Abstract
The addition of chemotherapeutic agents to ionizing radiation has improved survival in many malignancies. Cure rates may be further improved by adding novel targeted agents to current radiotherapy or radiochemotherapy regimens. Despite promising laboratory data, progress in the clinical development of new drugs with radiation has been limited. To define and address the problems involved, a collaborative effort between individuals within the translational research program of the Radiation Oncology Therapy Group and the National Cancer Institute was established. We discerned challenges to drug development with radiation including: 1) the limited relevance of preclinical work, 2) the pharmaceutical industry’s diminished interest, and 3) the important individual skills and institutional commitments required to ensure a successful program. The differences between early-phase trial designs with and without radiation are noted as substantial. The traditional endpoints for early-phase clinical trials—acute toxicity and maximum-tolerated dose—are of limited value when combining targeted agents with radiation. Furthermore, response rate is not a useful surrogate marker of activity in radiation combination trials.Consequently, a risk-stratified model for drug-dose escalation with radiation is proposed, based upon the known and estimated adverse effects. The guidelines discuss new clinical trial designs, such as the time-to-event continual reassessment method design for phase I trials, randomized phase II “screening” trials, and the use of surrogate endpoints, such as pathological response. It is hoped that by providing a clear pathway, this article will accelerate the rate of drug development with radiation.
This article outlines strategies for the design of early-phase clinical trials of radiation sensitizers. It is the result of a collaborative project involving experts from the National Cancer Institute (NCI) and the Radiation Therapy Oncology Group (RTOG). Two complimentary articles that present sometimes contrasting opinions (1, 2) are recommended to the reader.
Importance of Drug Development With Radiation Therapy and Potential Impact
Radiation therapy plays a key role in cancer management, in both the definitive and palliative setting; however, local failure remains a cause of morbidity and mortality. Randomized trials have demonstrated that delivering systemic therapy concurrently with radiation improves both local control and overall survival in many cancer types without excessive toxicity (Table 1).
Table 1.
Combination of radiation and systemic therapy, level 1 evidence*
| Primary | Systemic agent | Advantage of combined treatment compared with radiation alone | References |
|---|---|---|---|
| Glioblastoma (brain) | Temozolomide | Improved OS | (3) |
| Head and neck | Cisplatin, cetuximab | Improved OS | (4), (5) |
| Lung | Cisplatin | Improved OS | (6) |
| Esophagus | 5FU + cisplatin | Improved OS | (7) |
| Stomach | 5FU + leucovorin | Improved OS compared with no treatment | (8) |
| Rectum | 5FU | Improved OS | (9) |
| Anus | 5FU + mitomycin | Improved local control | (10) |
| Cervix | Cisplatin | Improved OS | (11) |
| Prostate | Androgen deprivation therapy | Improved OS | (12) |
| Bladder | 5FU + mitomycin | Improved local control | (13) |
* OS = overall survival; 5FU = 5-fluorouracil.
Classically, radiosensitizers were considered those drugs whose sole action was to decrease cancer cell survival by changing the slope of the radiation survival curve following exposure to ionizing radiation. Despite much research, few such drugs have entered clinical use. Radiation response modifiers in use today have single-agent activity against particular tumors (eg, cisplatin, 5-fluorouracil, temozolomide), with the underlying rationale for the drug–radiation combination going beyond classic radiosensitization (14).
Over the past decade, a large number of molecular agents that target cellular survival and growth signaling pathways have been developed. Many of these enhance the effect of ionizing radiation in the laboratory (Supplementary Table 1, available online). It is anticipated that these agents will enhance tumor control when combined with radiation therapy in human subjects. Cetuximab, a targeted agent, was effective when combined with radiation in a phase III trial (15).
The majority of targeted agents are cytostatic, or only mildly cytotoxic; hence, prolonged administration may be required to achieve clinical benefit. Furthermore, with the notable exception of adjuvant trastuzumab in breast cancer, this broad class of agents has had only a modest effect on overall survival so far. Combining these agents with radiation therapy, however, has the potential to improve cure rates and long-term overall survival. When cetuximab is combined with radiation therapy in head and neck cancer, it produces a 10% absolute improvement in 3-year survival (15), whereas in the absence of radiation, the same drug produces only a transient improvement in overall survival for a variety of cancer types (16–18).
Despite the promise of combined radiation and systemic treatments, progress has been slow. During the past decade, only two new agents, temozolomide (chemotherapeutic) and cetuximab (monoclonal antibody), have improved survival when combined with radiation therapy. We briefly review the development of three classes of radiation response modifiers.
Limited Success: Targeting Hypoxic Cells
A large number of clinical trials were performed based upon preclinical work demonstrating that nitroimidazoles overcome the radioresistance of hypoxic cancer cells. Results were disappointing; trials demonstrated only a modest benefit. There are multiple explanations (19–23):
Animal models did not accurately reflect acute and/or chronic hypoxia seen in human tumors.
There was an inability to assess tumor hypoxia and enrich trials with hypoxic tumors.
There were unexpected side effects; neuropathies prevented the use of a therapeutic dose.
Hypoxia may be an imperfect target because the majority of tumor cells are not profoundly hypoxic and partial reoxygenation occurs during fractionated radiation therapy (24). Further, the addition of chemotherapy to radiation may negate the radioprotective effect of hypoxia (25).
Exaggerated expectations and lack of industry support led to many small, underpowered trials being performed. Following the disappointing results, many lost interest in these agents, although nimorazole is routinely used in Denmark based upon a positive meta-analysis (23).
An alternative approach is the use of hypoxic cytotoxins such as tirapazamine that specifically destroy hypoxic cells. Although effective in vivo (26,27), tirapazamine failed to improve tumor control when combined with chemoradiation in large clinical trials (28,29). Recently it has been reported that tirapazamine induces vascular dysfunction and paradoxically worsens hypoxia at the center of tumors (30–32). Nonetheless, newer agents that exploit tumor hypoxia are under continued development (33).
Past Successes: Traditional Cytotoxics
Combining cytotoxic agents from a variety of classes with radiation improves survival in many cancers compared with radiation therapy alone (Table 1). When trying to understand why these agents succeeded but the hypoxia sensitizers did not, one must consider some key differences. The cytotoxics had been fully developed as active single agents prior to being combined with radiation. As a result, their pharmacokinetics, side-effect profiles, and appropriate clinical doses were already well understood. Significantly, some of the drugs had large pharmaceutical companies providing logistic and financial support. Their comparatively broad-spectrum cytotoxic action, although potentially narrowing the therapeutic window, actually proved to be advantageous, with no need to preselect tumor subtypes. Remarkably, despite decades of work, many fundamental questions concerning radiation response modification remain unanswered: What is the true target for these drugs? What is the optimal scheduling with radiation? Are certain tumors especially sensitive to the drug–radiation combination? Is the combination truly synergistic or merely additive? Our lack of success in resolving these issues does not appear to have hindered cytotoxics’ substantial impact on clinical outcomes.
Past Successes: Signal Transduction Inhibitors
The epidermal growth factor receptor (EGFR) is expressed in almost 100% of head and neck cancer, with the degree of overexpression correlating with survival (34,35). The receptor activates prosurvival pathways and may also promote DNA repair and angiogenesis (36,37). Preclinical studies showed that when cetuximab, an EGFR antagonist, is combined with radiation, cell proliferation is inhibited and apoptosis increased more than with either modality alone (38). Subsequently, a phase III trial demonstrated that cetuximab improves overall survival when combined with radiation in the treatment of locoregionally advanced squamous cell carcinoma of the head and neck compared with radiation alone (15). Unfortunately, EGFR inhibitors have not been as successful in other disease types (39,40). Further, even in this successful trial, the underlying hypothesis appears to be oversimplified: paradoxically it was the tumors with low EGFR expression that benefited most from cetuximab (41). There are also ongoing concerns about the toxicity of the combination (42).
Despite the above successes, enormous room for improvement remains because systemic agents worsen radiation side effects, and although cetuximab provides benefit, many EGFR-expressing tumors recur despite treatment. Few targets, furthermore, are as uniformly overexpressed as EGFR in head and neck cancers.
Current Status of Radiation Response Modifier Development in Radiation Oncology
There is limited drug development in the context of radiation oncology. A recent study found that only 30 phase I trials involving radiation are published per year (43), compared with almost 400 cancer-related, nonradiation, phase I studies. Clinical trials combining new agents with radiation therapy are often initiated only after a drug has been shown to have clinical activity as a single agent, on average 8 years into the drug’s lifetime. Hence the trial results typically become available when the drug’s patent is close to expiration, decreasing the company’s enthusiasm to fund additional indications. Industry’s reluctance to combine drugs with radiation early in development may also be related to fear of toxicity, resultant delay in drug approval, and possible bad publicity.
Identification of Novel Radiation Response Modifying Agents
The broad array of compounds that enhance tumor killing with radiation reflects the complexity of the cellular radiation response and the impact of the microenvironment. Although DNA appears to be the primary target of ionizing radiation, complex processes determine both the ability of the cell to repair DNA and the cells response to DNA damage. Evidence exists that apoptosis, necrosis, mitotic catastrophe and terminal differentiation all contribute, although the relative importance of each is unknown. A lack of complete mechanistic understanding is an impediment to developing effective radiation-enhancing agents, although chemotherapy drug development faces a similar situation. Critically, we need to better differentiate better between normal and neoplastic tissue, but we lack a complete understanding of why many anticancer agents (including radiation) already demonstrate relative selectivity for cancer cells.
An alternative approach is to investigate and overcome the mechanisms of radiation resistance. This approach has been fruitfully pursued for chemotherapeutic (44) and biological agents (45,46). Studies investigating secondary resistance to systemic agents have informed the field about membrane transporters, alternative survival pathways, and the cancer cell genome’s plasticity and its consequent ability to overcome external stresses. More recently, scientists have been trying to anticipate mechanisms of resistance prior to a drug’s clinical launch (47). Much of the preclinical work that has been undertaken into radiation resistance has examined single molecules and/or genes, but clearly, radiation resistance is a multifactorial phenomenon. Factors responsible for radioresistance include both the microenvironment (eg, hypoxia, immune status) and intrinsic cancer cell biology (eg, PI3K pathway activation, cancer stem cell persistence, p53 loss, enhanced DNA repair). Defining the interactions of radiation with these pathways may help development of effective radiation-enhancing agents.
The current NCI approach to development of radiation-enhancing agents is as follows: Investigational Drug Branch senior investigators, who act as the project leads, identify promising agents in coordination with program officials of the Radiation Research Program. They contact the agent source (eg, pharmaceutical company, biotech company) to initiate the material transfer agreement to start preclinical screening experiments by NCI’s Molecular Radiation Therapeutics Branch and/or interested extramural investigators. This collaborative effort enables the novel agent to move quickly to phase I development in conjunction with radiation around the time when the same agent is being tested in other phase II or phase I combination studies.
The Role of Preclinical Studies in Radiation Oncology Drug Development
In vitro and in vivo preclinical studies assess the ability of targeted agents to modulate the effects of ionizing radiation on tumors or tumor cells. Mechanistic studies performed include quantitation of DNA damage, modification of signaling pathways, understanding of microenvironmental perturbation, and investigation of the mechanism of cell death.
The relevance of preclinical work to success in clinical trials in both medical and radiation oncology is unclear, although the number of null phase III trials performed is not reassuring. Possible reasons for discordance between the lab and the clinic include difficulty in modeling the microenvironment and/or immune response, the heterogeneous nature of solid tumors (48,49), the use of fast-growing and sensitive cell lines, unrealistic drug concentrations, and aggressive interpretation of data (50). Methods to try and increase biological relevance include the use of multiple cell lines and/or models, clonogenic rather than colorimetric proliferative in vitro assays, orthotopic xenograft in in vivo models rather than nonorthotopic models, tumors established and passaged in vivo rather than from long-term in vitro cultures, and rigorous statistical analysis and adoption of clinically achievable drug concentrations.
Despite their limitations, preclinical studies are relatively quick and inexpensive, allow demonstration of radiosensitization, and may suggest mechanism of action. Furthermore, testing in different models contributes to an understanding of which variables contribute to likelihood of response (eg, HER2 amplification with trastuzumab, Ras mutations with cetuximab). Preclinical studies can also facilitate the development of endpoints and biomarkers that subsequently can be incorporated into clinical studies (51).
In summary, there is a great need to develop better preclinical models. The results of current preclinical studies alone cannot be used to determine which agents should be studied in early-phase clinical trials in combination with radiation. At present, in vitro studies in two, or preferably three, relevant cell lines are suggested as a minimum; in addition, in the case of targeted therapies, cell lines should be chosen to assess the importance of the target, preferably using isogenic cell lines that differ only with respect to target expression. When the experimental agent is expected to influence the microenvironment, in vivo studies are necessary.
The Role of Preclinical Studies in Predicting Toxicity
There are limited preclinical models of radiation toxicity, and even those that exist are imperfect. Amifostine, for instance, is an effective esophageal radioprotectant in vivo (30) but failed in clinical trials (52). Gemcitabine radiosensitized normal tissues in humans much more than was expected (53,54) based upon preclinical studies (55,56). One reason for the limited value of preclinical models may be the endpoints used. Animal studies typically use objective histological changes as an endpoint, whereas human trials rely on physician-rated and patient-reported subjective complaints (Supplementary Table 2, available online). For instance, with radiation-induced esophagitis, it is not known whether histological changes correlate with the clinical outcomes of odynphagia and dysphagia; rather, it is likely that these symptoms are the result of a combination of mucositis, smooth muscle dysfunction, and neuropathy.
Purpose of Preclinical Studies of Radiation Response Modifiers
Preclinical studies of radiation response modifiers have multiple possible purposes. These include:
The demonstration of efficacy. Does the drug radiosensitize tumor cells at achievable concentrations?
Exploring the mechanisms of radiation modification. For example, is the agent acting upon tumor cells or the microenvironment?
Defining a target and/or signature (biomarker) that defines which tumors are best radiosensitized and could potentially be used in personalized trials as inclusion criteria.
Investigating scheduling issues.
Investigating normal tissue responses. Is the drug widening the therapeutic window or merely shifting both the normal and tumor radiation dose–response curves to a similar extent? Might there be volume effects?
Facilitation of the development of biomarkers (biochemical, imaging) that could be incorporated into clinical trials as surrogates of response.
Challenges of Clinical Drug Development in Oncology and Unique Issues in Early-Phase Radiation Oncology Clinical Trials
Efficient drug development with or without radiation demands that disparate groups (clinicians, cancer centers, cooperative groups, institutional review boards, national regulatory agencies, and pharmaceutical companies) work harmoniously together. Challenges with anticancer drug phase I development in general include difficulty in the selection of the best compounds from a given drug class and difficulty in choosing the most efficient trial designs (57,58). The inadequacy of preclinical assays fosters overregulation, slowing the drug development process (59). As more anticancer agents receive US Food and Drug Administration approval and the standard of care improves, the bar is inevitably set ever higher, making the endorsement of newer, potentially more-potent agents more difficult (60). Many of these issues were addressed in the US Food and Drug Administration’s Critical Path Initiative (61); suggested solutions include the implementation of nontraditional enrollment schedules, use of biomarkers and functional imaging, and adjustment of regulatory requirements (62–69). An underlying theme already being adopted is the need to go beyond the traditional early-phase trial objectives of toxicity and pharmacokinetics to include identification of sensitive tumor types and proof that the new agent is “hitting the target.” For example, nitroimidazole hypoxic sensitizers may have proven successful had trial enrollment been limited to hypoxic tumors.
There are unique biological, clinical, and logistical challenges that characterize “personalized” drug development with radiation that may be above and beyond that for the drug alone.
Expertise
Designing and conducting high-quality clinical trials requires biological understanding, broad clinical experience, and institutional commitment to ensure that there is the appropriate regulatory infrastructure, as well as statistical and financial support. Close contacts with the pharmaceutical industry as well as with NCI programs such as the Cancer Therapy Evaluation Program (CTEP) and the Radiation Research Program are needed to ensure drug supply. A concern for the future development of drug–radiation combinations is the limited number of academic clinicians and a decrease in the number of radiation biologists (70,71).
Feasibility
Of the many promising drugs in development, logistic and financial pressures dictate that only a fraction will be tested with radiation therapy in clinical trials. However, there is no consensus regarding the experimental data required prior to proceeding with a clinical radiation–drug trial, nor is there a mechanism for prioritizing which drugs should be pushed forward. This lack of clarity slows the process at all steps for all involved—investigators, the pharmaceutical industry, and regulatory bodies.
Incomplete Understanding of the Mechanisms Through Which Radiation Therapy Controls Tumors
Although there is broad consensus that DNA damage plays a central role in radiation-induced cell death, the relative importance of other pathways, such as ceramide, immunostimulation, and radiation-induced vascular damage, remains uncertain. For example, when mammalian target of rapamycin (mTOR) inhibitors are combined with radiation, different model systems have provided conflicting mechanisms of action; some models show that the drugs radiosensitize through increased direct tumor kill, whereas other models suggest the drugs radiosensitize by attacking the vasculature (72–74). There are similar types of knowledge gaps for all types of cancer therapy.
In the clinic the most troublesome, radioresistant tumors tend to be of large diameter. Currently these are typically treated with daily-fractionated radiation for several weeks (75). The tumors studied in animal models are small by contrast and usually treated over a period of a few days, which means that the mechanisms operating in the latter setting may differ substantially from the former.
An important contrast between using a drug alone or as a radiation modifier is that the maximum tolerated dose of an investigational agent may not necessarily be the biologically optimum dose in combination with radiation (76). One pragmatic solution may be to investigate two dose levels in phase II clinical trials—one at or close to the maximum tolerated dose and the other somewhat lower. Variation in dose scheduling is worthy of exploration and problematic to model in animal models.
The Efficacy of Radiation Therapy Alone
Radiation is highly effective at shrinking tumors; hence, response rate is usually not a useful surrogate marker of activity in radiation combination trials. A more appropriate activity endpoint for radiation trials may be progression-free survival, but this is often difficult to objectively assess. Imaging endpoints (such as fluorodeoxyglucose uptake) show promise, but they require validation and standardization across institutions. In the neoadjuvant setting, a pathological complete response may be a very useful endpoint in patients with certain tumors (eg, esophagus, rectum). In view of the substantial tumor shrinkage rates seen with radiation therapy alone, randomized phase II trials can be particularly valuable when combining investigational agents with radiotherapy or radiochemotherapy. If short-term endpoints are proven reliable indicators of ultimate benefit with respect to clinical endpoints such as survival, then clinical trial designs that screen multiple agents to select the most promising for phase III trials may be possible.
Nonbiological Factors That Contribute to Clinical Outcome
Outcome following radiation therapy is dependent on spatial and technical factors not directly related to tumor biology, such as size of the target, proximity of the tumor to sensitive normal tissues, accuracy of target volume definition, patient immobilization, and dose and fractionation of radiation. Inadequate quality assurance and lack of consistency in radiation delivery has led to the conclusions obtained from large clinical trials being questioned (77–83). In the randomized RTOG trial 9704, which evaluated adjuvant chemo-radiation in pancreatic cancer, subtle protocol violations in target definition influenced both toxicity and survival (84). All the above factors should be considered when interpreting the results of clinical trials, but this information is not always reported. Clinical trials involving intensity-modulated radiation therapy (IMRT), in which the radiation dose is sculpted around critical structures, add a new degree of potential confounding factors, as demonstrated by a dose-escalation trial in prostate cancer in which the target volume was defined differently for different dose levels (85). Hence the importance of quality assurance in clinical trials cannot be overemphasized, and the quality assurance programs within the cooperative group trials supported by CTEP are an excellent investment.
Radiation-Induced Toxicity is Biphasic
Toxicity following radiation typically follows a biphasic course: early toxicity usually starts within the first 2 weeks and is generally defined as occurring within 3 months of commencing radiation therapy; late toxicity can occur months to years following completion of therapy. Although early toxicity is bothersome, it is generally reversible. Late toxicity is typically irreversible, although some studies suggest this may not be so (86). The choice between early and late toxicity endpoints in early-phase clinical trials needs to be decided pragmatically, balancing feasibility with what is most relevant for patient outcomes.
Phase I trials rarely include a randomized comparison group; therefore, investigators should be careful not to attribute to the investigational agent adverse effects that are expected to occur with radiotherapy alone. A helpful benchmark is the report of a recent NCI workshop that summarized the most serious and/or dose-limiting adverse effects of radiation therapy to different organs (75).
In phase I trials of investigational agents that do not involve radiation therapy, the dose-limiting toxicity is usually apparent within a few days or weeks, generally before the next cycle is due. In trials involving radiation therapy, however, it may be appropriate to wait longer, possibly 2 to 3 months, before escalating or de-escalating the dose of the systemic agent, although this interrupts continuity of patient accrual. For greater efficiency, therefore, consideration should be given to a “ping-pong” study design, wherein a cohort testing drug B is enrolled while awaiting evaluation of the cohort that received a certain dose of drug A (87).
Questions Addressed by Phase I Radiation Trials
Phase I trials in radiation oncology often have a different purpose than medical oncology trials. Phase I trials in medical oncology are frequently the “first-in-human” experience for the agent; toxicity may be unpredictable, and pharmacokinetic and biomarker studies are essential. On the other hand, most phase I trials in radiation oncology involve agents that have already been through phase I and/or phase II testing. Their toxicity is known, and complete pharmacokinetic studies are unnecessary. The primary purpose of the trial is to define the extent of toxicity within the region irradiated. Consequently, radiation oncology phase I trials are organ-specific and are comparatively safe (43).
Patients in phase I radiation trials may receive full-dose radiation treatment; therefore there is no need to restrict enrollment to patients lacking therapeutic options. Conversely, in most medical oncology early-phase trials, participants have often received several lines of treatment and may lack further therapeutic options.
As an illustrative example, we examined early-phase clinical studies for gemcitabine. Phase I studies of gemcitabine without radiation demonstrated that the maximum tolerated dose was dependent upon infusion duration and frequency but independent of the tumor type. Qualitatively, toxicities were broadly similar across different anatomic sites of disease (88). In contrast, phase I trials combining gemcitabine with ionizing radiation demonstrated that dose-limiting toxicities (DLTs) were predictable and very dependent upon the organ (and normal tissues) being irradiated. For example, the DLTs for pancreatic irradiation were nausea, anorexia, and abdominal pain, whereas for lung irradiation, the DLTs were pneumonitis, esophagitis, and dermatitis. The maximum tolerated dose of gemcitabine also varied greatly, ranging from 10mg/m2/week for mouth and throat cancers (53) to up to 600mg/m2/week in the brain (89). These observations emphasize the need for an organ-specific approach to phase I radiation combination trials.
New Opportunity: Combining Radiation Modifiers With Hypofractionated Radiation Therapy
Traditionally, radiation therapy was delivered in daily fractions of about 2 gray each, with definitive treatments lasting 6 to 8 weeks. Advances in the precision of radiation delivery have led to the increasing use of hypofractionated treatments (fewer but larger fractions, typically five or less). Early results are encouraging, although long-term toxicity outcomes are limited (90–93). It is unknown how radiosensitizers will influence clinical outcomes of hypofractionated radiation. In vitro clonogenic cell survival curves typically show increasing divergence between control cells and those treated with a radiation sensitizer as radiation dose increases, suggesting that radiosensitizers may be especially effective with large fraction sizes. Conversely, hypofractionated schedules present fewer opportunities for the drug to enhance the radiation effect, and large fraction sizes may induce different biological effects than smaller fractions, with some evidence suggesting that large fractions principally target the vasculature (94,95). Further, different kinds of molecular targeted agents may radiosensitze differently depending on the fraction size. Nonetheless, theoretical models predict that systemic agents will enhance outcomes when combined with hypofractionated regimens (25).
New Opportunity: Combining Immunomodulators With Radiation Therapy
Immune cells with antigen-presenting effector and suppressor functions heavily infiltrate human tumors. These cells have a major effect on the clinical attributes of human cancer, underlined by the recent successes of immunotherapies in prostate cancer and melanoma (96). Local radiation stimulates the immune system, initiating a cascading innate and adaptive immune attack on the tumor (97). There are currently a number of ongoing clinical trials combining modern immunotherapies with radiation therapy; early results are promising (98).
Recommendations
We emphasize that, given the limited ability of preclinical models to predict clinical outcome, these are not formal guidelines. Nonetheless, we suggest that during early stages of development a series of critical questions should be addressed (Box 1). Strategies for the efficient development of radiosensitizers must include the careful choice of agent with known pharmacokinetic properties and proven activity in certain tumor types or based on molecular target expression.
Box 1. . Considerations in the design of preclinical experiments and early phase clinical trials combining radiation with a targeted agent. Some points have been adapted from the Investigational Drug Steering Committee recommendations for the design of phase I studies (99).
Choice of systemic agent: key questions
1. Is the agent obtainable?
2. Are pharmacokinetics known? Known penetration into tumor type?
3. Is systemic side effect profile understood? Does toxicity overlap with radiation toxicity?
4. Does agent have single-agent activity in this tumor type? This should not limit the use of novel agents without single-agent activity if there is compelling preclinical data.
5. Are there subsets of tumors based on either histology or molecular profile that are especially sensitive or resistant to the agent?
6. Is there evidence for tumor radiosensitization by this class of agent?
7. Is there evidence for normal-tissue radiosensitization by this class of agent?
8. Are funding agencies interested in the drug’s development?
Choice of tumor type: key questions
1. What is role of radiation therapy (RT) currently—curative or palliative? This affects total radiation dose, fractionation, importance of acute-vs-chronic toxicity, and definition of dose-limiting toxicity (DLT).
2. What is long-term tumor control rate with RT alone?
3. Is RT normally combined with a systemic agent? If so,
a. Are there subpopulations and/or clinical scenarios in which radiation is used alone (eg, recurrent disease)?
b. Is it practical to combine radiation + accepted systemic agent + investigational agent?
4. Are pre- and/or midtreatment biopsies obtainable? What other correlative studies are feasible?
5. Are the majority of patients suitable for enrollment into a clinical trial (eg, have sufficient performance status)?
6. Are there a sufficient number of patients to ensure robust enrollment?
Preclinical RT drug development
1. There is good rationale for combining many developing classes of biological agent with radiation.
2. In vitro (clonogenic survival) and in vivo experiments are complimentary; whereas the former inform regarding intrinsic tumor effects, the latter inform regarding microenvironmental effects. Nonclonogenic assays have a very limited cell survival range and hence may be limited when examining radiation modification because of the substantial cell killing by radiation alone.
3. Efforts should be made to define mechanism of radiation modification.
4. Where possible preclinical models should explore a range of cell lines and models with the aim to establish which tumor subtypes respond best to the radiation–drug combination.
5. When considering microenvironment effects, efforts should be made to use orthotopic xenograft models.
6. Scheduling should be broadly investigated, although it is appreciated that it is not simple to translate a successful preclinical schedule for use in the clinic.
7. Efforts should be made to investigate normal tissue response, with the understanding that preclinical models are of limited validity, especially for late toxicities.
8. Efforts should be made to consider exploring biomarkers that may subsequently be incorporated into clinical trials.
Design of early-phase trials (both phase I and II)
1. Radiation doses, volumes, fractionation schemes, and quality assurance controls need to be carefully defined in both the protocol and the subsequent publications.
2. An independent medical monitor should be assigned to each trial to adjudicate attribution of DLTs. The monitor should be knowledgeable about expected radiation side effects in the disease site. In multicenter trials, the monitor will most likely be a member of the data safety monitoring board; in academic studies, the monitor will be appointed by either the clinical trials office or the institutional review board.
3. The incorporation of novel functional imaging endpoints into early-phase trials may be relevant. For certain classes of agents, novel tracers may provide evidence of proof of principle, evidence of target inhibition, or early hints of activity (eg, 18F-fluoromisonidazole, positron emission tomography; however, subsequent validation of novel imaging endpoints is required.
4. Incorporation of pre- and midtreatment biopsies (100) may aid in determining predictors of response. However, performing midtreatment biopsies may be impractical and/or unsafe in some settings. An alternative still in development is functional imaging to assess response.
5. Consider pragmatic solutions to speeding up drug development including combining different pathologies located within the same organ into a single trial, such as brain metastases with glial tumors. Although this approach involves only a single institutional review board and Investigational New Drug application to the US Food and Drug Administration, it lays the foundation for two subsequent phase II trials.
6. Effort should be made to establish an extant standard of care for radiation therapy trials (dosing schedule, use of combined chemotherapy) to facilitate multi-institution trials.
Phase I clinical trials
1. Trials should be organ specific, although not necessarily disease specific.
2. Primary endpoint of a phase I trial is usually an assessment of acute toxicity with the goal of identifying a recommended phase II dose.
3. Toxicity remains a relevant endpoint, as does defining a maximum tolerated dose, recognizing that the recommended dose and biologically active dose may be different from the maximum tolerated dose.
4. The definition of DLT needs to be very carefully considered and is dependent on clinical context. Many radiation trials are performed in locally advanced disease where the intent is curative; in such cases, severe transient toxicity may be considered acceptable because it resolves. Likewise grade 3 diarrhea is an accepted consequence of radiation therapy to the abdomen in the absence of any drug, and hence should not be scored as a DLT.
5. Intrapatient dose escalation is not appropriate.
6. In some circumstances, defining a maximal dose based on toxicity may not be appropriate (eg, for agents associated with very minimal expected toxicity or for agents for which escalation beyond a given dose may not be feasible because of absorption, volume, or financial constraints); in such cases, consideration could be given to defining a maximally potent dose.
7. Speed of dose escalation should be determined based upon expected toxicity and the degree of uncertainty involved. When toxicity is likely, a more protracted step-wise approach is recommended [eg, prior evidence of excessive toxicity when this agent was combined with radiation, overlapping toxicities with radiation, use in organ in which radiation alone is already very toxic (101), first agent in class to be combined with radiation].
8. To shorten the drug development process, there is generally no need to incorporate pharmacokinetic studies into a radiation phase I trial if these have already been performed because ionizing radiation does not influence drug absorption, excretion, or distribution, unless directed at the gastrointestinal tract. (Limited studies may be useful if a new drug schedule is used.)
9. Utilize preclinical and/or clinical data when planning the trial:
a. Single agent pharmacokinetic data will help decide whether to use continuous dosing during radiation therapy vs once-a-week dosing.
b. Data will show agent’s ability to modulate a molecular target that is relevant to radiation response.
c. Safety data will be avaialable for drug when used in the absence of radiation.
10. Consider use of full-dose radiation, with escalating doses of drug, vs fixed dose of drug and escalating radiation dose.
11. Continuing evaluation and refinement of novel trial designs (especially the time-to-event continual reassessment method vs traditional designs (3+3) is critical, especially when radiation is used with molecularly targeted agents.
Phase II clinical trials:
1. Perform randomized phase II trials in place of single-arm phase II studies.
2. Consider multiple arm trials. Options include the “pick the winner” design, testing multiple agents within the same trial with the goal of choosing one or more to take forward to phase III based on observed response and other criteria, and designs with multiple agents and a standard therapy control group, with pilot efficacy testing of each against the control.
3. Where feasible and appropriate, incorporate biomarkers into trials. Options include trials “enriched” with patients most likely to respond based on prerandomization marker screen or screening and randomization of all patients stratified by marker status.
Trials need to be designed based upon all the preclinical and clinical data available. If the drug has already completed phase I evaluation, the preclinical criterion for entering a radiation modifier trial may simply be demonstration of in vitro efficacy. Considerations such as dosing interval and scheduling drug administration vs radiation delivery need to be based upon the pharmacokinetic and biologic properties of the drug in humans.
Choice of Endpoints
The incorporation of secondary endpoints into phase I trials is increasingly practiced (Table 2), adding mechanistic understanding and facilitating their inclusion into subsequent phase II trials. Pre- and midtreatment tumor biopsies, when available, are the most useful endpoints but are rarely feasible. Post-treatment biopsies are less useful because they may simply reflect necrosis. Post-treatment, anatomical image–based measurements suffer from the limitation that tumor regression is expected irrespective of any drug action. Surrogates for post-treatment biopsies, such as functional imaging and circulating tumor cells, have much potential but have yet to be fully explored.
Table 2.
Endpoints for early phase (I, II) clinical trials in radiation oncology*
| Endpoint | Example | Advantages | Disadvantages |
|---|---|---|---|
| Toxicity | |||
| Toxicity acute | Mucositis (head and neck cancer); diarrhea (prostate cancer) | Requires short follow-up. Often used to determine MTD. | Reversible and may not correlate with chronic toxicity, which may be more clinically important endpoint. |
| Toxicity late | Bowel stricture (abdominal radiation), cognitive decline (brain tumors), fibrosis | Frequently irreversible, clinically important. | Many subjects die prior to developing late toxicity, often considered too late an event to be used to determine MTD. |
| Mechanism | |||
| Target and pathway engagement (surrogate tissue) | Skin biopsy (gefitinib), peripheral blood mononuclear cells (vorinostat), hair (PI3K) | Fairly easily obtainable. May provide biological insight into normal tissue. | May not reflect target inhibition in tumor (43). |
| Target and pathway engagement (tumor tissue) | Immunohistochemistry for pathway activation | May provide biological insight into tumor tissue. | Post treatment biopsy and/or resection rarely possible (rectal cancer an exemption). |
| Clinical efficacy | |||
| Overall survival | — | Potentially assesses safety and efficacy. | Long follow-up may be required. |
| Local-regional control | Lung cancer | Requires smaller sample size. Overcomes crossover problem. | Difficult to define in certain areas (eg, brain). May not correlate with overall survival. Analysis and interpretation may be complicated by competing events (eg, distant failures). Most tumors shrink after RT; therefore difficult to assess added benefit of drug. Introduction of bias, based upon evaluation frequency. |
| Biological activity | |||
| Pathological response | Rectal cancer | Hard endpoint. Translational research can be incorporated. | Post-treatment biopsy and/or resection not possible for most cancers. |
| Progression-free survival | — | An earlier endpoint than overall survival. | Requires validation for each cancer site. Introduction of bias, based upon evaluation frequency. |
| Systemic biomarkers of clinical efficacy | PSA, β-HCG | Allows for rapid assessment of treatment efficacy | Requires formal validation. Sample timing and preparation critical. |
| Novel biological imaging | Post-treatment PET scan | Noninvasive. Rapidly emerging technology. | Problematic to standardize between institutions. Not fully reproducible |
| Miscellaneous | |||
| Systemic biomarkers of clinical toxicity | TGF-β1, IL-6 | Lack clinical meaning | Requires formal validation. Sample timing and preparation critical. |
| Circulating tumor cells | — | Fairly easily obtainable | A developing technology. Reflects disease within and outside of RT field. |
| Quality of life measure and neurocognitive testing | — | Reflect patient experienced symptoms. | Requires validation. Compliance poor in sicker patients. |
* Some information extracted from LoRusso et al. (102). HCG = human chorionic gonadotropin;. IL-6 = interleukin 6; MTD = maximum tolerated dose; PET = positron emission tomography; PI3K = phosphoinositide 3 kinase inhibitor, PSA = prostate-specific antigen; RT = radiation therapy; TGF-β1 = transforming growth factor beta 1.
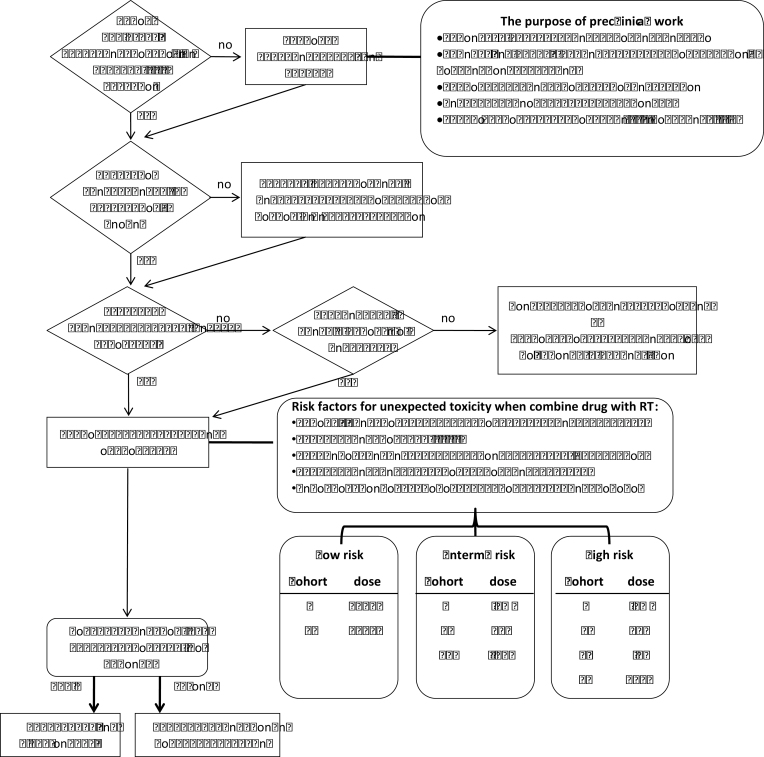
The design of the dose-escalation scheme should reflect the expected normal tissue toxicity of the combination and previous clinical experience with the systemic agent (Figure 1). When combining radiation with a drug known to significantly enhance normal tumor injury, the trial may best be considered high risk, and careful dose escalation is indicated; conversely when combining minimally toxic radiosurgery with a low-toxicity biologic agent, a more aggressive escalation is appropriate. Other factors to consider when assessing risk include overlapping toxicities between the systemic agent and radiation therapy, use in an organ in which radiation alone is already very toxic (43), status as first agent in class to be combined with radiation, and combined use of three modalities (ie, radiation, molecular agent, and chemotherapy).
Figure 1.
Suggested pathway for the early clinical development of radiation response modifiers. *The percentage values for dose escalation between different cohorts according to perceived risk refer to percentage of the standard systemic dose when the drug is used as a single agent. These values are suggestive only. RT = radiation therapy.
Trial Design: Statistical Considerations
Although acute toxicity remains the standard endpoint for radiation oncology phase I trials, the use of broader endpoints incorporating late toxicity should be considered. Typical designs [3 + 3, continual reassessment method (58,103), and others] dictate that enrollment be suspended while a given dose is evaluated with respect to dose-limiting toxicities. This means that the evaluation of all patients in the dose cohort must be completed, which works satisfactorily for relatively short-term acute toxicities relevant to drug dosing but clearly is impractical for incorporating late toxicities. Thus, more recent designs extended for this purpose are of particular interest to radiation oncology. The most relevant design is the time-to-event continual reassessment method (TITE-CRM) (104,105). In the TITE-CRM, the original continual reassessment method approach (a design that posits a dose–response curve with initial parameters and sequentially updates the curve as patient experiences are obtained) is modified to incorporate both early and late toxicities. Specifically, the TITE-CRM design permits enrollment of new patients while previous patients remain under observation for DLTs and facilitates updating a given patient’s status if a late toxicity occurs, thus influencing the DLT estimate. This is accomplished by defining a toxicity observation time window, with patients followed for a period of time free of a DLT contributing partial information to the current dose evaluation based on the length of their follow-up. Additional constraints to control dose escalation and increase safety can be incorporated. For example, in the implementation of the design for RTOG 0813, a phase I trial of radiation dose escalation in stereotactic lung radiotherapy, escalation is restricted to one predetermined dose step, and no patient can be assigned a higher dose than previously used until there is 1 year of cumulative observation at the current dose. Although this trial addressed radiation dose escalation specifically, the approach fits well into combined agent trials, and in fact, there may be more credible information in this instance for the hypothetical dose–response curve. One critical issue of this design is logistical management because the number of patients per dose-level is not predetermined prior to protocol activation and current patient status must be regularly incorporated. Experience with RTOG 0813 shows that this is feasible if appropriate oversight systems are in place. Also, although it is a promising approach and is gaining wider use, the TITE-CRM design has some limitations, and research continues on potential improvements (106). First, the approach depends on various modeling assumptions being valid—for example, distribution of time to toxicity events and the assumed length of the DLT window—and these parameters should be substantiated accordingly when using the design. Second, a primary limitation in practice has been the circumstance of overly rapid accrual, so that DLT-free information amassing at a given dose is among many patients but few with adequately long follow-up to warrant dose escalation (106). Proposed modifications appear to aid in addressing this problem and should strongly be considered (106).
A number of additional alternative phase I designs may also be considered (1), but those that permit intrapatient dose escalation are likely not appropriate because of the cumulative nature of radiation-induced toxicity and potential for late toxicities. Finally, in any phase I design selected, when writing the protocol, careful consideration must be given to therapeutic intent (curative vs palliative) and toxicity induced by radiation alone when defining dose-limiting toxicity (eg, in a trial combining a systemic agent with abdominal radiation, it may be inappropriate to consider grade 3 acute diarrhea as a dose-limiting toxicity).
In phase II trials, there has been something of a sea change in preferred design, with the hopes that failure of agents and/or regimens in phase III trials based on provocative but apparently misleading signals that arose in phase II can be reduced. The principal changes are the inclusion of a concurrent control group and randomization (63,107,108), an approach formally adopted by the Clinical Trial Design Task Force of the NCI Investigational Drug Steering Committee for combination (eg, radiation + drug) trials (109). Journal editors have likewise called for more substantive reports of promising treatments at this pilot efficacy stage, with single-arm, historically controlled trials reserved for some special circumstances (110). We note that the phase II randomized trials now widely favored differ from so-called selection designs (111–113), where several candidate experimental regimens are evaluated concurrently and the best is chosen without regard to type 1 error control or even direct comparative testing. Rather, the currently favored designs are pilot efficacy trials with a standard therapy control group (124), and this approach seems particularly relevant for combination trials with radiation therapy because techniques for the latter continue to advance, rendering historical control data less relevant. Similarly, other factors, such as the growth-inhibiting activity of many new agents (as opposed to traditional cytotoxic effects) and the advent of new molecularly defined disease classes to which agents are targeted, point to a need for a concurrent comparison group for reliable activity assessment. The potential benefits of randomized phase II trials come at the expense of larger sample size and collection of control group data that may be of limited interest (115), and it is yet to be established whether the rate of phase III success will be improved with this approach. Nonetheless, this is a reasonable expectation based on the aforementioned considerations, and there are additional benefits to controlled trials introduced at this earlier stage, such as biomarker discovery and validation (116). Finally, we note that distinguishing features of a randomized phase II trial relative to a definitive phase III evaluation include 1) a biological or clinical activity endpoint rather than an endpoint with unequivocal clinical benefit thus intended to change clinical practice and 2) less-stringent error control and power requirements that are sufficient to inform the decision to pursue and the design for a phase III evaluation.
Once one chooses to implement a randomized phase II design, a number of additional design questions immediately arise. These include 1) the allocation ratio, or whether the randomization should be unbalanced or even dynamically adaptive to observed response, 2) the number of treatment arms and a possible strategy for discontinuing unpromising arms, and 3) whether biomarkers putatively indicative of response should be incorporated into the entry screen or evaluation of therapy response.
When allocating treatment in large, randomized phase II studies and in phase III studies, one may wish to use a unequal allocation ratio [eg, a ratio of 3:2 as in the UK CHART trial (117)], favoring allocation to the experimental arm to obtain more information on the experimental agent and possibly increase appeal of the study. If the allocation ratio is less than 2:1, the increase in sample size required to achieve the same statistical power as for 1:1 allocation will be very modest. Alternatively, the allocation ratio may be altered as response information accrues, an example of a so-called adaptive design. This idea, which has garnered much recent attention, was actually proposed more than four decades ago in the context of clinical trials (118) and is currently associated with a Bayesian statistical approach, which provides a convenient structure for adapting the allocation ratio. Although there are several proposed advantages of adaptive randomization (119), standard designs with fixed allocation ratios can be shown to be more efficient statistically (120,121) and thus offer the most practical approach in many instances.
A related major consideration concerns multiple treatment arms. There is obvious sample size efficiency in a common shared control arm for several experimental arm comparisons as well as logistical gains from setting up one trial rather than several, and a number of the more innovative Bayesian designs focus on this aspect. These are among the points raised by Parmar et al. in their review, and they call for a more effective screening program for anticancer treatment agents, accompanied by a specific frequentist multiarm screening concept that they have implemented (100). This once again is an idea with developments and proposals dating back some time (101,123). One extra-statistical challenge involves complexities of negotiation with multiple industry interests in a single trial (100). One specific consideration in relation to radiation therapy trials is the long latency of late radiation toxicities and the general focus on therapeutic ratio, which makes dynamic trial designs more challenging because important adverse event data for a given treatment may not be acquired until relatively late in the trial.
A final consideration is whether and how to incorporate biomarkers into the trial. When biologically supported, a narrowly focused trial on those most likely to respond or experience a large benefit is an efficient approach. However, a trial with restrictive marker-based entry criteria may leave questions unanswered regarding the clinical utility of the marker and whether benefit is truly restricted to marker-positive patients (124). In the restricted case, generally referred to as an enrichment design, one screens potential entrants for the marker and randomizes only those who are positive (125), thus determining response in this class of patients. In the broader design, both marker-positive and marker-negative patients are included, with randomization within marker strata to the same comparative arms, which permits evaluation of the putative predictive effect of the marker. Additional considerations regarding marker testing in clinical trials are discussed in the reviews by Simon and Freidlin (126,127). Finally, the adaptive concept can be combined with marker-based trials, leading to a host of new design types (128). The I-SPY breast cancer trials (129), which enable the rapid assessment of novel phase II drugs and the identification of effective drugs and drug combinations in different breast cancer subtypes, are an interesting example in practice. In general, the advantages and limitations of many new proposed trial designs in the context of biomarkers must be carefully evaluated (128). More research is required to understand where there may be appropriate application of novel designs to drug–radiation combinations.
Conclusions
For many common cancers, adding novel targeted agents to radiotherapy or radio-chemotherapy may increase cure rates. New approaches include combining radiosensitizers with hypofractionated radiation schedules and integrating immunomodulators with radiation therapy. Researchers should not be overly reliant on in vivo models, with more emphasis placed on the incorporation of correlative endpoints into early-phase clinical studies. Further work is required on the development and validation of preclinical models and the application nof the scientific method to determine the optimal path for the early-phase clinical development of radiation sensitizers.
Funding
YRL was supported by the ASCO Cancer Foundation Young Investigator Award. AC was supported by the National Cancer Institute, National Institutes of Health (R01CA108633, RC2CA148190, the Brain Tumor Funders Collaborative Group). The National Cancer Institute supports the Translational Research Program of the Radiation Therapy Oncology Group (RTOG) (grants U10 CA21661, U10 CA37422, and U10 CA32115). The Kimmel Cancer Center at Thomas Jefferson University is supported by the National Institutes of Health Cancer Center Core grant (P30CA56036).
Supplementary Material
Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or the ASCO Conquer Cancer Foundation.
B. Vikram, N. Takebe, B. Freidlin, and C. N. Coleman contributed to this work in their personal capacities, and the opinions herein do not necessarily reflect the official position of the National Institutes of Health or the Department of Health and Human Services.
Y. Lawrence has received grant support from Merck & Co., Inc. and Wyeth. M. Machtay has received speaking honoraria/consulting fees from BMS, Imclone, and Eli Lilly (regarding Cetuximab) S. M. Bentzen has received consulting fees from AstraZeneca.
References
- 1. Harrington KJ, Billingham LJ, Brunner TB, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br J Cancer. 2011; 105(5):628–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colevas AD, Brown JM, Hahn S, et al. Development of investigational radiation modifiers. J Natl Cancer Inst. 2003; 95(9):646–651 [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987 [DOI] [PubMed] [Google Scholar]
- 4. Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009; 92(1):4–14 [DOI] [PubMed] [Google Scholar]
- 5. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006; 354(6): 567 [DOI] [PubMed] [Google Scholar]
- 6. Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992; 326(8):524–530 [DOI] [PubMed] [Google Scholar]
- 7. Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA. 1999; 281(17):1623–1627 [DOI] [PubMed] [Google Scholar]
- 8. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345(10):725–730 [DOI] [PubMed] [Google Scholar]
- 9. Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991; 324(11):709–715 [DOI] [PubMed] [Google Scholar]
- 10. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996; 348(9034):1049–1054 [PubMed] [Google Scholar]
- 11. Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008; 26(35):5802–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 2009; 337(5):295–300 [DOI] [PubMed] [Google Scholar]
- 13. James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012; 366(16):1477–1488 [DOI] [PubMed] [Google Scholar]
- 14. Bentzen S, Harari P, Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat Rev Clin Oncol. 2007; 4(3):172–180 [DOI] [PubMed] [Google Scholar]
- 15. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006; 354(6):567–578 [DOI] [PubMed] [Google Scholar]
- 16. Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007; 357(20):2040–2048 [DOI] [PubMed] [Google Scholar]
- 17. Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009; 373(9674):1525–1531 [DOI] [PubMed] [Google Scholar]
- 18. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008; 359(11):1116–1127 [DOI] [PubMed] [Google Scholar]
- 19. Brown JM. The hypoxic cell: a target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999; 59(23):5863–5870 [PubMed] [Google Scholar]
- 20. Hoogsteen IJ, Marres HA, van der Kogel AJ, Kaanders JH. The hypoxic tumour microenvironment, patient selection and hypoxia-modifying treatments. Clin Oncol (R Coll Radiol). 2007; 19(6):385–396 [DOI] [PubMed] [Google Scholar]
- 21. Kaanders JH, Bussink J, van der Kogel AJ. Clinical studies of hypoxia modification in radiotherapy. Semin Radiat Oncol. 2004; 14(3):233–240 [DOI] [PubMed] [Google Scholar]
- 22. McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol). 2007; 19(6):427–442 [DOI] [PubMed] [Google Scholar]
- 23. Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007; 25(26):4066–4074 [DOI] [PubMed] [Google Scholar]
- 24. Kallman RF, Dorie MJ. Tumor oxygenation and reoxygenation during radiation therapy: their importance in predicting tumor response. Int J Radiat Oncol Biol Phys. 1986; 12(4):681–685 [DOI] [PubMed] [Google Scholar]
- 25. Ang KK. More Lessons learned from the suffocation of hypoxia. J Clin Oncol. 2010; 28(18):2941–2943 [DOI] [PubMed] [Google Scholar]
- 26. Brown JM, Lemmon MJ. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 1990; 50(24):7745–7749 [PubMed] [Google Scholar]
- 27. Brown JM, Marilyn JL. Tumor hypoxia can be exploited to preferentially sensitize tumors to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1991; 20(3):457–461 [DOI] [PubMed] [Google Scholar]
- 28. Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010; 28(18):2989–2995 [DOI] [PubMed] [Google Scholar]
- 29. Williamson SK, Crowley JJ, Lara PN, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005; 23(36):9097–9104 [DOI] [PubMed] [Google Scholar]
- 30. Huxham LA, Kyle AH, Baker JH, McNicol KL, Minchinton AI. Exploring vascular dysfunction caused by tirapazamine. Microvasc Res. 2008; 75(2):247–255 [DOI] [PubMed] [Google Scholar]
- 31. Huxham LA, Kyle AH, Baker JH, McNicol KL, Minchinton AI. Tirapazamine causes vascular dysfunction in HCT-116 tumour xenografts. Radiother Oncol. 2006; 78(2):138–145 [DOI] [PubMed] [Google Scholar]
- 32. Bains LJ, Baker JH, Kyle AH, Minchinton AI, Reinsberg SA. Detecting vascular-targeting effects of the hypoxic cytotoxin tirapazamine in tumor xenografts using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009; 74(3):957–965 [DOI] [PubMed] [Google Scholar]
- 33. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011; 11(6):393–410 [DOI] [PubMed] [Google Scholar]
- 34. Dassonville O, Formento JL, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol. 1993; 11(10):1873–1878 [DOI] [PubMed] [Google Scholar]
- 35. Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002; 62(24):7350–7356 [PubMed] [Google Scholar]
- 36. Huang S-M, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000; 6(6):2166–2174 [PubMed] [Google Scholar]
- 37. Klaus D, Claus M, Hans-Peter R. Inhibition of radiation-induced EGFR nuclear import by C225 (cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005; 76(2):157–161 [DOI] [PubMed] [Google Scholar]
- 38. Bonner JA, Raisch KP, Trummell HQ, et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J Clin Oncol. 2000; 18(suppl1):47s–53 [PubMed] [Google Scholar]
- 39. Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B Trial 30407. J Clin Oncol. 2011; 29(23):3120–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010; 98(1):93–99 [DOI] [PubMed] [Google Scholar]
- 41. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010; 11(1):21–28 [DOI] [PubMed] [Google Scholar]
- 42. Giro C, Berger B, Bolke E, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 2009; 90(2):166–171 [DOI] [PubMed] [Google Scholar]
- 43. Glass C, Den R, Dicker AP, Lawrence YR. Toxicity of phase I radiation oncology trials: worldwide experience. Int J Radiat Oncol Biol Phys. 2010; 78(3)(suppl):s65 [Google Scholar]
- 44. Goldie JH, Coldman AJ. Drug Resistance in Cancer: Mechanisms and Models. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 45. Stegmeier F, Warmuth M, Sellers WR, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Ther. 2010. 87(5):543–552 [DOI] [PubMed] [Google Scholar]
- 46. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008; 8(8):592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hait WN. Targeted cancer therapeutics. Cancer Res. 2009; 69(4):1263–1267 [DOI] [PubMed] [Google Scholar]
- 48. Heppner GH. Tumor heterogeneity. Cancer Res. 1984; 44(6):2259–2265 [PubMed] [Google Scholar]
- 49. Axelson H, Fredlund E, Ovenberger M, Landberg G, Pahlman S. Hypoxia-induced dedifferentiation of tumor cells—mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol. 2005; 16(4–5): 554–563 [DOI] [PubMed] [Google Scholar]
- 50. Linder S, Shoshan M. Is translational research compatible with preclinical publication strategies? Radiat Oncol. 2006; 1 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001; 7(9):987–989 [DOI] [PubMed] [Google Scholar]
- 52. Movsas B. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation Therapy Oncology Group Trial 98-01. J Clin Oncol. 2005; 23(10):2145–2154 [DOI] [PubMed] [Google Scholar]
- 53. Eisbruch A, Shewach DS, Bradford CR, et al. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: a phase I trial and intracellular drug incorporation study. J Clin Oncol. 2001; 19(3):792–799 [DOI] [PubMed] [Google Scholar]
- 54. van Putten JWG, Price A, van der Leest AHD, Gregor A, Little FA, Groen HJM. A phase I study of gemcitabine with concurrent radiotherapy in stage III, locally advanced non-small cell lung cancer. . Clin Cancer Res. 2003; 9(7):2472–2477 [PubMed] [Google Scholar]
- 55. Milas L, Fujii T, Hunter N, et al. Enhancement of tumor radioresponse in vivo by gemcitabine. Cancer Res. 1999; 59(1):107–114 [PubMed] [Google Scholar]
- 56. Gregoire V, Beauduin M, Rosier JF, et al. Kinetics of mouse jejunum radiosensitization by 2’,2’-difluorodeoxycytidine (gemcitabine) and its relationship with pharmacodynamics of DNA synthesis inhibition and cell cycle redistribution in crypt cells. Br J Cancer. 1997; 76(10):1315–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol. 2006; 24(27):4426–4433 [DOI] [PubMed] [Google Scholar]
- 58. O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990; 46(1):33–48 [PubMed] [Google Scholar]
- 59. Albani S, Prakken B. The advancement of translational medicine—from regional challenges to global solutions. Nat Med. 2009; 15(9):1006–1009 [DOI] [PubMed] [Google Scholar]
- 60. Borad MJ, Von Hoff DD. Clinical trial designs for more rapid proof-of-principle and approval. In: Neidel S, ed. Cancer Drug Design and Discovery. 1sted. Waltham, MA: Academic Press; 2007; 53–87 [Google Scholar]
- 61. Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008; 59 1–12 [DOI] [PubMed] [Google Scholar]
- 62. Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010. 16;(6):1745–1755 [DOI] [PubMed] [Google Scholar]
- 63. Seymour L, Ivy SP, Sargent D, et al. The design of phase II clinical trials testing cancer therapeutics: consensus recommendations from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res. 2010; 16(6):1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Forster MD, Saijo N, Seymour L, Calvert H. Performing phase I clinical trials of anticancer agents: perspectives from within the European Union and Japan. Clin Cancer Res. 2010. 16(6):1737–1744 [DOI] [PubMed] [Google Scholar]
- 65. Bates SE. Phase I clinical trials: overcoming barriers. Clin Cancer Res. 2010. 16(6):1709 [DOI] [PubMed] [Google Scholar]
- 66. Rock EP, Molloy VJ, Humphrey JS. GCP data quality for early clinical development. Clin Cancer Res. 2010. 16(6):1756–1763 [DOI] [PubMed] [Google Scholar]
- 67. LoRusso PM, Boerner SA, Seymour L. An overview of the optimal planning, design, and conduct of phase I studies of new therapeutics. Clin Cancer Res. 2010. 16(6):1710–1718 [DOI] [PubMed] [Google Scholar]
- 68. Senderowicz AM. Information needed to conduct first-in-human oncology trials in the United States: a view from a former FDA medical reviewer. Clin Cancer Res. 2010. 16(6):1719–1725 [DOI] [PubMed] [Google Scholar]
- 69. Ivy SP, Siu LL, Garrett-Mayer E, Rubinstein L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the National Cancer Institute investigational drug steering committee. Clin Cancer Res. 2010;16(6):1726–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosenstein BS, Held KD, Rockwell S, Williams JP, Zeman EM. American Society for Radiation Oncology (ASTRO) survey of radiation biology educators in U.S. and Canadian radiation oncology residency programs. Int J Radiat Oncol Biol Phys. 2009; 75(3):896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wasserman TH, Smith SM, Powell SN. The growth of academic radiation oncology: a survey of endowed professorships in radiation oncology. Int J Radiat Oncol Biol Phys. 2009; 74(2):338–340 [DOI] [PubMed] [Google Scholar]
- 72. Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006; 66(20):10040–10047 [DOI] [PubMed] [Google Scholar]
- 73. Jaboin JJ, Shinohara ET, Moretti L, Yang ES, Kaminski JM, Lu B. The role of mTOR inhibition in augmenting radiation induced autophagy. Technol Cancer Res Treat. 2007; 6(5):443–447 [DOI] [PubMed] [Google Scholar]
- 74. Shinohara E, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005; 24(35):5414–5422 [DOI] [PubMed] [Google Scholar]
- 75. Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology. Part 2. State of the science by anatomic site. Oncology (Williston Park). 2009; 23(4):380–385 [PubMed] [Google Scholar]
- 76. Maity A, Bernhard EJ. Modulating tumor vasculature through signaling inhibition to improve cytotoxic therapy. Cancer Res. 2010; 70(6):2141–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. FitzGerald TJ, Urie M, Ulin K, et al. Processes for quality improvements in radiation oncology clinical trials. Int J Radiat Oncol Biol Phys. 2008; 71(1)(suppl. 1):S76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Justin EB, Joachim Y. Quality of radiotherapy reporting in randomized controlled trials of Hodgkin’s lymphoma and non-Hodgkin’s lymphoma: a systematic review. Int J Radiat Oncol Biol Phys. 2009; 73(2):492–498 [DOI] [PubMed] [Google Scholar]
- 79. Morris SL, Beasley M, Leslie M. Chemotherapy for pancreatic cancer. N Engl J Med. 2004; 350(26):2713–2715 [DOI] [PubMed] [Google Scholar]
- 80. Bydder S, Spry N. Chemotherapy for pancreatic cancer. N Engl J Med. 2004; 350(26):2713–2715 [PubMed] [Google Scholar]
- 81. Crane CH, Ben-Josef E, Small W., Jr Chemotherapy for pancreatic cancer. N Engl J Med. 2004; 350(26):2713–2715 [PubMed] [Google Scholar]
- 82. Rischin D, Peters L, Fisher R, et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol. 2005; 23(1):79 [DOI] [PubMed] [Google Scholar]
- 83. Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total- nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997; 15(8):2769–2779 [DOI] [PubMed] [Google Scholar]
- 84. Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704-A phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2012;82(2):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Michael JZ, Didier C, Zvi F, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999; 85(11):2460–2468 [DOI] [PubMed] [Google Scholar]
- 86. Delanian S, Porcher Rl, Rudant Jrm, Lefaix J-L. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005; 23(34):8570–8579 [DOI] [PubMed] [Google Scholar]
- 87. Choy H, Jain AK, Moughan J, et al. RTOG 0017: a phase I trial of concurrent gemcitabine/carboplatin or gemcitabine/paclitaxel and radiation therapy (“ping-pong trial”) followed by adjuvant chemotherapy for patients with favorable prognosis inoperable stage IIIA/B non-small cell lung cancer. J Thorac Oncol. 2009; 4(1):80–86 [DOI] [PubMed] [Google Scholar]
- 88. Kaye SB. Gemcitabine: current status of phase I and II trials. J Clin Oncol. 1994; 12(8):1527–1531 [DOI] [PubMed] [Google Scholar]
- 89. Huang YJ, Wu YL, Xie SX, Yang JJ, Huang YS, Liao RQ. Weekly gemcitabine as a radiosensitiser for the treatment of brain metastases in patients with non-small cell lung cancer: phase I trial. Chin Med J (Engl). 2007; 120(6):458–462 [PubMed] [Google Scholar]
- 90. Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade cliomas. J Clin Oncol. 2010;28(18):3048–3053.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010; 303(11):1070–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008; 112(3):650–658 [DOI] [PubMed] [Google Scholar]
- 93. Milano M, Constine L, Okunieff P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol. 2008; 3(1):36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005; 8(2):89–91 [DOI] [PubMed] [Google Scholar]
- 95. Acker JC, Marks LB, Spencer DP, et al. Serial in vivo observations of cerebral vasculature after treatment with a large single fraction of radiation. Radiat Res. 1998; 149(4):350–359 [PubMed] [Google Scholar]
- 96. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012; 12(4):298–306 [DOI] [PubMed] [Google Scholar]
- 97. Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011; 71(7):2488–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012; 366(10):925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brown A, Wendler D, Camphausen K, Miller F, Citrin D. Performing nondiagnostic research biopsies in irradiated tissue: a review of scientific, clinical, and ethical considerations. . J Clin Oncol. 2008; 26(24):3987–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Parmar MK, Barthel FM, Sydes M, et al. Speeding up the evaluation of new agents in cancer. J Natl Cancer Inst. 2008; 100(17):1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sargent DJ, Goldberg RM. A flexible design for multiple armed screening trials. Stat Med. 2001; 20(7):1051–1060 [DOI] [PubMed] [Google Scholar]
- 102. LoRusso PM, Boerner SA, Seymour L. An overview of the optimal planning, design, and conduct of phase I studies of new therapeutics. Clin Cancer Res. 2010. 16(6):1710–1718 [DOI] [PubMed] [Google Scholar]
- 103. Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995; 14(11):1149–1161 [DOI] [PubMed] [Google Scholar]
- 104. Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000; 56(4):1177–1182 [DOI] [PubMed] [Google Scholar]
- 105. Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol. 2006; 24(27):4426–4433 [DOI] [PubMed] [Google Scholar]
- 106. Polley MY. Practical modifications to the time-to-event continual reassessment method for phase I cancer trials with fast patient accrual and late-onset toxicities. Stat Med. 2011; 30(17):2130–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mandrekar SJ, Sargent DJ. Randomized phase II trials: time for a new era in clinical trial design. J Thorac Oncol. 2010; 5(7):932–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ratain MJ, Sargent DJ. Optimising the design of phase II oncology trials: the importance of randomisation. Eur J Cancer. 2009; 45(2):275–280 [DOI] [PubMed] [Google Scholar]
- 109. Seymour L, Ivy SP, Sargent D, et al. The design of phase II clinical trials testing cancer therapeutics: consensus recommendations from the Clinical Trial Design Task Force of the National Cancer Institute Investigational Drug Steering Committee. Clin Cancer Res. 2010; 16(6):1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cannistra SA. Phase II trials in journal of clinical oncology. . J Clin Oncol. 2009; 27(19):3073–3076 [DOI] [PubMed] [Google Scholar]
- 111. Liu PY, LeBlanc M, Desai M. False positive rates of randomized phase II designs. Control Clin Trials. 1999; 20(4):343–352 [DOI] [PubMed] [Google Scholar]
- 112. Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985; 69(12):1375–1381 [PubMed] [Google Scholar]
- 113. Steinberg SM, Venzon DJ. Early selection in a randomized phase II clinical trial. Stat Med. 2002; 21(12):1711–1726 [DOI] [PubMed] [Google Scholar]
- 114. Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005; 23(28):7199–7206 [DOI] [PubMed] [Google Scholar]
- 115. Tang H, Foster NR, Grothey A, Ansell SM, Goldberg RM, Sargent DJ. Comparison of error rates in single-arm versus randomized phase II cancer clinical trials. J Clin Oncol. 2010; 28(11):1936–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sharma MR, Stadler WM, Ratain MJ. Randomized phase II trials: a long-term investment with promising returns. J Natl Cancer Inst. 2011; 103(14):1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet. 1997; 350(9072): 161–165 [DOI] [PubMed] [Google Scholar]
- 118. Zelen M. Play the winner rule and the controlled clinical trial. J Am Stat Assoc. 1969; 64(325):131–146 [Google Scholar]
- 119. Berry DA. Adaptive clinical trials: the promise and the caution. J Clin Oncol. 2011; 29(6):606–9 [DOI] [PubMed] [Google Scholar]
- 120. Korn EL, Freidlin B. Outcome—adaptive randomization: is it useful? J Clin Oncol. 2011; 29(6):771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Korn EL, Freidlin B. Reply to Y. Yuan. et al. J Clin Oncol. 2011; 29(13):e393 [Google Scholar]
- 122. Freidlin B, Korn EL, Gray R, Martin A. Multi-arm clinical trials of new agents: some design considerations. Clin Cancer Res. 2008; 14(14): 4368–4371 [DOI] [PubMed] [Google Scholar]
- 123. Schaid DJ, Wieand SAM, Therneau TM. Optimal two-stage screening designs for survival comparisons. Biometrika. 1990; 77(3):507–513 [Google Scholar]
- 124. Mandrekar SJ, Sargent DJ. All-comers versus enrichment design strategy in phase II trials. J Thorac Oncol. 2011; 6(4):658–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004; 10(20):6759–6763 [DOI] [PubMed] [Google Scholar]
- 126. Freidlin B, McShane LM, Korn EL. Randomized clinical trials with biomarkers: design issues. J Natl Cancer Inst. 2010; 102(3):152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Simon R. Clinical trial designs for evaluating the medical utility of prognostic and predictive biomarkers in oncology. Per Med. 2010; 7(1): 33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Freidlin B, Korn EL. Biomarker-adaptive clinical trial designs. Pharma cogenomics. 2010; 11(12):1679–1682 [DOI] [PubMed] [Google Scholar]
- 129. Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009; 86(1):97–100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.