Abstract
ZD7288 has been widely used as a tool in the study of hyperpolarization-activated cyclic nucleotide-gated channels (HCN channels), and to test the relationships between HCN channels and heart and brain function. ZD7288 is widely considered a selective blocker of HCN currents. Here we show that ZD7288 inhibits not only HCN channel currents, but also Na+ currents in DRG neurons and ZD7288 was confirmed to inhibit Na+ current in HEK293 cells transfected with Nav1.4 plasmids. Thus our findings challenge the view that ZD7288 is a selective blocker of HCN channels. Conclusions about the role of NCN channels in neuronal function should be re-evaluated if based exclusively on the effect of ZD7288.
Keywords: ZD7288, HCN channel currents, Na+ currents, neuropathic pain, DRG
Introduction
Since Brown discovered that the mechanism by which adrenaline accelerates heart rate involves hyperpolarization-activated cation currents (HCN) in 1979,1 researchers started to look for the blockers of HCN. After more than 10 y, ZD7288 [4-(N-Ethyl-N-phenylamino)-1,2 dimethyl-6-(methylamino) pyrimidinium chloride ICI-D7288 N-Ethyl-1,6-dihydro-1,2-dimethyl-6-(methylimino)-N-phenyl-4-pyrimidinamine hydrochloride] was found to be a highly selective blocker of HCN.2 ZD7288 reduces heart rate without impairing cardiac function, and it also mediates analgesia in neuropathic pain,3 consistent with an action on HCN channels. Thus, ZD7288 has been widely used as a selective HCN inhibitor in physiological studies. Here, while investigating the effect of HCN currents on neuropathic pain, we discovered that ZD 7288 also blocks Na+ currents. This finding challenges the view that ZD7288 mediates analgesia via block of HCN channels.
Results
Identification of Na+ currents in DRG neurons
Na+ currents recorded in DRG neurons were identified by lidocaine, a special blocker of Na+ currents. As shown in Figure 1A, lidocaine almost completely inhibited sodium currents. The current inhibited could be recovered partially by washout. Protocols used to isolate sodium currents were identical to those described previously.5
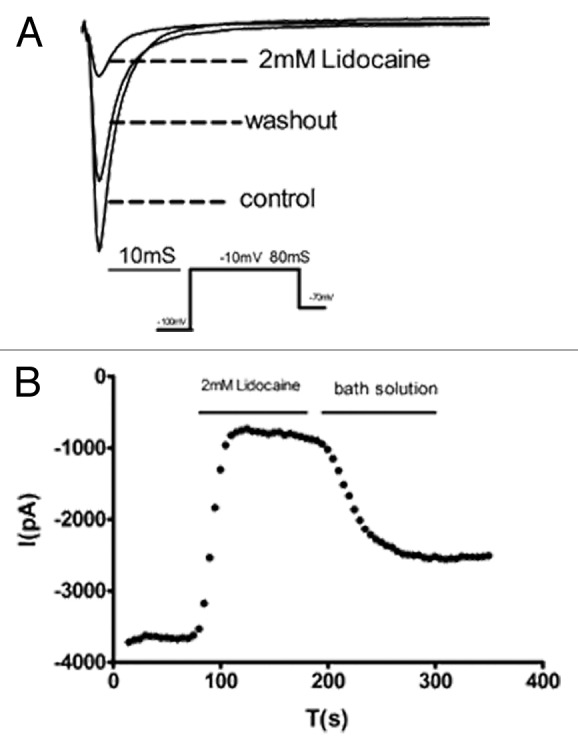
Figure 1. Na+ currents recorded in DRG neurons were identified by lidocaine, a selective blocker of sodium currents. (A) showed the Na+ currents recorded in a DRG neuron. Lidocaine almost completely inhibited the current, and the current could be recovered partially by washout. (B) Time course of current traces in the presence of Lidocaine (2 mM) recorded in response to 80 ms depolarizing pulses from -100 to -10 mV.
ZD7288 inhibits Na+ currents in DRG neurons
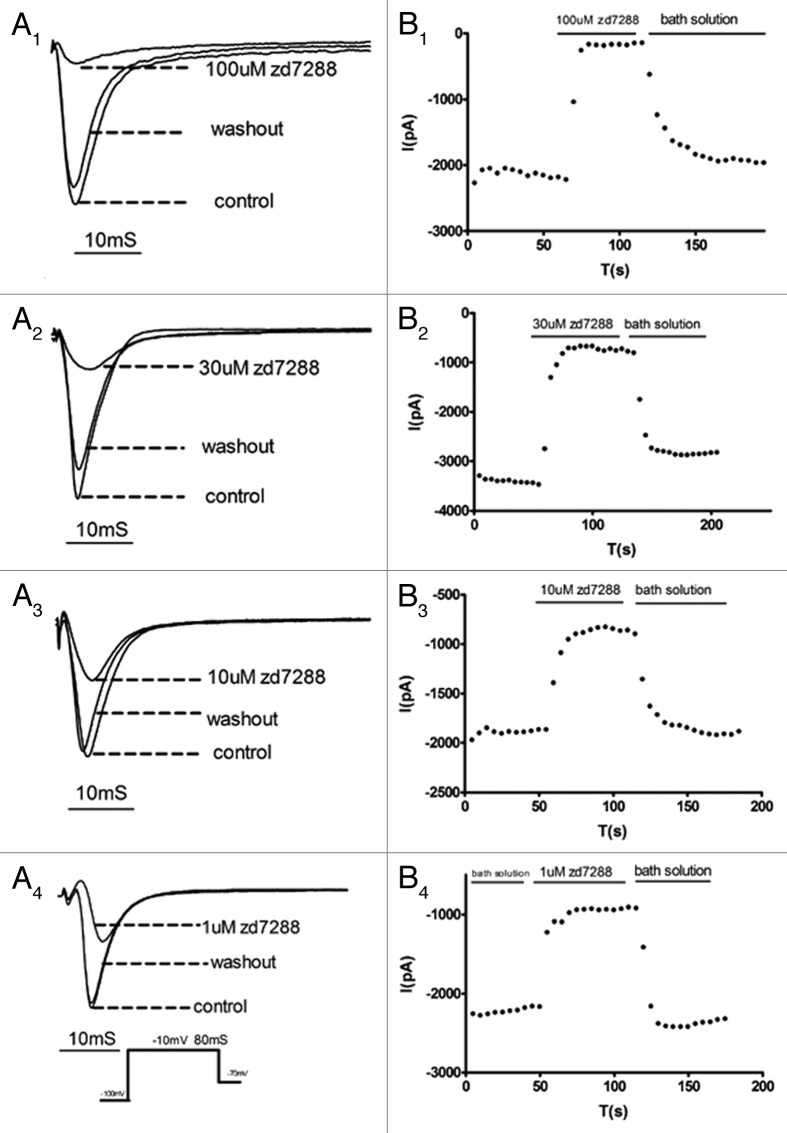
Na+ currents were recorded in DRG neurons as described above, and the effects of ZD7288 were investigated. We found that ZD7288 inhibited sodium currents and its effects could be recovered upon washout of ZD7288. Figure 2A1-A4 shows the inhibitory effect of ZD7288 on Na+ currents at different drug concentrations. We also measured the time course (Fig. 2B1-B4) of development of ZD7288 block at various drug concentrations at a test potential of -10 mV, showing that the rate of development of block increased with drug concentration. Based on the data of Figure 2, we constructed a dose response curve (Fig. 3) which, when fitted with the Hill equation, yielded an IC50 of 1.17 μM.
Figure 2. Inhibition of Na+ currents in DRG neurons by ZD7288. (A1-A4) Traces were recorded in the presence and absence of different concentrations of ZD7288 and after washout of ZD7288. Time course of peak current amplitudes in the presence of different concentrations [100, 30, 10 and 1 (μM)] of ZD7288 recorded in response to 80 ms depolarizing pulses from -100 to -10 mV.
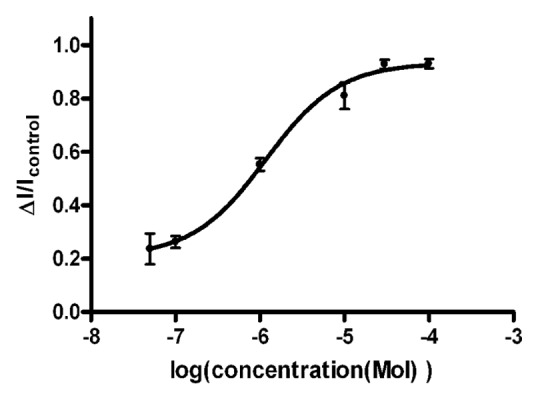
Figure 3. Concentration-effect curve for ZD 7288 inhibition of Na+ currents. Normalized current is plotted against the logarithm of ZD 7288 concentration (μM) [100 (n = 3), 30 (n = 3), 10 (n = 4), 1 (n = 3), 0.1(n = 4) and 0.05 (n = 4)]. The data were fitted with the Hill equation.
ZD7288 does inhibit HCN channel currents
To ensure that ZD7288 was indeed also a blocker of HCN channel currents, we determined the effect of this drug on HCN currents in DRG neurons. HCN channel whole cell current in DRG neurons was induced by giving a membrane potential step from a holding potential of -60 mV step to -140 mV for 700 ms and then back to -70 mV. Figure 4A shows the effect of increasing ZD7288 concentrations on native HCN currents in DRG neurons, thus confirming that this drug is indeed an HCN blocker. A fit to the dose response curve in Figure 4B revealed an IC50 of 15 μM, indicating that HCN channels are blocked less effectively than sodium channels.
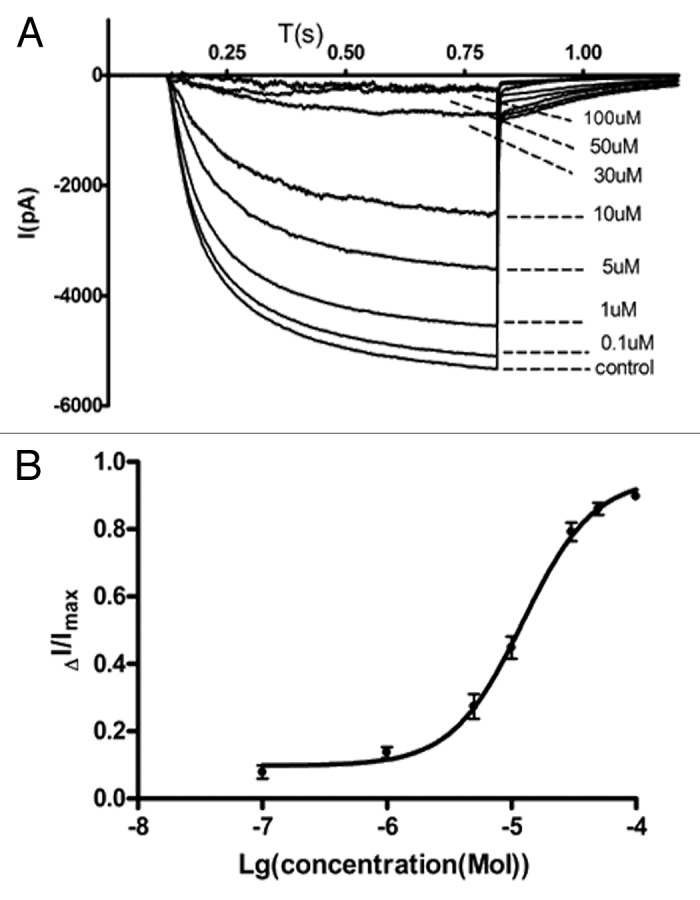
Figure 4. Inhibition of HCN current in DRG cells by ZD7288. (A) Traces were recorded in the absence and presence of ZD7288, at a range of concentrations r [0.1, 1, 5, 10, 30, 50, 100 (μM)]. DRG cells were whole-cell voltage clamped at -60 mV 100 ms and stepped to -140 mv for 700 ms and back to -70 mv for 300 ms to evoke HCN current. (B) Concentration-effect curve for ZD 7288 inhibition of HCN currents. Normalized current is plotted against the logarithm of ZD 7288 concentration (μM) [100 (n = 3), 30 (n = 4), 10 (n = 4), 1 (n = 3), 0.1(n = 3)]. The data were fitted with the Hill equation.
Inhibition of transiently expressed Nav1.4 channels by ZD7288
To confirm the inhibitory effects of ZD7288 on sodium channels, we transfected HEK293 with Nav1.4 plasmids along with a GFP marker. Nav1.4 channel currents were recorded as Figure 5 in absence, presence and following washout of 30μM ZD7288. As evident from Figure 5, ZD7288 also inhibited transiently expressed Na+ channels, and a concentration of 30 µM virtually eliminated all current activity. These data show that the skeletal muscle sodium channel isoform is blocked as potently as the native neuronal sodium channels in DRG neurons.
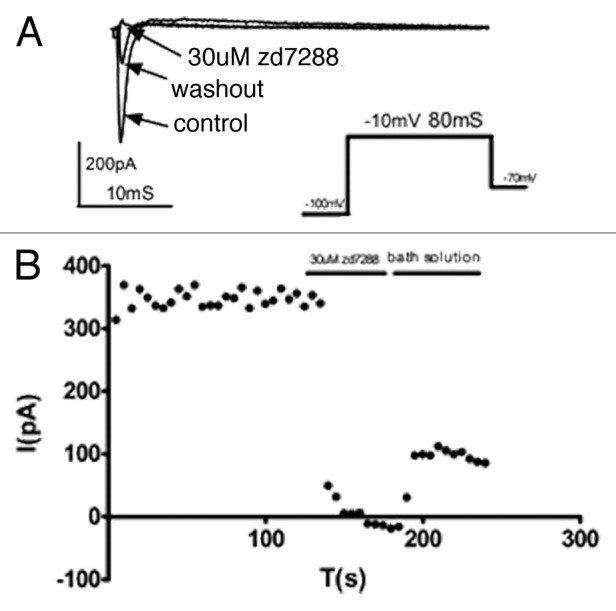
Figure 5. Inhibition of recombinant Nav1.4 channels by ZD7288. (A) pcDNA3.1-Nav1.4 plasmids were transfected into HEK293 cells with GFP plasmids, and Nav1.4 channel currents were recorded in the absence, presence and after washout of ZD7288 (30μM). (B) Time course of peak current amplitude in the presence of ZD7288 (30μM) recorded in response to 80 ms depolarizing pulses from -100 to -10 mV.
Discussion
Hyperpolarization-activated cation nonselective cyclic nucleotide-gated (HCN) channel currents were first functionally described as the “funny” current If in rabbit sinoatrial node6 and the “queer” current Iq in hippocampal pyramidal neurons.7 Subsequently, it was also demonstrated as Ih in a variety of peripheral sensory nerve preparations, such as dorsal root ganglia (DRG), rat and rabbit nodose ganglia and guinea-pig myenteric neurons.8 These excitatory currents push a cell toward the threshold of activation for action potentials, therefore play an important role in the modulation of heart rate and neuronal activities. Altered HCN currents in injured tissues may be an important mechanism underlying neuropathic pain, and drugs that modulate these currents may offer new therapeutic options.
In the past three decades, several molecules with heart rate-reducing properties have been developed and identified as HCN channel blockers. Early drugs identified as pure bradycardic agents include alinidine (ST567), ZD7288 and zatebradine (UL-FS49) and its derivatives; a more recently discovered drug is ivabradine (S16257)9 which has been used in the clinic (EMEA 2006). The main action of these substances is to induce a reduction of the diastolic depolarization slope by blocking HCN channel current.10 HCN channel blockers (i.e., ZD7288) also were found to mediate pain relief in rat models of neuropathic pain, presumably through block of Ih. While studying the blocking effect of ZD 7288 on Ih in DRG neurons, we found that ZD 7288 also blocks sodium channels. It has been shown that ZD 7288 also inhibits calcium channels,11,12 but only at very high concentrations (i.e., with an IC50 of over 100 μM). In our experiments, we found an IC50 for sodium channel block of less than 2 μM, which indicates a much greater sensitivity than that of HCN channels. Hence, it is likely that the analgesic effects of ZD7288 are mediated at least in part via sodium channel block rather than inhibition of HCN channels. At this point we did not fully explore the blocking mechanisms (i.e., whether the compound is a pore blocker, or a modifier of sodium channel gating). Nonetheless, conclusions based on functional/physiological studies based on ZD7288 should be re-evaluated for a possible involvement of sodium channels.
Materials and Methods
Animals
All experiments performed on animals were performed under protocols approved by the Animal Care and Use Committee of our university in compliance with the Guide for the Care and Use of Laboratory Animals provided by Province Disease Control center of Hubei. Male Wistar rats weighing 100 g were used in all experiments. The rats were provided by Province Disease Control center of Hubei and housed in separated cages with free access to water and food. The room temperature was kept at 25°C and under a normal light-dark cycle.
Preparation of Dorsal root ganglion cells
DRG neurons were isolated from Wistar rats (200 g) that were killed by cervical dislocation and subsequent decapitation. DRG neurons were prepared as previously described.4 Briefly, all DRG contained in the lumbar 5–6 region were minced for 6 min by micro scissors after dissection, then subjected to collagenase (2.5mg/mL, type I, invitrogen) and trypsin (1.25mg/mL, invitrogen) for 15 min at 37°C. The solution containing DRGs was stirred at a speed of 170 rpm and softly dispersed 40 times every 5 min. The enzymatic reaction was stopped when most cells were smooth and round. The reaction was stopped by washing the cells with DMEM containing 10% fetal bovine serum (FBS). Cells were collected by centrifugation at 1000 rpm for 6 min and then 200 uL fresh DMEM supplemented with 10% FBS was added to the cells. The cells were placed on poly-d-lysine (0.1mg/ml, sigma)-treated glass coverslips contained within the cell culture dish and kept in 5% CO2 incubator at 37 degrees. Patch clamp experiments were performed after 2–3 h.
Cell culture and transfection
Human embryonic kidney cells (HEK293) were cultured in DMEM containing 10% FBS, with a stable temperature of 37°C and 5% CO2. pcDNA3.1-Nav1.4 plasmid together with EGFP was transfected into HEK293 cells by Lipofection.
Whole-cell recording
Na+ current was measured by whole-cell patch clamp recording. Recordings were made with Axopatch 700B amplifier controlled by pClamp 10.0 (Molecular Devices). The output was filtered at 5 kHz and digitally sampled at 20 kHz using a DigitData 1440 Series interface (Molecular Devices). 80% of the series resistance was compensated by the analog circuitry. Data were recorded 10 min after whole-cell was established. Recordings were made at 25°C. Patch electrodes were fabricated with a PIP5 puller (HEKA) and the tip was heat-polished to a final tip resistance of 3–4 MΩ. Solution for sodium currents recording in DRG cells, the pipette contained (mM):CsF 140, NaCl 10, MgCl2 2, CaCl2 0.1, EGTA 1 and HEPES 10, PH was adjusted to 7.2 with CsOH. The bath solution contained (mM): NaCl 35, Choline chloride 105, CaCl2 1,MgCl2 1,CdCl2 0.1, TEA-Cl 20, HEPES 10, glucose 10, PH was adjusted to 7.4 with NaOH; For HCN channel currents recording in DRG cells, the pipette solution contained (mM): KCl 135, MgCl2 10, CaCl2 0.1, EGTA 1, HEPES 10, PH was adjusted to 7.2 with KOH. The bath solution contained (mM): NaCl 140, KCl 5, MgCl2 1, HEPES 10, CaCl2 2, D-glucose 10, PH was adjusted to 7.4 with NaOH. Solution for HEK293 patch, the pipette contained (mM): CsF 140, NaCl 10, EGTA 5 and HEPES 10. PH was adjusted to 7.2 with CsOH. Bath solution contained (mM): NaCl 140, glucose 5, MgCl2 1, KCl 3, CaCl2 1, HEPES 10. PH was adjusted to 7.4 with NaOH. All reagents were purchased from Sigma, otherwise indicated.
Data analysis
Voltage-clamp experimental data were analyzed using clampfit (V 10.0, Molecular Devices), sigmaplot 10.0 and GraphPad prism 4. Dose response curves were fitted with the Hill equation.
Acknowledgments
This research was supported by the National Natural Science foundation of China (grant # 30972848 and 81271234) and special funds of basic research operating expenses for universities of China. We would like to thank Professor Ding (HUST) for providing plasmids.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22209
References
- 1.Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–6. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- 2.Marshall PW, Rouse W, Briggs I, Hargreaves RB, Mills SD, McLoughlin BJ. ICI D7288, a novel sinoatrial node modulator. J Cardiovasc Pharmacol. 1993;21:902–6. doi: 10.1097/00005344-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Luo L, Chang L, Brown SM, Ao H, Lee DH, Higuera ES, et al. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience. 2007;144:1477–85. doi: 10.1016/j.neuroscience.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–52. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Fan N, Sikand P, Donnelly DF, Ma C, Lamotte RH. Increased Na+ and K+ currents in small mouse dorsal root ganglion neurons after ganglion compression. J Neurophysiol. 2011;106:211–8. doi: 10.1152/jn.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–6. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 8.Maher MP, Wu NT, Guo HQ, Dubin AE, Chaplan SR, Wickenden AD. HCN channels as targets for drug discovery. Comb Chem High Throughput Screen. 2009;12:64–72. doi: 10.2174/138620709787048028. [DOI] [PubMed] [Google Scholar]
- 9.Bucchi A, Barbuti A, Baruscotti M, DiFrancesco D. Heart rate reduction via selective ‘funny’ channel blockers. Curr Opin Pharmacol. 2007;7:208–13. doi: 10.1016/j.coph.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Baruscotti M, Bucchi A, Difrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Felix R, Sandoval A, Sánchez D, Gómora JC, De la Vega-Beltrán JL, Treviño CL, et al. ZD7288 inhibits low-threshold Ca(2+) channel activity and regulates sperm function. Biochem Biophys Res Commun. 2003;311:187–92. doi: 10.1016/j.bbrc.2003.09.197. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Alonso JL, Halliwell JV, Colino A. ZD 7288 inhibits T-type calcium current in rat hippocampal pyramidal cells. Neurosci Lett. 2008;439:275–80. doi: 10.1016/j.neulet.2008.05.016. [DOI] [PubMed] [Google Scholar]