Abstract
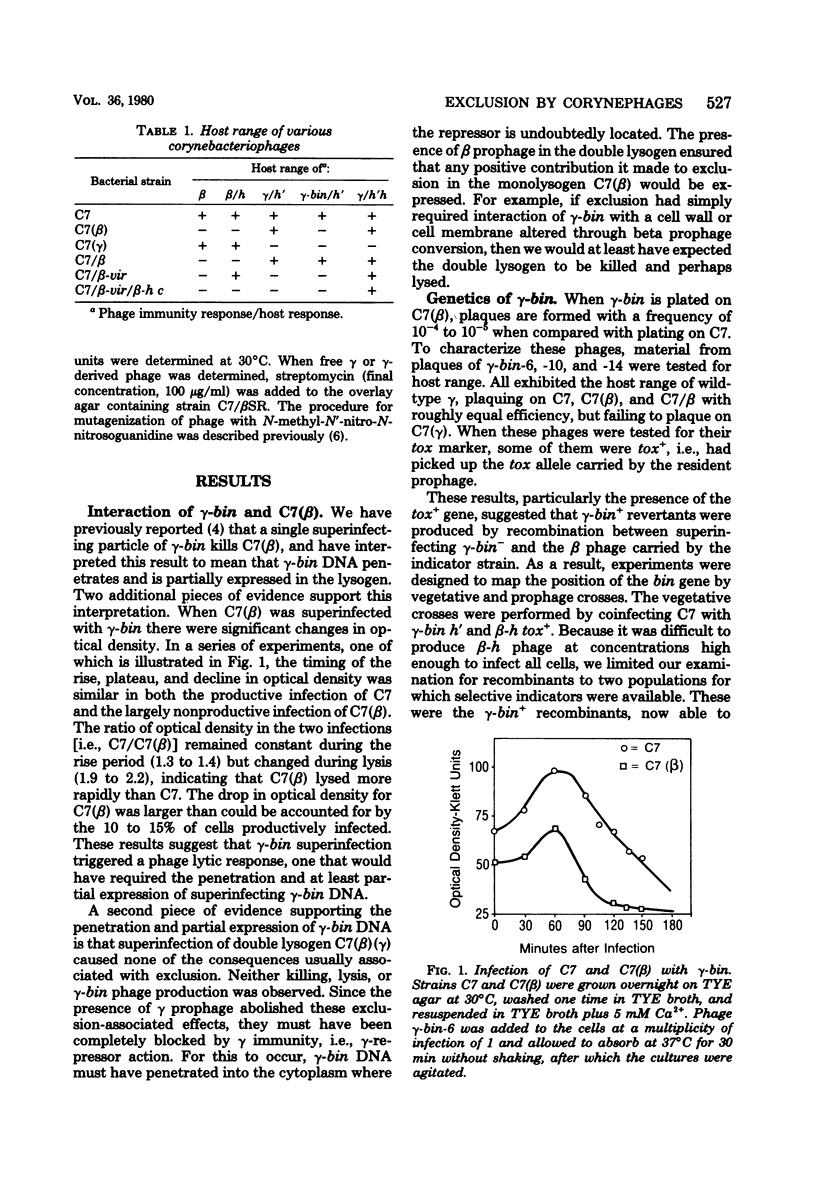
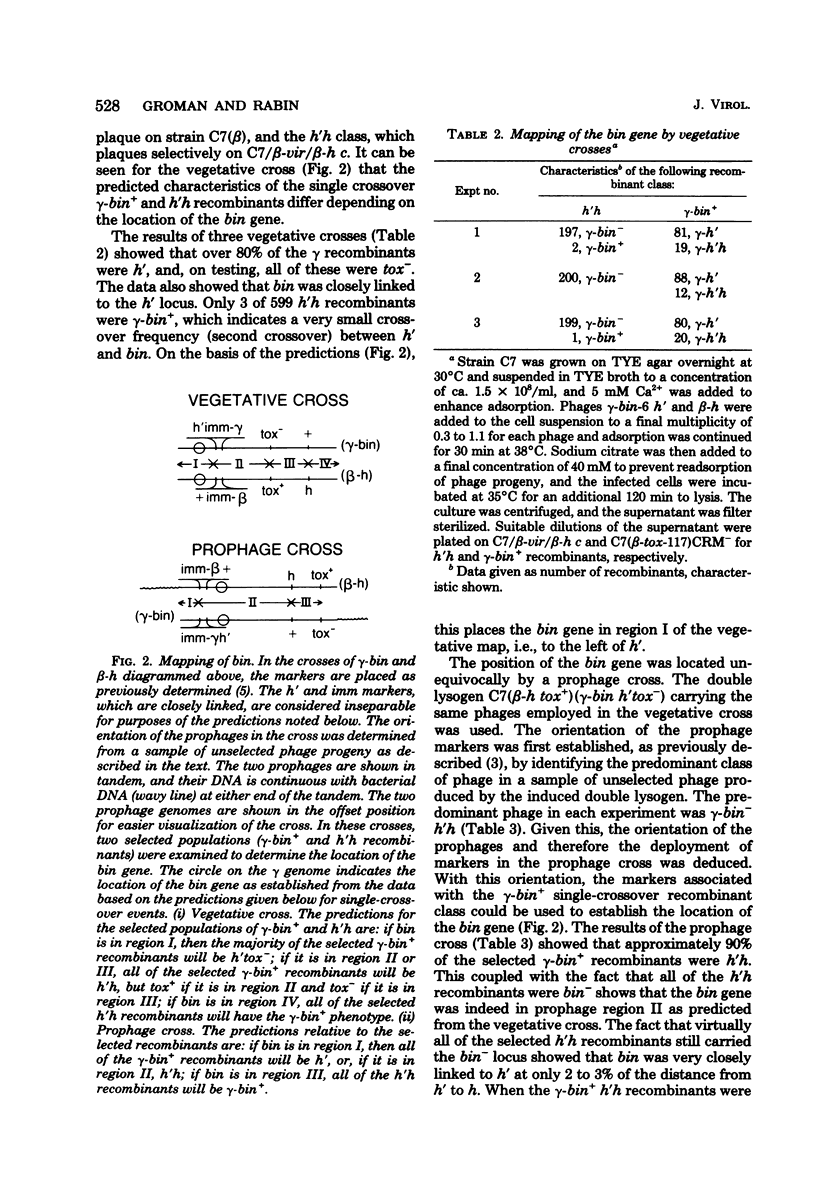
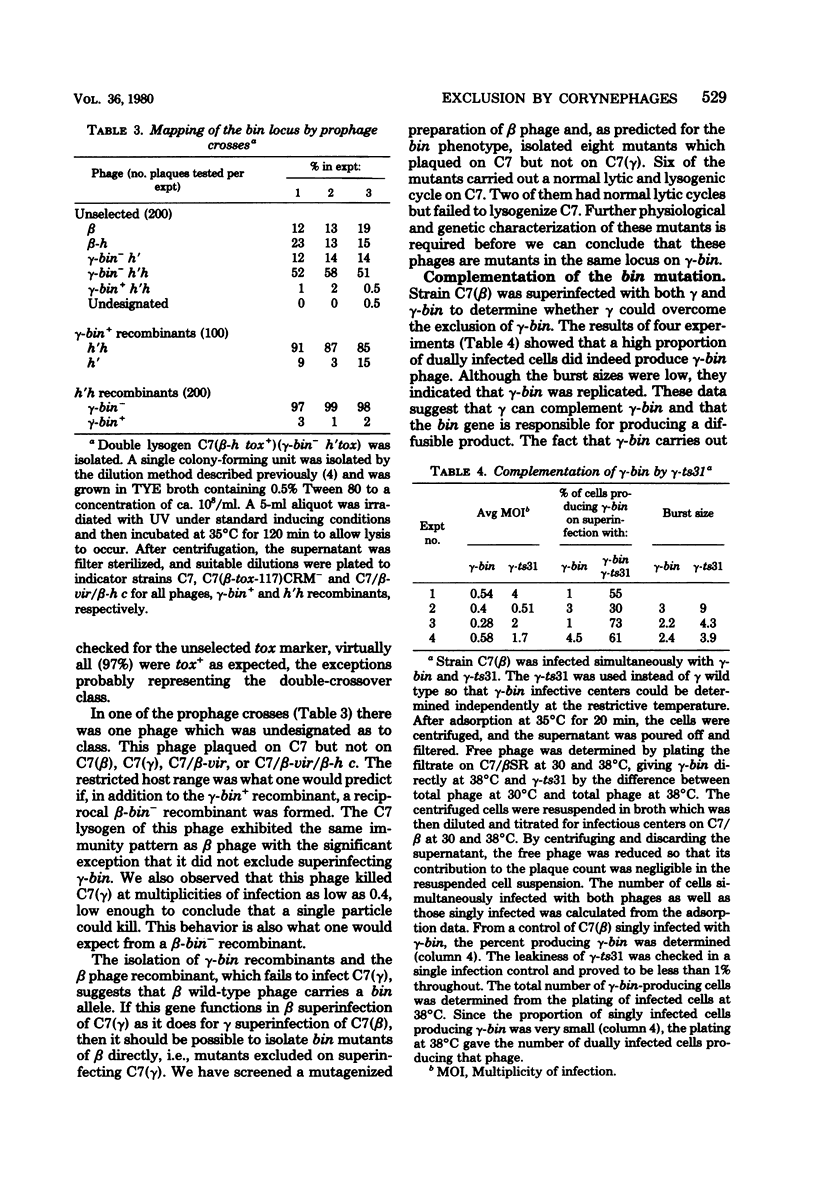
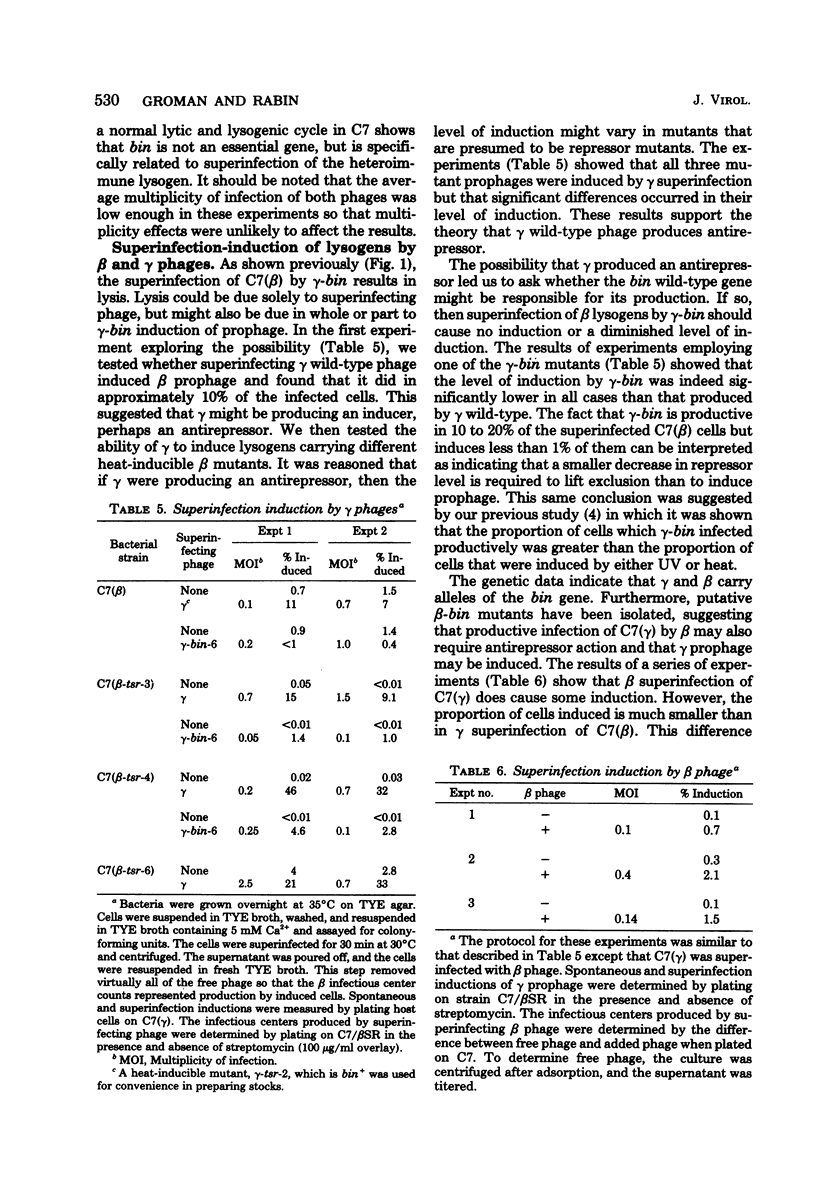
Superinfection of Corynebacterium diphtheriae C7(beta) by heteroimmune phage gamma is productive, whereas superinfection by gamma-bin mutants is for the most part nonproductive. Exclusion of gamma-bin phage occurred after its DNA had penetrated and was partially expressed in the heteroimmune lysogen. All of the infected cells were killed, and lysis was observed. The beta inhibitor causing exclusion was produced during the prophage state and appeared to be distinct from immune repressor. The ability of gamma-bin phage to superinfect C7(beta) productively could be restored by recombination with beta phage, indicating that both beta and gamma phages contain either indentical or similar alleles of the bin gene. The bin gene was mapped by vegetative and prophage crosses and found to be located in the region of the phage genome concerned with regulation. Both beta and gamma wild-type phages induced the resident prophage in a significant fraction of superinfeted heteroimmune lysogens. This, coupled with the fact that induction of C7(beta) abolished exclusion, suggests that the bin gene product acts as antirepressor, i.e., it reduces the level of heteroimmune repressor either directly or indirectly. The gamma-bin mutants either failed to produce antirepressor or did so with reduced efficiency. Antirepressor activity was negatively controlled by homoimmune repressor. The isolation of beta mutants that appeared bin-like suggests that beta and gamma phages contain homologous systems of exclusion and antiexclusion. Exclusion of gamm-bin by beta phage in gram-positive C. diphtheriae exhibited striking parallels to the sieB exclusion described for phages P22 and lambda in gram-negative organisms. The extended similarities of these coryngephages to lambda bacteriophage is noted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck G., Groman N., Falkow S. Relationship between beta converting and gamma non-converting corynebacteriophage DNA. Nature. 1978 Feb 16;271(5646):683–685. doi: 10.1038/271683a0. [DOI] [PubMed] [Google Scholar]
- Groman N., Laird W. Bacteriophage production by doubly lysogenic Corynebacterium diphtheriae. J Virol. 1977 Sep;23(3):592–598. doi: 10.1128/jvi.23.3.592-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N., Laird W. Heat-inducible mutants of corynebacteriophage. J Virol. 1977 Sep;23(3):587–591. doi: 10.1128/jvi.23.3.587-591.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N., Rabin M. Control of corynebacteriophage reproduction by heteroimmune repression. J Virol. 1978 Oct;28(1):28–33. doi: 10.1128/jvi.28.1.28-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Isolation and characterization of tox mutants of corynebacteriophage beta. J Virol. 1976 Jul;19(1):220–227. doi: 10.1128/jvi.19.1.220-227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Prophage map of converting corynebacteriophage beta. J Virol. 1976 Jul;19(1):208–219. doi: 10.1128/jvi.19.1.208-219.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Repression and immunity in Salmonella phages P22 and L: phage L lacks a functional secondary immunity system. Virology. 1978 Sep;89(2):618–622. doi: 10.1016/0042-6822(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Wright A., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology. 1974 Dec;62(2):367–384. doi: 10.1016/0042-6822(74)90399-7. [DOI] [PubMed] [Google Scholar]



