Actual value is not provided because of the Centers for Medicare and Medicaid Services’ prohibition against publication of groups with less than 11 patients.
Discussion
Although prostate cancer treatment with PRT was roughly 70% more expensive than IMRT, we found only a modest associated reduction in genitourinary toxicity for patients undergoing PRT compared with IMRT at 6 months post-treatment and no difference at 12 months. Gastrointestinal and other toxicity were not statistically significantly different for PRT compared with IMRT at either 6 or 12 months post-treatment.
We found that many patients traveled substantial distances to undergo PRT. In fact, some patients traveled past one PRT center, sometimes in their home state, to receive treatment at a more distant PRT center. Because PRT treatment involves 7 to 9 weeks of daily treatment, such travel often involves relocating for the duration of the treatment, so patients may incur substantial out-of-pocket costs. This is perhaps an extreme example of an indirect cost associated with cancer care (26). Thus, the adoption pattern of PRT reflects a tiered system of access to cancer care; one level involving most Americans who travel locally for cancer care, and another level where a select group of patients can afford to travel nationally to obtain the treatments that are perceived to be “best.”
The long distances traveled by some patients highlight the importance of examining a national sample. The Chronic Condition Warehouse includes comprehensive Medicare claims for all enrollees nationwide with prostate cancer. Therefore, in contrast with prior studies (13,14), we were able to include six treatment facilities rather than a single center.
Regarding toxicity, it is plausible that differences between PRT and IMRT would be limited to early genitourinary side effects. In prior studies, the only improvement in radiation dose distribution for PRT compared with IMRT was a reduction in the amount of bladder exposed to low and intermediate levels of radiation (27,28). Because the amount of bladder exposed to low doses of radiation predicts early toxicity (29), the reduction of radiation to the bladder may be responsible for the transient improvement in 6-month toxicity associated with PRT.
Our findings on toxicity should be considered in conjunction with our findings on cost. We found that Medicare’s reimbursement per patient for PRT was 1.7 times that of IMRT. The relative reimbursement of new medical technologies needs to be considered carefully so that physicians and hospitals do not have a financial incentive to adopt a technology before supporting evidence is available.
There were several limitations to our study, including the lack of some treatment-related information and patient-reported outcome data. We do not know radiation dose and field size; it is possible that IMRT patients may have received a higher dose or nodal radiotherapy, which could explain the increase in 6-month toxicity. Furthermore, the grading of toxicity is unreliable using Medicare claims. Our analysis detected moderate to severe toxicities that often required direct medical intervention. As a result, our analysis was relatively specific in its detection of toxicity. Unfortunately, we were unable to reliably detect milder and more common toxicities from treatment, such as mild to moderate proctitis or cystitis that did not require medical intervention. Therefore, it is possible that PRT may reduce these more mild to moderate toxicities compared with IMRT. Prospective studies of quality of life based on patient-reported outcomes are also needed in order to fully evaluate whether the increased expense of new radiation technologies is justified.
Although we excluded patients with a diagnosis of metastatic disease, other staging data were not available. Only 12 months of follow-up were available, so further analyses of longer-term outcomes concerning both toxicity and cancer control are warranted. In addition, patients were not randomized; however, the large pool of controls allowed us to match PRT patients very closely with respect to observed risk factors.
Our study investigated a comprehensive and clinically relevant set of procedure and diagnosis claims to assess toxicity. Because the complication rate from IMRT has been reportedly low (12), we felt that a method of measuring complications that erred on the side of sensitivity was required to truly assess for any subtle differences in treatment-related outcomes.
Although we were unable to adjust for unknown risk factors, any bias that may not have been accounted for in our analysis is likely to be in favor of PRT. For example, IMRT sometimes involves radiation to the entire pelvis, whereas PRT involves only treatment of the prostate. Thus, the patients in our study who underwent IMRT were more likely to receive regional radiation, placing them at increased risk for toxicity (30). Because PRT is performed in only a few medical centers, there is perhaps more uniform technique, including the use of prostate and rectal immobilization with an endorectal balloon. IMRT is widely performed, potentially increasing the variability of treatment, which may increase the likelihood for treatment-related toxicity.
Therefore, our finding of a modest and transient benefit to the use of PRT compared with IMRT for prostate cancer indicates that the likelihood of a true clinically significant benefit with the use of PRT is low in this population. Nonetheless, prospective, randomized trials are needed to confirm our finding of transient improvement in toxicity with PRT compared with IMRT without a long-term effect.
This study represents the most robust comparison of early toxicity for PRT vs IMRT for prostate cancer to date. In a national sample of Medicare beneficiaries, PRT was rare and expensive and was associated with only a modest and transient reduction in genitourinary toxicity. Continued longitudinal study of the comparative effectiveness of PRT compared with IMRT is needed before widespread application of PRT for prostate cancer can be justified.
Funding
This work was supported by the National Cancer Institute, National Institutes of Health (R01CA149045). JBY is also supported by CTSA grant KL2 RR024138 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Supplementary Material
The study sponsor (NIH) did not play a role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007; 25(24):3603–3608 [DOI] [PubMed] [Google Scholar]
- 3. Kagan AR, Schulz RJ. Proton-beam therapy for prostate cancer. Cancer J.. 2010; 16(5):405–409 [DOI] [PubMed] [Google Scholar]
- 4. Dvorak T, Wazer DE. Evaluation of potential proton therapy utilization in a market-based environment. JACR. 2010; 7(7):522–528 [DOI] [PubMed] [Google Scholar]
- 5.National Association for Proton Therapy. http://proton-therapy.org Accessed September 1, 2011.
- 6. Schippers JM, Lomax AJ. Emerging technologies in proton therapy. Acta Oncol.. 2011; 50(6):838–850 [DOI] [PubMed] [Google Scholar]
- 7.Institute for Clinical and Economic Review. Brachytherapy and Proton Beam Therapy for Treatment of Clinically-Localized, Low-Risk Prostate Cancer Final Appraisal Document. Boston, MA: Institute for Clinical and Economic Review, 2008.
- 8.Institute for Clinical and Economic Review. Intensity Modulated Radiation Therapy (IMRT) for Localized Prostate Cancer Final Appraisal Document. Boston, MA: Institute for Clinical and Economic Review; 2007.
- 9. Vargas C, Fryer A, Mahajan C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys.. 2008; 70(3):744–751 [DOI] [PubMed] [Google Scholar]
- 10. Mendenhall NP, Li Z, Hoppe BS, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012; 82(1):213–221 [DOI] [PubMed] [Google Scholar]
- 11. Nihei K, Ogino T, Onozawa M, et al. Multi-institutional phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys.. 2011; 81(2): 390–396 [DOI] [PubMed] [Google Scholar]
- 12. Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin Radiat Oncol. 2008; 18(1):48–57 [DOI] [PubMed] [Google Scholar]
- 13. Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012; 307(15):1611–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim S, Shen S, Moore DF, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. XXXX; 60(5):908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper MM. The Dartmouth Atlas of Health Care: what is it telling us? Health Syst Rev. 1996; 29(3):44–45, 47 [PubMed] [Google Scholar]
- 16. Wennberg JE, Fisher ES, Goodman DC, Skinner JS. Tracking the Care of Patients with Severe Chronic Illness—The Dartmouth Atlas of Health Care 2008. Hanover, NH: Dartmouth Institute for Health Policy and Clinical Practice Center for Health Policy Research; 2008. [PubMed] [Google Scholar]
- 17. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med.. 2002; 346(15):1138–1144 [DOI] [PubMed] [Google Scholar]
- 18. Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys.. 2011; 81(4):e325–e334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002; 40(8 Suppl):IV-62–8 [DOI] [PubMed] [Google Scholar]
- 20. Berge V, Thompson T, Blackman D. Additional surgical intervention after radical prostatectomy, radiation therapy, androgen-deprivation therapy, or watchful waiting. Eur Urol.. 2007; 52(4):1036–1043 [DOI] [PubMed] [Google Scholar]
- 21. Chen AB, D’Amico AV, Neville BA, Earle CC. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol. 2006; 24(33):5298–5304 [DOI] [PubMed] [Google Scholar]
- 22. Hu JC, Wang Q, Pashos CL, Lipsitz SR, Keating NL. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008; 26(14):2278–2284 [DOI] [PubMed] [Google Scholar]
- 23. Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med.. 2010; 363(19): 1822–1832 [DOI] [PubMed] [Google Scholar]
- 24. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998; 36(1):8–27 [DOI] [PubMed] [Google Scholar]
- 25. Rubin DB. Bias reduction using Mahalanobis-metric matching. Biometrics. 1980; 36(2):293–298 [Google Scholar]
- 26. Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007; 99(1):14–23 [DOI] [PubMed] [Google Scholar]
- 27. Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys.. 2007; 69(2):444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mock U, Bogner J, Georg D, Auberger T, Potter R. Comparative treatment planning on localized prostate carcinoma conformal photon- versus proton-based radiotherapy. Strahlenther Onkol.. 2005; 181(7):448–455 [DOI] [PubMed] [Google Scholar]
- 29. Karlsdottir A, Johannessen DC, Muren LP, Wentzel-Larsen T, Dahl O. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol.. 2004; 71(1):43–53 [DOI] [PubMed] [Google Scholar]
- 30. Sanguineti G, Endres EJ, Parker BC, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol.. 2008; 47(2):301–310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.