Abstract
Of the three principal conformations of acid-sensing ion channels (ASICs)—closed, open and desensitized—only the atomic structure of the desensitized conformation had been known. Two recent papers report the crystal structure of chicken ASIC1 in complex with the spider toxin psalmotoxin 1, and one of these studies finds that, depending on the pH, channels are in two different open conformations. Compared with the desensitized conformation, toxin binding induces only subtle structural changes in the lower part of the large extracellular domain but a complete rearrangement of the two transmembrane domains (TMDs), suggesting that desensitization gating (the transition from open to desensitized) is mainly associated with conformational rearrangements of the TMDs. Moreover, the study reveals how two different arrangements of the TMDs in the open state give rise to ion pores with different selectivity for monovalent cations.
Keywords: acid-sensing ion channel, ASIC, desensitization, epithelial Na+ channel, ion selectivity, pore structure
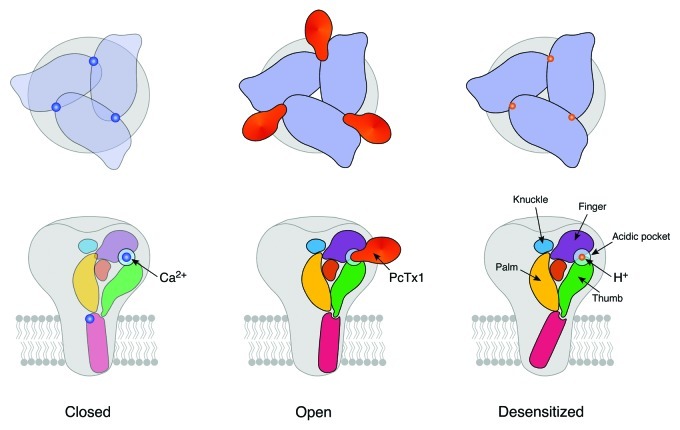
Acid-sensing ion channels (ASICs) are ligand-gated ion channels with the simplest extracellular ligand one can imagine—protons.1 ASICs have recently received considerable interest because their activation may contribute to nociception2 and several neuropathologies, including ischemic neurodegeneration during stroke3 and axonal degeneration in autoimmune neuroinflammation.4 Understanding ASIC gating (the transitions between closed, open and desensitized states; Fig. 1) at the molecular level may help to modulate ASIC activity during these pathologic situations, for example by stabilizing the non-conducting closed or desensitized states. Drug design to this end would greatly benefit from atomic resolution structures of these conformations. While, five years ago, the crystal structure of the supposedly desensitized conformation of chicken ASIC1 (cASIC1) had been resolved at 1.9 Å5, the crystal structures of resting (closed) and open conformations remained unknown. In a recent study, Baconguis and Gouaux6 reported the crystal structure of cASIC1 in complex with the spider toxin psalmotoxin1 (PcTx1) and found that the channels were in an open conformation. Even more exciting, at two different pH, channels crystallized in two different open conformations with different ion selectivities.
Figure 1. Schematic outline of the three principal conformations of ASIC1. Top, top views; bottom, side views. It appears that the acidic pocket at subunit interfaces is key to ASIC gating. Binding of PcTx1 to the acidic pocket opens the cASIC pore (center), and it has been proposed5 that binding of H+ does the same. In contrast, in the closed state a Ca2+ ion might neutralize the negative charges within the acidic pocket, stabilizing the closed state (left row).7,33 The structure of the ECD is very similar in open and desensitized conformations, whereas the structure of the TMDs differs markedly. Structures of ECD and TMDs in the closed conformation are currently unknown. Only one of the two open state conformations is shown.
Raising the H+ concentration 25-fold (from pH 7.4 to 6.0) fully activates ASIC1.5,7 In the continued presence of H+ the channel desensitizes. In the desensitized state, the channel has H+ bound but the ion pore does not conduct and cannot be opened by further increasing the H+ concentration. PcTx1 from the venom of the tarantula Psalmopoeus cambridgei is a gating modifier of ASICs8 and inhibits rat ASIC1a9 by stabilizing the desensitized conformation and trapping channels in the desensitized state.8 In contrast, PcTx1 opens rat ASIC1b and cASIC1,10,11 suggesting that it stabilizes the open conformation of these ASICs.
The cASIC1-PcTx1 Complex
In a study that appeared just a few weeks in advance of that by Baconguis and Gouaux,6 Dawson and colleagues12 also crystallized cASIC1 in complex with PcTx1, at pH 5.5 and at a medium resolution of 3.0 Å. They used a non-functional version of cASIC with truncated termini, ΔcASIC1 (26–463), however, which perturbs the structure of the transmembrane domains (TMDs),13 complicating the decision whether the pore is open or not. Using the apo cASIC1 structure (the initial high-resolution structure of the desensitized state), the structure of cASIC1 in complex with PcTx1 was determined12 and found to be almost identical to the apo structure.5 Therefore, Dawson et al.12 concluded that cASIC1 in complex with PcTx1 was in the desensitized conformation—a conclusion that turned out to be wrong (see below).
Irrespective of the conformation of the channel, the study allowed the identification of the toxin binding site and the molecular interactions of toxin and channel.12 Three PcTx1 molecules per cASIC trimer were bound in cavities known as the acidic pockets far (45 Å) from the TMDs (Fig. 2). The acidic pockets have been proposed as the ligand-binding domains (LBDs) of ASICs5 and binding of PcTx1, an agonist of cASIC1,11 in those pockets is consistent with this idea. Three independent molecular docking studies had already proposed the same PcTx1 binding site,14-16 but the precise molecular interactions between residues of the toxin and the channel predicted by these docking studies differ considerably from those revealed by crystallization,12 highlighting that toxin binding induces conformational changes of the channel and the toxin.12 In fact, a study using high-resolution NMR spectroscopy found that structural flexibility of a K+ channel and a scorpion toxin represents an important determinant for the high specificity of toxin–channel interactions.17
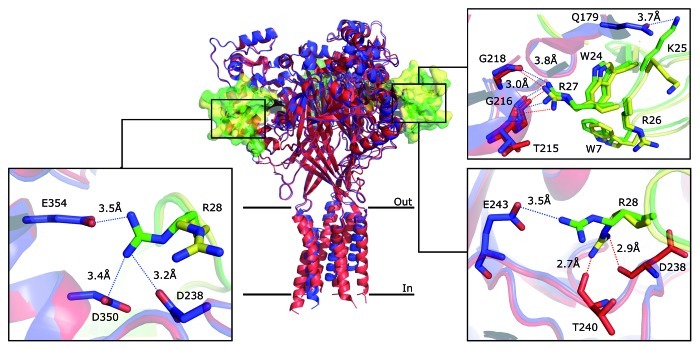
Figure 2. Molecular interactions between PcTx1 and cASIC1. The superposition of the structure obtained by Dawson et al. (PDB ID 3S3X, blue) and the low-pH structure obtained by Baconguis and Gouaux (PDB ID 4FZ0, red) is shown in cartoon representation. PcTx1 is shown in solvent-accessible surface representation (Dawson in green and Baconguis in yellow). The discrepancies of the two structures concerning their molecular interactions are illustrated in boxes; for details see text. Blue dashed lines indicate the possible hydrogen bonds in the structure obtained by Dawson et al. and red dashed lines in the structure obtained by Baconguis and Gouaux.
Dawson et al.12 noted that PcTx1 binding induced only subtle conformational changes in cASIC1: a slight shift (by 1.3 Å) of α-helices 4 and 5 and an unallocated electron density in the extracellular domain (ECD) that might originate from a cation bound in the central vestibule of the channel. They proposed that the nanomolar affinity of PcTx1 is mainly determined by strong hydrophobic interactions with cASIC1, whereas the specificity of the binding comes from a cluster of basic residues, which is flexible in solution18 and extends deeply into the acidic pocket.12 In fact, the hydrophobic patch on PcTx1 seals the basic cluster enhancing the electrostatic interactions with acidic residues of cASIC1.12
The cASIC1-PcTx1 complex is not the first crystal of a complex of the LBD of an ion channel with a toxin. Previously, complexes of α-cobratoxin (α-Ctx) and α-conotoxins with the acetylcholine binding protein (AchBP),19-21 a homolog of the LBD of nicotinic acetylcholine receptors (nAchR), and of α-bungarotoxin (α-Btx) with a single ECD of nAchR α122 had been crystallized, revealing that these toxins deeply bind into the LBDs at subunit interfaces via extensive hydrophobic interactions, which are complemented by hydrogen bonding and electrostatic and cation-π interactions.19-21,23 These toxins behave either like antagonists stabilizing the resting state of AchBP and nAchR α1 or like inhibitors stabilizing the desensitized state. Thus, there are many parallels in the molecular interactions of these toxins with nAchRs and of PcTx1 with cASIC.
In their study, Baconguis and Gouaux6 also crystallized a complex of cASIC1 with PcTx1 but used functional ΔcASIC1 (14–463), with only a slight truncation of the N-terminus. Moreover, they crystallized complexes at two different pH (pH 7.25 and 5.5) at similar resolution as Dawson et al. (3.3 and 2.8 Å, respectively). Thus, crystals obtained at pH 5.5 are directly comparable between the two studies, except that the structures of the TMDs are expected to be perturbed in ΔcASIC1 (26–463) used by Dawson et al.12 Indeed, the toxin-binding site is identical in the two studies with a few discrepancies concerning the molecular interactions between residues of the toxin and the channel (Fig. 2). Briefly, both studies find important contributions of hydrophobic PcTx1 residues Trp 7 and Trp 24 and basic residues Arg 26, Arg 27 and Arg 28. A previous site-directed mutagenesis study confirmed the importance of Trp 24, Arg 26 and Arg 27.16 Trp 24 and Arg 26 are equally oriented in both crystals. Arg 27, however, makes a weak H-bond with Gly218 of cASIC1 in the structure obtained by Dawson et al. (3.8 Å distance) whereas this interaction is strong in the structure obtained by Baconguis and Gouaux (3.0 Å distance, Fig. 2), making the position of Arg 27 energetically more favorable in the latter structure. More strikingly, the position of Arg 28 is different in the two crystals. Dawson et al. report that Arg 28 deeply penetrates the acidic pocket to form H-bonds with Glu 243.12 But Arg 28 of only two of the three PcTx1 molecules in this complex have the reported orientation, while Arg 28 of the third toxin molecule points away from Glu 243, making weak H-bonds with Asp 238, Asp 350 and Glu 354. Moreover, in the crystals obtained by Baconguis and Gouaux, Arg 28 has a completely different orientation and interacts with backbone oxygens of Asp 238 and Thr 240 of cASIC1 (Fig. 2).6 Whether these discrepancies mean that Arg 28 has some conformational flexibility and may make different, mutually exclusive contacts with cASIC1 residues is an open question, which can be addressed by site-directed mutagenesis, however. According to available site-directed mutagenesis data,16 Lys 25 of PcTx1 does not make an important contribution to binding of PcTx1 to cASIC1. In agreement, in the crystal obtained by Dawson et al., Lys 25 points away from the pocket and makes a weak H-bond with Gln 179 (3.7 Å distance). In contrast, in the crystal obtained by Baconguis and Gouaux, Lys 25 points into the pocket where it is not stabilized by H-bonds, however (Fig. 2). Thus, the position of Lys 25 seems to be energetically more favorable in the crystal obtained by Dawson et al.12
The Structures Of Two Open ASIC Pores
Similar to a previous study,11 Baconguis and Gouaux found that in the presence of PcTx1, functional ΔcASIC1 (14–463) did not completely desensitize but conducted steady-state currents at pH 7.25 and at pH 5.5.6 Thus, in the presence of PcTx1 a subpopulation of the channels was in the open and another (and larger) subpopulation in the desensitized conformation. Fortunately, it was the subpopulation in the open conformation that formed crystals (see below). Current-voltage relationships of ΔcASIC1 (14–463) in the presence of PcTx1 and different cations revealed that at pH 7.25, open channels were unselective for monovalent cations whereas at pH 5.5 they were Na+-selective (PNa/PK = 10/1). Since the typical open ASIC pore is selective for Na+ over K+ (PNa/PK ~10),24 only at low pH PcTx1 stabilized the typical Na+-selective open state, whereas at high pH it stabilized an atypical unselective open state. Solely for shark ASIC1b it had previously been reported that it carries sustained unselective cation currents at neutral pH.25 The related bile-acid sensitive ion channel (BASIC), however, also has a dynamic selectivity.26
Compared with Dawson et al.,12 Baconguis and Gouaux6 had the advantage that they had a structure with (likely) unperturbed TMDs, which adopted dramatically different conformations than in the desensitized state,13 rendering the ion pore open! At high pH (unselective state), the symmetric open pore is stabilized by sparse inter-subunit and hydrophobic contacts between TMD1 and TMD2 and, compared with the desensitized conformation, many intra- and inter-subunit interactions between TMD1 and TMD2 are disrupted. In contrast, at low pH (Na+-selective state), TMD2 of one subunit is shifted by approximately four residues toward the extracellular side of the membrane relative to the other two subunits, conferring axial asymmetry onto the pore. There are extensive interactions between TMD1 and TMD2 in the low-pH structure, rendering asymmetric pore formation favorable. Apart from the asymmetry, a second surprise of the low-pH pore was the partial exposure of TMD1 of one subunit to the ion pore6; previously mainly TMD2 had been implicated in pore formation.24,27 At high pH, the open pore has its smallest diameter of ~10 Å near Asp 433, whereas at low pH, it has an elliptical shape with dimensions of 4–5 Å by 7–10 Å at its most constricted part near Leu 440, thus two turns below the smallest constriction at high pH.6 The authors suggest that monovalent cations permeate the channel in a fully or a partially hydrated state.6 Thus, TMDs adopt strikingly different conformations in the open and desensitized conformations, as it had previously been proposed based on the accessibility of TMD residues to cysteine-reactive reagents.27 Moreover, conformations of the TMDs in the two open conformations are also strikingly different, with the unselective open pore having a more simple architecture than the Na+-selective pore. Interestingly, evolutionary old relatives of ASICs, peptide gated channels from the cnidarian Hydra, have an unselective ion pore.28 Future studies will show whether their open pore, and thus perhaps the primordial ASIC pore, has a similar structure as the unselective ASIC pore revealed by Baconguis and Gouaux.6
The Structure Of The ECD In The Open Conformation
What are the structural changes in the ECD that differentiate open and desensitized conformations? The ECD of each subunit consists of five subdomains, the palm, finger, thumb and knuckle domains and a β-ball domain, which are linked to the TMDs via an apparently flexible wrist.5 In the complex structure, the lower palm domain and the wrist slightly rotate around an axis situated below the scaffold, separating the thumb and palm domains of adjacent subunits by a few Å and enlarging the central vestibule.6 The separation of thumb and palm is illustrated by the distance between the Cα atoms of Asn 357 (thumb) and Arg 85 (palm), which increased from 8 Å in the desensitized conformation to 11 Å in the open conformations, respectively.6 The enlargement of the central vestibule is illustrated by the distance between the Cα atoms of Val 75 (β-sheet 1 of the palm) of the three subunits, which increased from ~7 Å in the desensitized conformation to 11 and 12 Å in the selective and unselective states, respectively.6 Moreover, in the β1-β2 linker of the palm domain the peptide bond between Thr 84 and Arg 85 flips by ~180°. Similarly striking conformational changes were found in the β11-β12 linker,6 which is in close contact to the β1-β2 linker. Previous studies already highlighted a crucial role of these linker regions, which lie at the outer surface of the palm, for desensitization gating.29,30 These changes are similar in both open structures, the low-pH, Na+-selective and the high-pH, unselective structure. Overall, one notes only slight conformational changes that differentiate the ECD in the open and desensitized structures.
Comparing the structure obtained by Dawson et al.12 with the structures obtained by Baconguis and Gouaux,6 one realizes that these conformational changes, namely a separation of thumb and palm, an enlargement of the central vestibule and structural changes in the β1-β2 and β11-β12 linkers, are also present in the structure obtained by Dawson et al.12 The fact that Dawson et al.12 did not mention those changes nicely illustrates how subtle the structural differences between open and desensitized conformations really are. If you do not know that you are looking at the open state conformation of the ECD, you do not realize it! Thus, it appears that desensitization gating of ASICs is mainly associated with a conformational rearrangement of the TMDs, as it has previously also been proposed for the nAChR.23
Baconguis and Gouaux6 propose the following scenario for cASIC1 gating: The upper palm and knuckle domains provide a structural scaffold that is virtually identical in open and desensitized conformations. In contrast, the lower palm domain slightly moves, via the wrist inducing radial and rotational movements of the TMDs. Finger and thumb, which flank the palm domain and make major contributions to the acidic pocket, bind PcTx1 and presumably also H+, thereby modulating movements of the lower palm domain. It should be emphasized that this scenario is derived from a comparison of open and desensitized structures (Fig. 1), and thus provides information mainly on desensitization gating of cASIC1. Movements accompanying activation gating remain unknown.
Why does, at different pH, the open cASIC pore adopt two different conformations with different ion selectivities? In a crystal, it is not possible to “see” the additional proton(s) that are bound at low-pH and stabilize the Na+-selective pore. Moreover, the structure of the ECD is virtually identical in the two open state conformations. Baconguis and Gouaux6 propose that at low pH, Glu 80 gets protonated, allowing contraction of the central vestibule. Glu 80 had previously been involved in desensitization gating,31 underscoring its importance. But at present, the residues that are differentially protonated in the low- and high-pH structures remain unknown, leaving the forces that differentiate the Na+-selective from the unselective open conformation mysterious.
Finally, how do the two pore structures of cASIC in complex with PcTx1 relate to the cASIC pore that is gated open by H+? While the low-pH, selective pore may well correspond to the cASIC pore that is opened by H+ in the absence of PcTx1, this is less likely for the unselective pore. Some ASICs have a dynamic selectivity that changes from Na+-selective to unselective, but usually the unselective state is reached at lower (and not higher) pH than the selective one,25,32 so that Glu 80 should be protonated and the central vestibule contracted. It has been proposed that for some ASICs the desensitized state is unstable, and that channels reopen to an unselective open state.30 It is unknown why this happens only at low pH but the β1-β2 and β11-β12 linkers had been implicated in reopening of the ASIC1 pore.30 In summary, while the exact relation of the two open state structures reported by Baconguis and Gouaux6 to the physiological ASIC pore is unclear, they will certainly instruct in many laboratories further structure-function studies, which will give us a clearer picture of the structure of the conductive ASIC pore that is opened by H+.
Acknowledgments
We thank Dominik Wiemuth for comments on the manuscript. S.G. is supported by grants from the Deutsche Forschungsgemeinschaft (GR1771/3-5).
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22154
References
- 1.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 2.Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–58. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–9. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 5.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–23. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 6.Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature. 2012;489:400–5. doi: 10.1038/nature11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babini E, Paukert M, Geisler HS, Gründer S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Kalbacher H, Gründer S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol. 2005;126:71–9. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, et al. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–21. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Kalbacher H, Gründer S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol. 2006;127:267–76. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samways DS, Harkins AB, Egan TM. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. 2009;45:319–25. doi: 10.1016/j.ceca.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson RJ, Benz J, Stohler P, Tetaz T, Joseph C, Huber S, et al. Structure of the acid-sensing ion channel 1 in complex with the gating modifier Psalmotoxin 1. Nat Commun. 2012;3:936. doi: 10.1038/ncomms1917. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietra F. Docking and MD simulations of the interaction of the tarantula peptide psalmotoxin-1 with ASIC1a channels using a homology model. J Chem Inf Model. 2009;49:972–7. doi: 10.1021/ci800463h. [DOI] [PubMed] [Google Scholar]
- 15.Qadri YJ, Berdiev BK, Song Y, Lippton HL, Fuller CM, Benos DJ. Psalmotoxin-1 docking to human acid-sensing ion channel-1. J Biol Chem. 2009;284:17625–33. doi: 10.1074/jbc.M109.003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saez NJ, Mobli M, Bieri M, Chassagnon IR, Malde AK, Gamsjaeger R, et al. A dynamic pharmacophore drives the interaction between Psalmotoxin-1 and the putative drug target acid-sensing ion channel 1a. Mol Pharmacol. 2011;80:796–808. doi: 10.1124/mol.111.072207. [DOI] [PubMed] [Google Scholar]
- 17.Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, et al. Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature. 2006;440:959–62. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]
- 18.Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci. 2003;12:1332–43. doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourne Y, Talley TT, Hansen SB, Taylor P, Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. EMBO J. 2005;24:1512–22. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celie PH, Kasheverov IE, Mordvintsev DY, Hogg RC, van Nierop P, van Elk R, et al. Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an alpha-conotoxin PnIA variant. Nat Struct Mol Biol. 2005;12:582–8. doi: 10.1038/nsmb951. [DOI] [PubMed] [Google Scholar]
- 21.Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–46. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10:953–62. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- 23.Dutertre S, Lewis RJ. Toxin insights into nicotinic acetylcholine receptors. Biochem Pharmacol. 2006;72:661–70. doi: 10.1016/j.bcp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Gründer S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- 25.Springauf A, Gründer S. An acid-sensing ion channel from shark (Squalus acanthias) mediates transient and sustained responses to protons. J Physiol. 2010;588:809–20. doi: 10.1113/jphysiol.2009.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiemuth D, Gründer S. A single amino acid tunes Ca2+ inhibition of brain liver intestine Na+ channel (BLINaC) J Biol Chem. 2010;285:30404–10. doi: 10.1074/jbc.M110.153064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Yang Y, Canessa CM. Outlines of the pore in open and closed conformations describe the gating mechanism of ASIC1. Nat Commun. 2011;2:399. doi: 10.1038/ncomms1409. [DOI] [PubMed] [Google Scholar]
- 28.Dürrnagel S, Kuhn A, Tsiairis CD, Williamson M, Kalbacher H, Grimmelikhuijzen CJ, et al. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J Biol Chem. 2010;285:11958–65. doi: 10.1074/jbc.M109.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Yang Y, Canessa CM. Asn415 in the beta11-beta12 linker decreases proton-dependent desensitization of ASIC1. J Biol Chem. 2010;285:31285–91. doi: 10.1074/jbc.M110.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Springauf A, Bresenitz P, Gründer S. The interaction between two extracellular linker regions controls sustained opening of acid-sensing ion channel 1. J Biol Chem. 2011;286:24374–84. doi: 10.1074/jbc.M111.230797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cushman KA, Marsh-Haffner J, Adelman JP, McCleskey EW. A conformation change in the extracellular domain that accompanies desensitization of acid-sensing ion channel (ASIC) 3. J Gen Physiol. 2007;129:345–50. doi: 10.1085/jgp.200709757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, et al. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–83. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 33.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/S0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]