Abstract
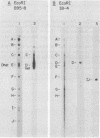
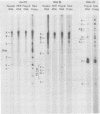
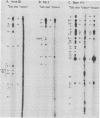
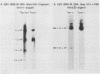
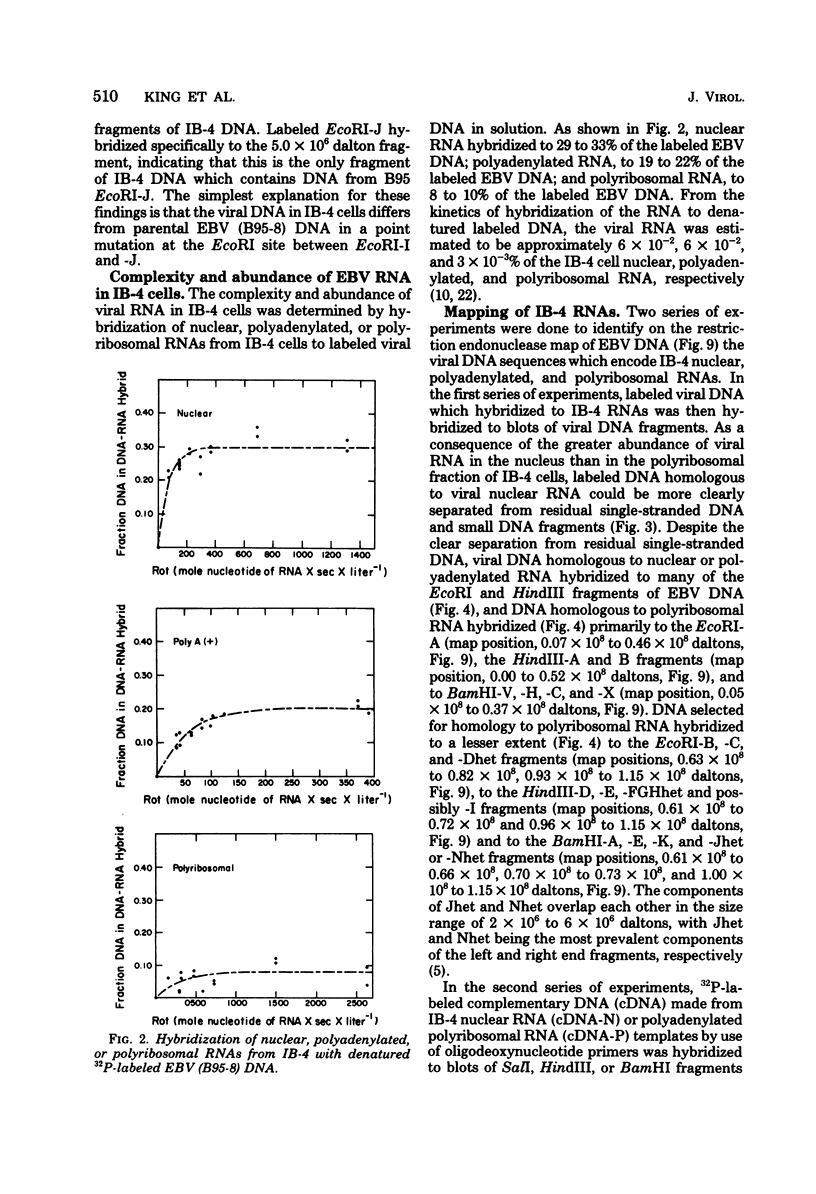
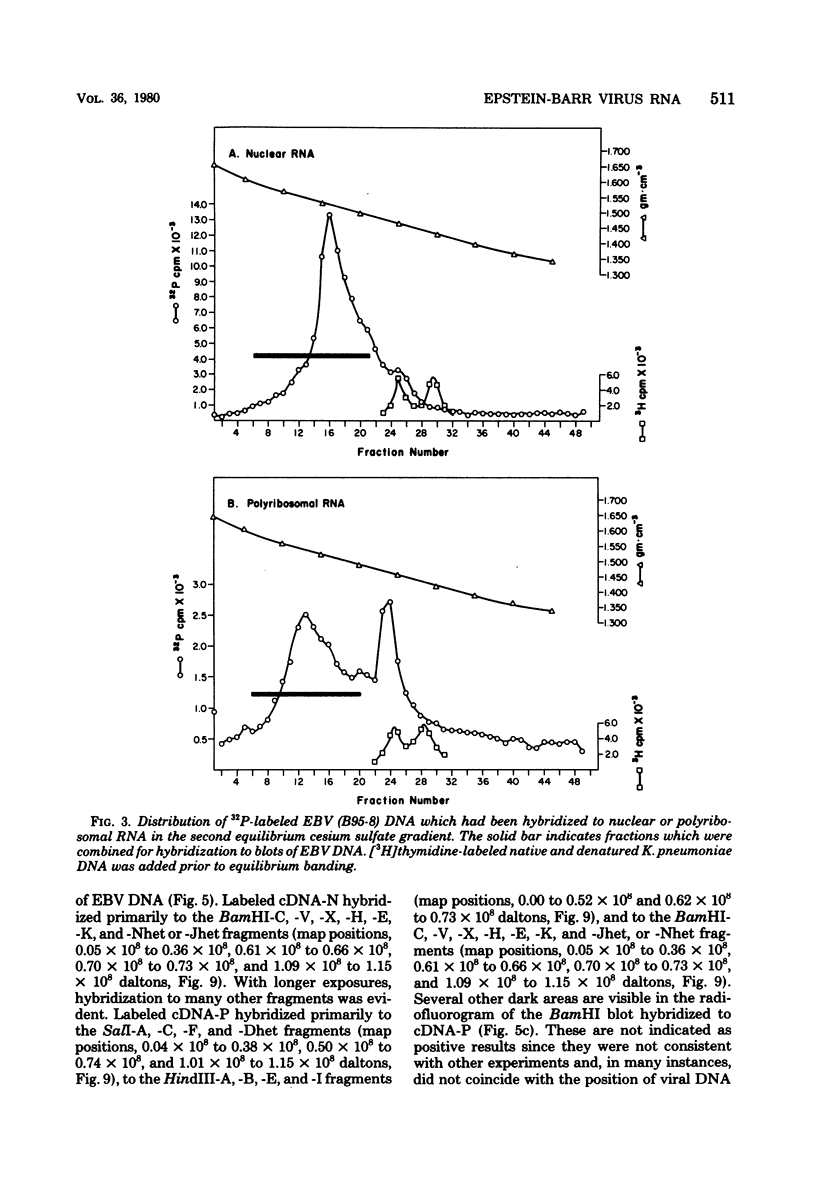
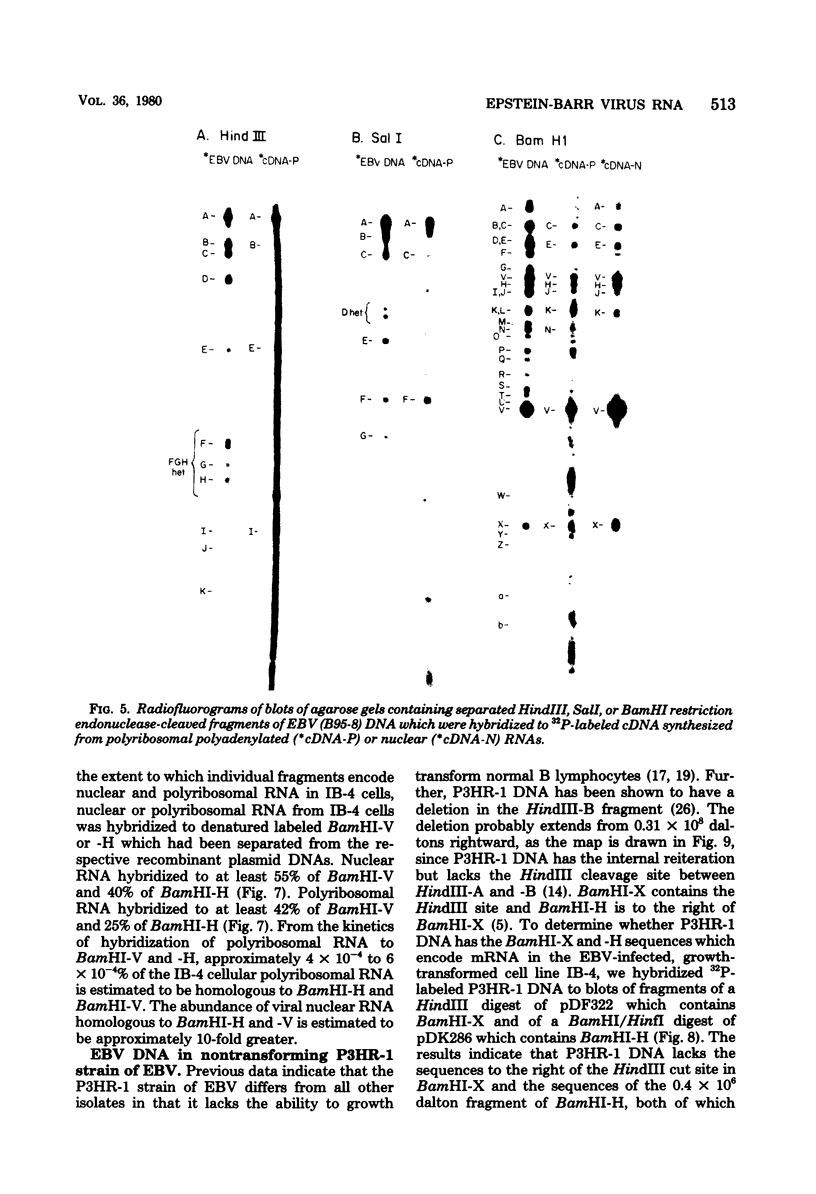
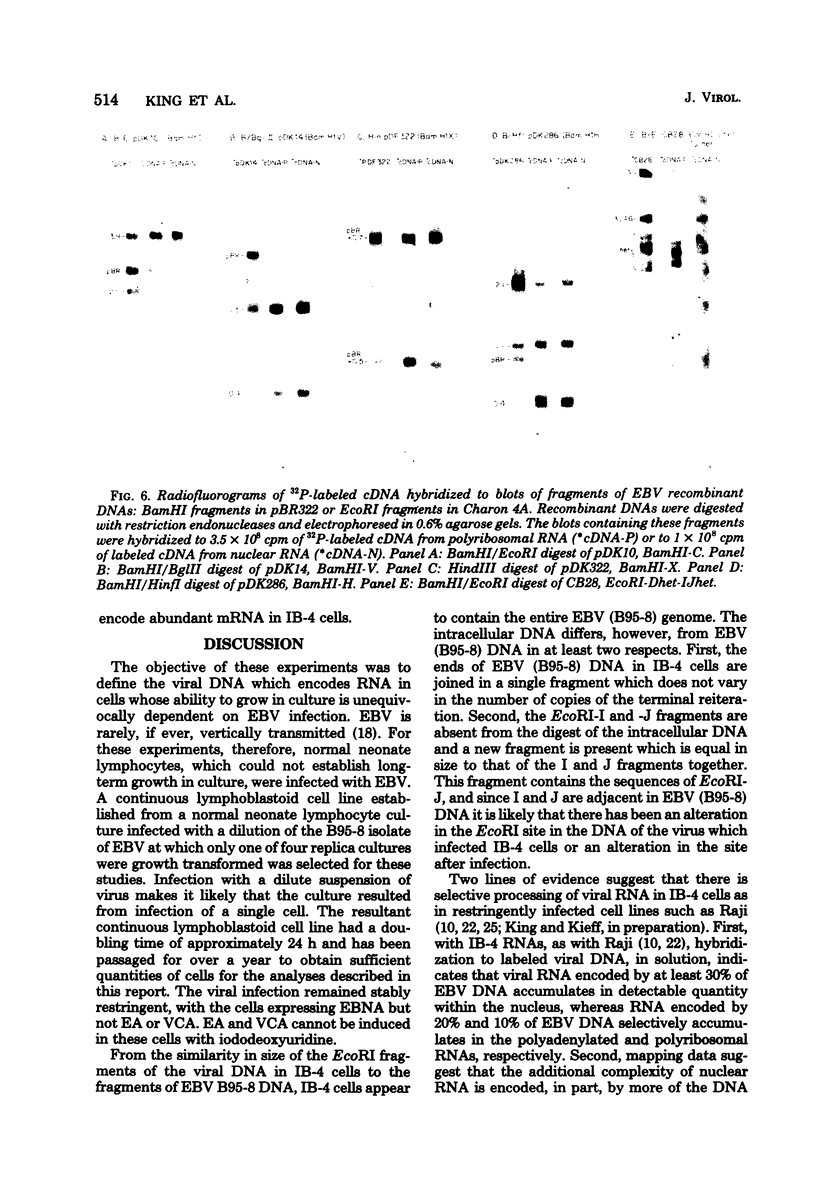
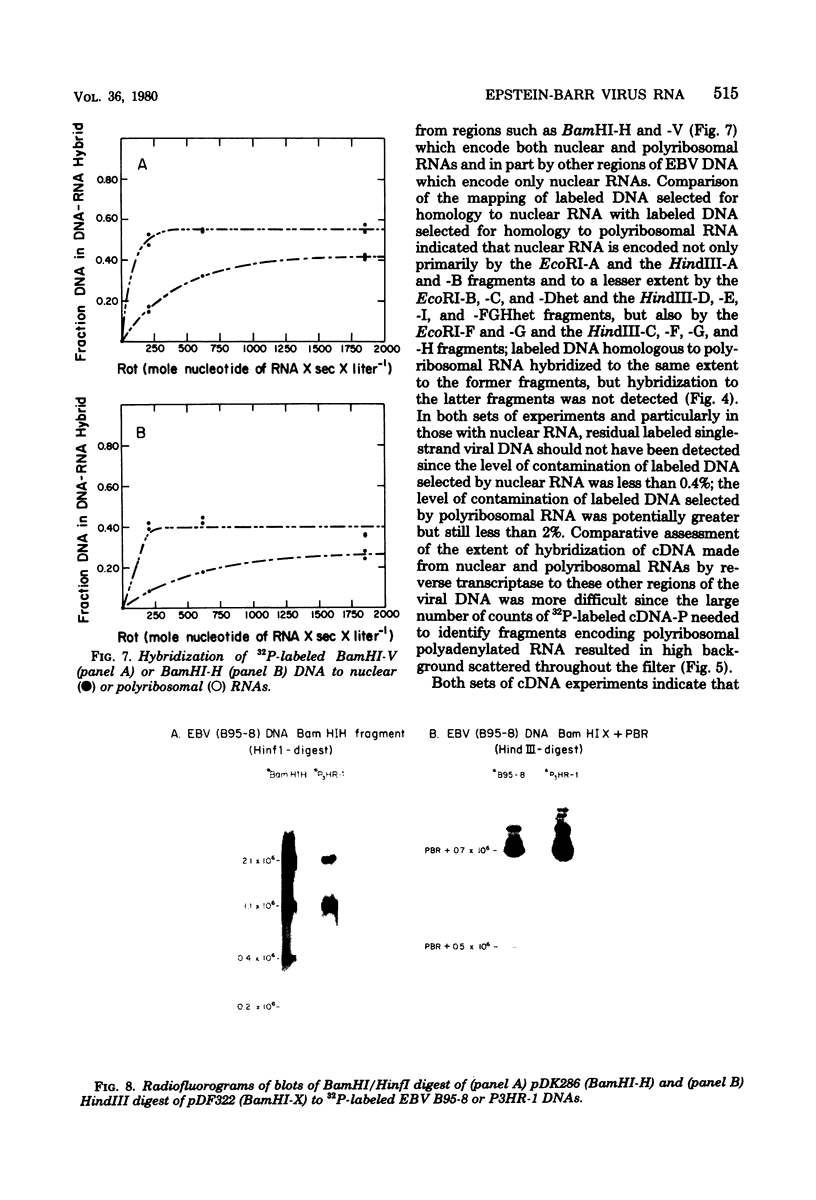
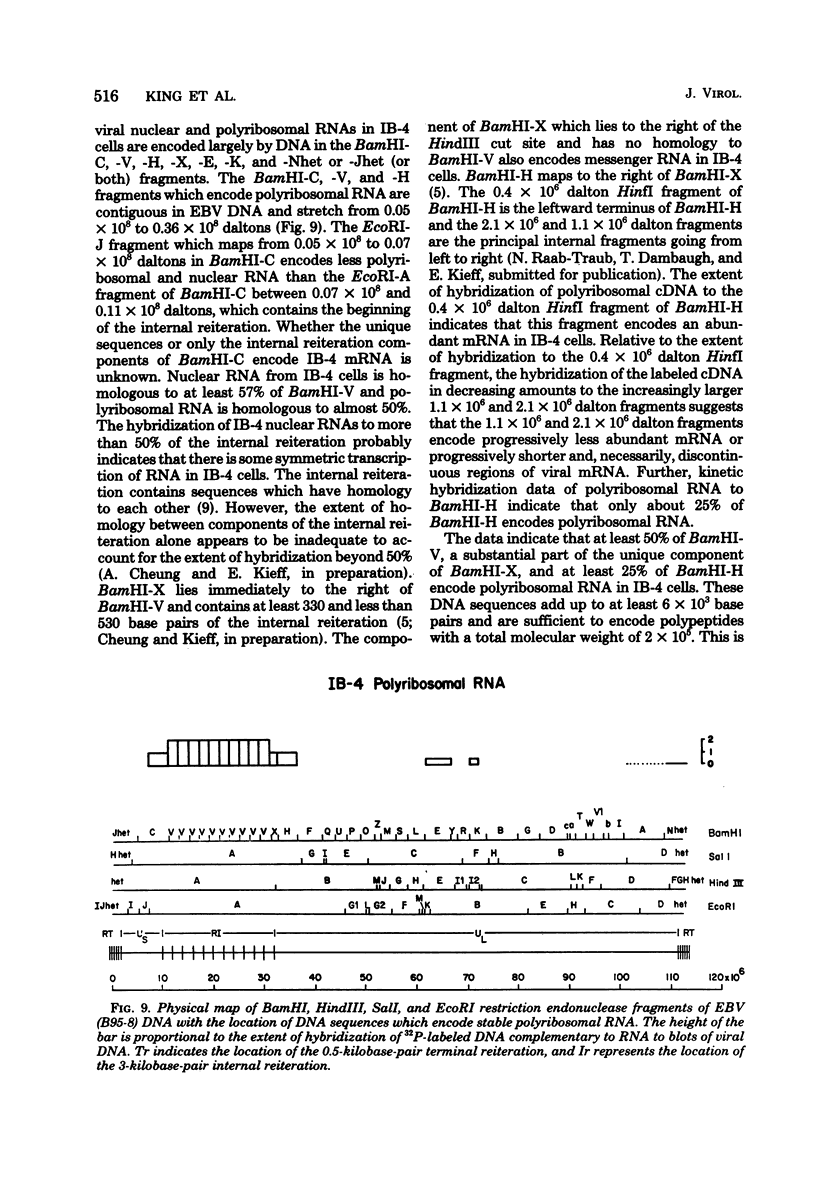
A continuous lymphoblastoid cell line, IB-4, was established by infection and growth transformation of normal neonatal B lymphocytes with the B95-8 isolate of Epstein-Barr virus (EBV). The IB-4 cells contained the intranuclear antigen, EBNA, but not early antigen, EA. The fragments produced by the digestion of intracellular episomal viral DNA (density, 1.700 to 1.720 g/cm3) with EcoRI restriction endonuclease were identical in size to the A, B, C, E, F, G, and H fragments of virion DNA. As expected from the previous observation that episomal intracellular DNA is circular, the fragment containing the rightward terminal sequences of EBV DNA in IB-4 cells was larger than the corresponding fragment of linear viral DNA, probably as a consequence of covalent linkage to the leftward terminal fragment. Also, two fragments, EcoRI-I and -J, which were adjacent to each other in the virion DNA, were absent from the intracellular DNA. The labeled EcoRI-J of viral DNA hybridized instead to a new fragment equal in size to EcoRI-I and -J combined. Analysis of viral RNA in IB-4 cells showed that RNAs encoded by more than 30% of the viral DNA comprised approximately 0.06% of the nuclear RNA, whereas RNAs encoded by 20% and 10% of the viral DNA comprised approximately 0.06% and 0.003% of the polyadenylated and polyribosomal RNAs, respectively. Viral mRNA (polyribosomal RNA) was encoded by DNA which mapped at 0.05 x 10(8) to 0.36 x 10(8) daltons and to a lesser extent by DNAs which mapped at 0.62 x 10(8) to 0.67 x 10(8), 0.70 x 10(8) to 0.73 x 10(8), and 1.13 x 10(8) to 1.15 x 10(8) daltons in the B95-8 genome. The most agundant nuclear viral RNAs were encoded primarily by DNA which mapped at the same loci; but RNAs encoded by many other fragments of viral DNA could also be detected among nuclear RNAs. Viral mRNA(s) (polyribosomal) was encoded by about 40% of the internal reiteration and by 25% of the BamHI-H fragments which mapped from 0.32 x 10(8) to 0.36 x 10(8) daltons, nuclear RNAs were encoded by at least 57% of the internal reiteration and 40% of BamHI-H. These data indicate that there is selective accumulation of some viral RNAs within the nucleus of IB-4 cells and that there is selective post-transcriptional processing of these RNAs. Finer mapping of the DNA which encodes mRNA (polyribosomal) in IB-4 cells indicated that some of this DNA is deleted in the DNA of the P3 HR-1 virus, the only isolate of EBV which cannot initiate growth transformation. These data, therefore, support the hypothesis that expression of this region of EBV genome is important for growth transformation or for the maintenance of restrigent infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Bjursell G., Kaschka-Dierich C., Lindahl T. Circular Epstein-Barr virus genomes of reduced size in a human lymphoid cell line of infectious mononucleosis origin. J Virol. 1977 May;22(2):373–380. doi: 10.1128/jvi.22.2.373-380.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain B., Pshyk K. Enhanced reactivity in mixed leukocyte cultures after separation of mononuclear cells on Ficoll-Hypaque. Transplant Proc. 1972 Jun;4(2):163–164. [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Henle W., Henle G., Hewetson J. F., Kaplan H. S. Surface marker characteristics and Epstein-Barr virus studies of two established North American Burkitt's lymphoma cell lines. Proc Natl Acad Sci U S A. 1976 Jan;73(1):228–232. doi: 10.1073/pnas.73.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. IV. Linkage map of restriction enzyme fragments of the B95-8 and W91 strains of Epstein-Barr Virus. J Virol. 1978 Nov;28(2):524–542. doi: 10.1128/jvi.28.2.524-542.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. VI. Mapping of the internal tandem reiteration. J Virol. 1979 Aug;31(2):315–324. doi: 10.1128/jvi.31.2.315-324.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. D. Epstein-Barr virus-specific RNA. I. Analysis of viral RNA in cellular extracts and in the polyribosomal fraction of permissive and nonpermissive lymphoblastoid cell lines. J Virol. 1976 May;18(2):518–525. doi: 10.1128/jvi.18.2.518-525.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. DNA of Epstein-Barr virus. II. Comparison of the molecular weights of restriction endonuclease fragments of the DNA of Epstein-Barr virus strains and identification of end fragments of the B95-8 strain. J Virol. 1977 Aug;23(2):421–429. doi: 10.1128/jvi.23.2.421-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Kieff E., Given D., Powell A. L., King W., Dambaugh T., Raab-Traub N. Epstein-Barr virus: structure of the viral DNA and analysis of viral RNA in infected cells. Biochim Biophys Acta. 1979 Nov 30;560(3):355–373. doi: 10.1016/0304-419x(79)90009-x. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G. Human lymphoblastoid cell lines and Epstein-Barr virus: a review of their interrelationships and their relevance to the etiology of leukoproliferative states in man. Yale J Biol Med. 1971 Jun;43(6):358–384. [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K. High-frequency establishment of human immunoglobulin-producing lymphoblastoid lines from normal and malignant lymphoid tissue and peripheral blood. Int J Cancer. 1971 Nov 15;8(3):432–442. doi: 10.1002/ijc.2910080311. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973 Jul 14;2(7820):93–94. doi: 10.1016/s0140-6736(73)93286-8. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Powell A. L., King W., Kieff E. Epstein-Barr virus-specific RNA. III. Mapping of DNA encoding viral RNA in restringent infection. J Virol. 1979 Jan;29(1):261–274. doi: 10.1128/jvi.29.1.261-274.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Pritchett R., Kieff E. DNA of Epstein-Barr virus. III. Identification of restriction enzyme fragments that contain DNA sequences which differ among strains of Epstein-Barr virus. J Virol. 1978 Aug;27(2):388–398. doi: 10.1128/jvi.27.2.388-398.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]