Abstract
NaV1.5 is a mechanosensitive voltage-gated Na+ channel encoded by the gene SCN5A, expressed in cardiac myocytes and required for phase 0 of the cardiac action potential (AP). In the cardiomyocyte, ranolazine inhibits depolarizing Na+ current and delayed rectifier (IKr) currents. Recently, ranolazine was also shown to be an inhibitor of NaV1.5 mechanosensitivity. Stretch also accelerates the firing frequency of the SA node, and fluid shear stress increases the beating rate of cultured cardiomyocytes in vitro. However, no cultured cell platform exists currently for examination of spontaneous electrical activity in response to mechanical stimulation. In the present study, flow of solution over atrial myocyte-derived HL-1 cultured cells was used to study shear stress mechanosensitivity of Na+ current and spontaneous, endogenous rhythmic action potentials. In voltage-clamped HL-1 cells, bath flow increased peak Na+ current by 14 ± 5%. In current-clamped cells, bath flow increased the frequency and decay rate of AP by 27 ± 12% and 18 ± 4%, respectively. Ranolazine blocked both responses to shear stress. This study suggests that cultured HL-1 cells are a viable in vitro model for detailed study of the effects of mechanical stimulation on spontaneous cardiac action potentials. Inhibition of the frequency and decay rate of action potentials in HL-1 cells are potential mechanisms behind the antiarrhythmic effect of ranolazine.
Keywords: electrophysiology, mechanosensitivity, ion channels, drugs, myocytes
Introduction
NaV1.5 is a sodium-selective voltage-gated ion channel present in various mechanically active cells, such as cardiac myocytes, human intestinal smooth muscle cells, and interstitial cells of Cajal. Mutations in SCN5A, the gene encoding NaV1.5, are known to cause cardiac arrhythmias such as long QT 3 (LQT3) and Brugada syndromes.1 NaV1.5 has a well-defined role in the cardiac action potential, and electrophysiologic pathologies that result from NaV1.5 abnormalities have solid molecular and physiological foundations.1,2
Ion channel mechanosensitivity has been well documented, but most studies focus on non-voltage gated ion channel mechanosensitivity. Not until recently has evidence emerged to suggest that mammalian voltage-gated ion channels such as NaV1.5,3,4 shaker5 and the L-type Ca2+ channel are mechanosensitive.6-8 While the actin cytoskeleton contributes to NaV1.5 mechanosensitivity,9 the NaV1.5 α-subunit is mechanosensitive as a stand-alone unit.10 We have recently shown that ranolazine inhibits the excitatory component of NaV1.5 mechanosensitivity.11 However, the roles of voltage-gated ion channel mechanosensitivity in physiology and ranolazine-mediated inhibition of NaV1.5 in the heart are unclear.
Mutations in SCN5A and dysfunction of NaV1.5 are associated with clinical pathologies rooted in cardiac mechano-electrical feedback (MEF) dysfunction. For example, in both familial and idiopathic-dilated cardiomyopathy, SCN5A mutations are the only α-subunit channelopathies described12; all other mutations implicated in idiopathic-dilated cardiomyopathy lie in genes that code for structural proteins. Several of these structural proteins associate with or are important in NaV1.5 mechanosensitivity, including telethonin,13 dystrophin14 and syntrophin.15 In addition to inhibiting the excitatory aspects of NaV1.5 mechanosensitivity,11 ranolazine has shown promise for the treatment of abnormalities leading to heart failure.16 These studies provide rationale and a potential mechanism for structural or mechanical abnormalities in cardiac disease. Another potential example involves stretch of the pulmonary veins, which may serve to trigger and/or propagate atrial fibrillation.17 Pulmonary veins contain interstitial cells of Cajal-like cells. Interstitial cells of Cajal of the human gut express NaV1.5 and are mechanosensitive.18,19 Interestingly, ranolazine was effective in suppression of excitatory Na+ channel activity in pulmonary vein sleeves.20
In our recent study, ranolazine was found to abolish pressure- and flow-induced mechanical activation of voltage shifts or increased peak current, respectively.11 The correlation of these changes, as seen by voltage clamp electrophysiology, with spontaneous action potentials has been limited. Ranolazine was reported to reduce action potential frequency and TTX-resistant and -sensitive currents in rat DRG neurons.21 Furthermore, optical mapping of ventricular myocytes under intermittent shear stress22,23 lacks accompanying electrophysiological data. These studies on mechanosensitivity have utilized either dissociated native cells or transfected cultured cells that generally lack required components.
There were two aims in this study. First, we sought to determine whether HL-1 cultured cells, derived from murine atrial myocytes and known to exhibit spontaneous electro-mechanical rhythmic activity, would be sensitive to mechanical stimulation. Second, to determine whether this mechanical sensitivity would be responsive to ranolazine, we examined the sensitivity of spontaneous action potentials to flow of extracellular solution in current-clamp mode and correlated this shear stress-mediated sensitivity with that of voltage-clamped Na+ currents in the same cells. We found rhythmic action potentials and whole cell Na+ currents to be mechanosensitive and mechanosensitivity of either to be inhibited by ranolazine.
Results
Na+ current is required for action potentials in HL-1 cultured atrial myocytes
Sodium current contributes to action potentials (AP) in cardiac smooth muscle. Additionally, the currents in the murine atrial myocyte cell line HL-1 have been shown previously to have genotypic, phenotypic, and electrophysiologic properties similar to adult atrial cardiomyocytes.24 Inward currents recorded from a voltage-clamped HL-1 cell dialyzed with Cs+- rich solution resembled the sodium current prevalent in native cardiomyocytes with a similar voltage-dependence of activation11 (Fig. 1A and B). HL-1 cells plated on coverslips formed networks, and a majority of cells exhibited rhythmic contractions. Replacement of extracellular Na+ with N-methyl-D-glucamine (NMDG+) abolished action potential spikes in HL-1 (Fig. 1C), an effect partially reversible but persistent after 40 min, suggesting the Na+ current was a prerequisite for action potential oscillations.
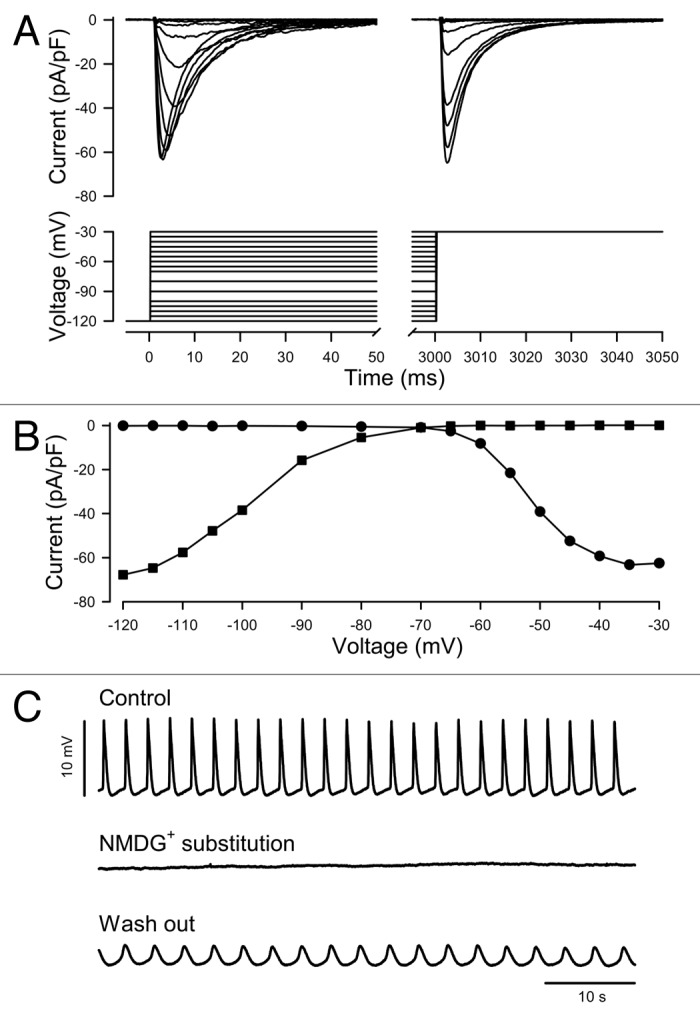
Figure 1. Voltage dependent sodium current contributes to spontaneous, rhythmic action potentials in untransfected HL-1 cultured atrial myocytes. (A) Representative family of current traces from a voltage-clamped cell dialyzed with Cs+ rich solution and elicited by the voltage protocol shown (inset). (B) Current-voltage relationship of the traces shown in (A). (C) Spontaneous rhythmic action potentials recorded sequentially in current-clamp mode from a cell which produced Na+ current similar to (A). Extracellular solution contained the principal charge carrier Na+ (Control, t = set as 0 min, baseline = -32 mV), NMDG+ (NMDG+ substitution, t = 2 min, resting potential = -44 mV), then Na+ (Wash out, t = 40 min, resting potential = -31 mV).
Ranolazine inhibits the mechanical activation of peak Na+ current induced by bath flow in HL-1 cells
Ranolazine can inhibit peak and persistent Na+ current of NaV1.5.25 Having tested the effect of ranolazine on the mechanosensitivity of Na+ channels in dissociated murine cardiomyocytes and SCN5A-transfected HEK293 cells,11 we confirmed its effect on HL-1 cells. Shear stress by flow of control solution at 10 mL/min led to a 14 ± 5% increase in peak Na+ current (control, -9.8 ± 2.0 pA/pF; flow, -11.2 ± 2.4 pA/pF; n = 5, p < 0.05, Figure 2). In the same cells, 50 µM ranolazine blocked peak Na+ current by 47 ± 3% (control, -9.8 ± 2.0 pA/pF; ranolazine, -5.0 ± 0.9 pA/pF; n = 5, p < 0.05) and inhibited the peak current activated by flow (ranolazine, -5.0 ± 0.9 pA/pF; flow with ranolazine, -4.9 ± 0.9 pA; n = 5, p > 0.05, Fig. 2), suggesting that ranolazine inhibits shear-induced mechanosensitivity of endogenous Na+ current in HL-1 as in dissociated myocytes or SCN5A-transfected HEK cells.
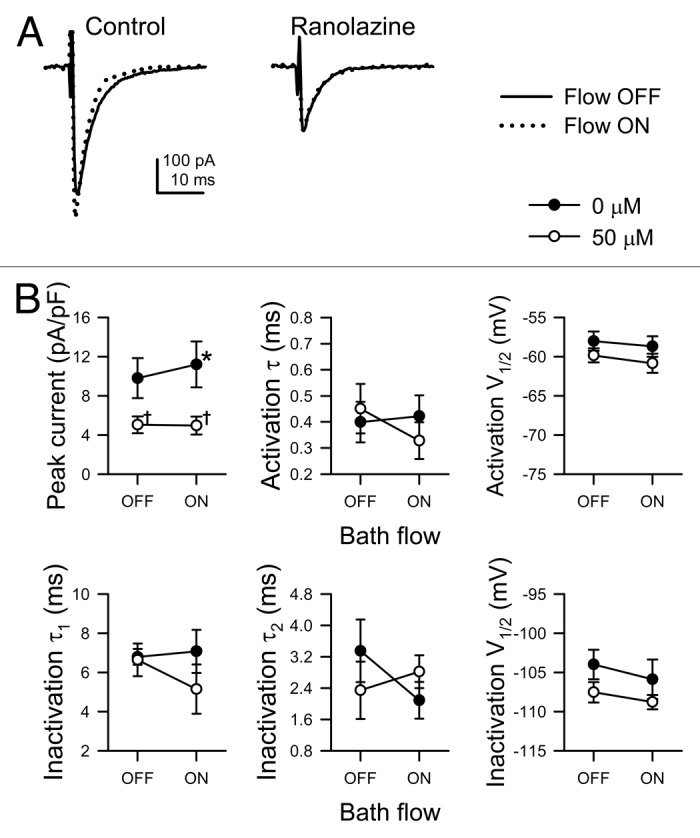
Figure 2. Ranolazine blocks the mechanosensitive response and peak currents of endogenous Na+ channels in HL-1 cells. (A) Single Na+ current traces recorded by whole cell voltage-clamp from HL-1 cells, elicited by stepping to -30 mV from -120 mV before (black traces, Flow OFF) or during (gray traces, Flow ON) bath flow, produced by rinsing solution through the recording chamber at 10 mL/min in the absence (Control, 0 µmol/L) or presence (Ranolazine, 50 µM) of drug. (B) Mean parameters of HL-1 Na+ current in response to solution flow with or without ranolazine. From top left to bottom right, peak current density, time constant (τ) of activation, V1/2 of steady-state activation, slow time constant of inactivation (τ1), fast time constant of inactivation (τ2), and V1/2 of steady-state inactivation. (n = 5; *p < 0.05 compared with 0 mL/min flow, †p < 0.05 compared with 0 µM ranolazine, and p > 0.05 interaction between bath flow and ranolazine blockade by two-way ANOVA with Bonferroni multiple comparisons posttest).
Ranolazine inhibits mechanical activation of rhythmic action potentials in HL-1 cells
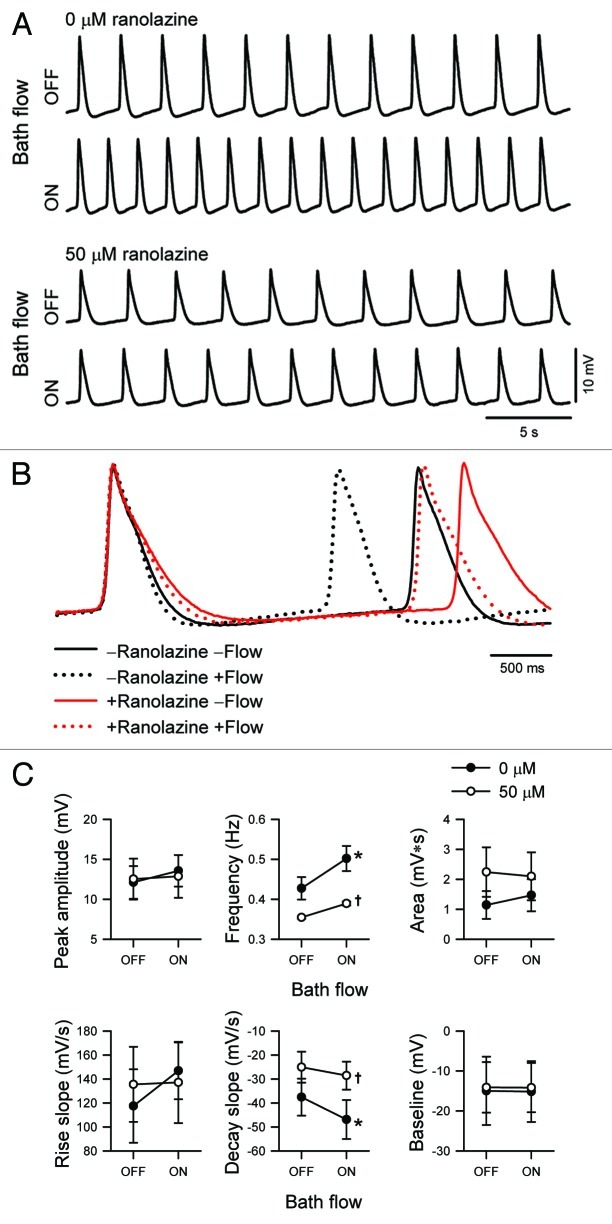
We tested the effect of ranolazine on AP using current-clamp whole-cell electrophysiology. Flow of drug-free solution significantly increased the rate of AP decay by 27 ± 12% (control, -37.6 ± 0.8; flow, -46.9 ± 0.8 mV/s; n = 4, p < 0.05) and increased AP frequency by 18 ± 4% (control, 0.43 ± 0.03; flow, 0.50 ± 0.03 Hz; n = 4, p < 0.05, Fig. 3). After incubation with ranolazine (50 µM) for 4.2 ± 0.8 min, the slope of decay decreased 32 ± 12% to -25.1 ± 0.6 mV/s, and frequency slowed 16 ± 5% to 0.35 Hz (n = 4, p < 0.05 to control). However, myocytes exposed to ranolazine were less responsive to mechanical stimulus, as decay slope increased only 17 ± 6% (ranolazine, -25.1 ± 0.6 mV/s; flow with ranolazine, -28.6 ± 0.6 mV/s; n = 4, p > 0.05), and frequency increased only 10 ± 2% (ranolazine, 0.35 ± 0.01 Hz; flow with ranolazine, 0.38 ± 0.01 Hz; n = 4, p > 0.05 to no drug with flow or p < 0.05 to drug without flow by two-way repeated-measures ANOVA with Bonferroni multiple comparisons posttest). No significant changes to peak amplitude, area, rise slope, or baseline (Fig. 3C) nor to antipeak amplitude (data not shown) were observed in response to flow and/or drug.
Figure 3. Ranolazine inhibits the flow-induced response of rhythmic action potentials in HL-1 atrial myocytes. (A) Rhythmic action potentials recorded sequentially from a single HL-1 atrial myocyte before (OFF) or during (ON) flow of extracellular solution without (0 μM) or with drug (50 μM ranolazine). From top to bottom, time elapsed was 0, 2, 12, and 14 min, and resting potential was -42, -40, -32, and -31 mV. (B)Action potentials from (A) were both normalized to and centered at peak. (C) Mean parameters of HL-1 action potentials in response to solution flow without (0 μM, filled circles) or with drug (50 μM ranolazine, empty circles). From top left to bottom right, peak amplitude, frequency, area between peak and baseline, rate of rise, rate of decay, baseline. (n = 4; *p < 0.05 compared with Flow OFF, †p < 0.05 compared with 0 µM Ranolazine, and p > 0.05 interaction between bath flow and ranolazine blockade by two-way ANOVA with Bonferroni multiple comparisons posttest).
Discussion
In this study, we report the following: first, the HL-1 cultured cell line, derived from murine atrial caridiomyocytes, express robust spontaneous rhythmic action potentials; second, shear stress by flow increases peak Na+ currents and action potential frequency, and it also accelerates the repolarization phase of the action potential; third, both the baseline current and flow-induced changes to the action potential were blocked by ranolazine.
Ranolazine prolongs repolarization and decreases frequency of the HL-1 spontaneous action potentials
The HL-1 cell line is a robust in vitro model for studying atrial myocyte electrophysiology. In the absence of shear stress, ranolazine extended the AP length via delayed repolarization, as demonstrated previously in dissociated myocytes.26,27 Rather than by block of late Na+ current which would shorten repolarization, AP extension due to prolongation of the repolarization phase is more likely due to block of IKr and/or NCX, two other known targets for ranolazine (IC50 ~10 and ~100 uM for ranolazine28).
Shear flow shortens repolarization and increases frequency of the HL-1 spontaneous action potentials
Faster AP frequency evoked by flow in HL-1 cells is similar to what has been reported on rat ventricular cardiomyocytes with optical mapping under intermittent parallel-plate shear stress.22,23 The effects of shear stress on HL-1 AP are not uniform across all AP parameters, such as resting potential, upstroke, plateau, or repolarization. Thus, it is less likely that non-selective cation stretch-sensitive channels (SACs) are involved in the shear response.29 Instead, voltage-gated ion channels may be responsible for shear sensitivity of HL-1 AP.
Ranolazine decreases the effects of shear flow on HL-1 spontaneous action potentials
Shortened repolarization and increased frequency, the responses of HL-1 AP to shear stress that were decreased by ranolazine, support the notion that ranolazine modulates cardiomyocyte mechanosensitivity.11 The voltage clamp data presented here show that NaV currents are present in HL-1 cells (Fig. 1) and that shear stress increases their peak currents (Fig. 2). These results agree with previous findings in NaV1.5-transfected HEK293 and freshly dissociated murine cardiomyocytes.11 However, under shear stress without ranolazine, we would have predicted that sodium channel mechanoactivation should depolarize the membrane, accelerate the early part of the upstroke, accelerate the initial part of the repolarization, and have a mixed effect on frequency (i.e., slower recovery from inactivation vs. a left-shifted window). Our results do not support this prediction; instead, the upstroke and early part of repolarization both overlap while frequency changes. We offer two potential explanations. First, the lack of depolarization with shear stress could be due to low availability of NaV current in resting HL-1 cells. While the resting membrane potential of HL-1 cells is -15 mV (Fig. 3C), the half-point of Na+ channel availability is -100 mV, and the window current is -70 mV (Fig. 1B). Therefore, we expect the availability of NaV1.5 at the resting potential to be small, which would limit the effects of mechanosensitivity on NaV channels at baseline. Second, we have shown previously that recovery from inactivation is delayed with stretch in the patch, possibly explaining the lack of effect on the AP upstroke as Na+ channel availability is decreased.3
While ranolazine and shear stress affect the same parameters of HL-1 AP, their effects are opposite. Furthermore, when ranolazine and shear stress are combined, ranolazine diminishes the effects of the latter on HL-1 AP. These data support the role of ranolazine as a blocker of mechanosensitivity and suggest that other targets of ranolazine may be involved in mechanosensitivity. Aside from NaV1.5, ranolazine is known to affect several other ion channels and transporters, including some possibly involved in the effects of shear stress.28
In summary, we show that the HL-1 cell line is a viable model for the study of atrial myocyte mechanosensitivity, that ranolazine and shear stress have opposite effects on spontaneous action potentials, and that ranolazine diminishes the effects of shear stress. We also show that Na+ currents in HL-1 cells are responsive to both ranolazine and shear stress and suggest other potential ranolazine targets of mechanosensitivity.
Methods
HL-1 cell culture
The HL-1 murine atrial myocyte cell line was maintained in accordance with established protocol.24 HL-1 cells grown on coverslips were transferred directly to a recording chamber filled with extracellular solution (below) without resuspension.
Data acquisition
Electrodes (Kimble KG12 glass) were pulled by a Sutter P97 puller (Sutter Instruments), fire polished to 2–5 MΩ resistance and coated with R6101 heat-cured compound. Whole cell data from HL-1 cells dialyzed with intracellular solution were recorded with an Axopatch200B patch clamp, CyberAmp320, Digidata 1322A, and pClamp9.2 software (Molecular Devices).
Voltage-clamp protocol
A single 18 sweep, 90 sec pulse protocol was designed to measure peak current, voltage dependence, and kinetics of activation and inactivation as described previously.11 Cells were held at -120 mV, stepped to test pulses from -130 mV to 30 mV at 5 or 10 mV intervals for 3 sec, then to -120 mV for 0.1 msec, and finally to a second test pulse at -30 mV for 100 ms. The sampling rate was 20 kHz, and intersweep time was 5 sec.
Current-clamp protocol
Current-clamp data were acquired in gap-free mode with no current injected. Data were sampled at 100 Hz and filtered at 40 Hz.
Solutions
The extracellular solution contained (in mM): 160 Cl−, 150 Na+ or NMDG+, 5 K+, 2.5 Ca2+, 10 HEPES, 5.5 glucose, and 0 or 0.05 ranolazine. pH was adjusted to 7.4 with NaOH. The intracellular solution contained (in mM): 125 CH3SO3−, 35 Cl−, 145 Cs+, 5 Na+, 5 Mg2+, 5 HEPES, 2 EGTA. pH was adjusted to 7.0 with CsOH. Osmolality was 290 mmol/kg. The predicted liquid junction potential of -12.7 mV was subtracted during analysis. All chemicals were purchased from Sigma-Aldrich.
Mechanical stimulation
Flow of solution through the bath chamber at 10 mL/min served as the mechanical stimulus as previously described.9,11 For voltage-clamp experiments, control currents were recorded in ranolazine-free solution after seal and access until a stable baseline was established. Next, the 0.7 mL elliptical chamber was flushed with ranolazine-free solution at 10 mL/min for the duration of the 90 sec recording to obtain bath flow data as a paired control. Upon the conclusion of the flow recording, extracellular solution plus ranolazine premixed to 50 µM was immediately washed into the chamber then allowed to incubate on cells for 4 min before the ranolazine data were recorded. Finally, the cells were perfused with ranolazine solution at 10 mL/min to determine the effect of ranolazine on mechanosensitivity of NaV1.5. For current-clamp experiments, continuous recordings were taken under stagnant control or flowing conditions for 4 or 1 min, respectively, alternately without or with ranolazine (50 μM) present.
Analysis
Data were analyzed with pClamp 10 (Molecular Devices), Microsoft Excel 2010, and SigmaPlot 12 (Systat Software). Spontaneous action potentials were analyzed by template search event detection in pClamp 10. Peak currents are expressed as a fraction of cell capacitance (pA/pF). Peak currents at test pulses 1 or 2 vs. the voltages of test pulse 1 determined the voltage dependence of activation or inactivation, respectively. Peak currents normalized to the equation 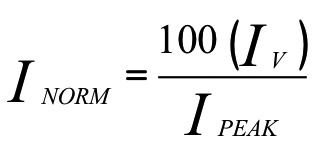 were fit with a sigmoid 3-parameter curve:
were fit with a sigmoid 3-parameter curve: 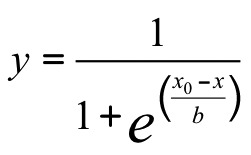 ,where x0 is V1/2, the voltage of half-activation or -inactivation. Currents that activated during the first 50 ms of test pulse 1 were fitted with a 3-term weighted exponential equation:
,where x0 is V1/2, the voltage of half-activation or -inactivation. Currents that activated during the first 50 ms of test pulse 1 were fitted with a 3-term weighted exponential equation: 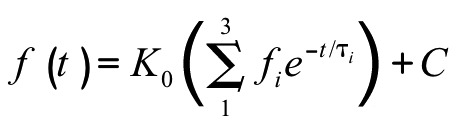 , in which three time constants represent one activation and two inactivation states of NaV1.5. Significance was assigned when p < 0.05 by two-way repeated measures ANOVA with Bonferroni multiple comparisons posttest (Prism 5, GraphPad Software).
, in which three time constants represent one activation and two inactivation states of NaV1.5. Significance was assigned when p < 0.05 by two-way repeated measures ANOVA with Bonferroni multiple comparisons posttest (Prism 5, GraphPad Software).
Acknowledgments
We thank Kristy Zodrow for secretarial assistance. This work was supported by NIDDK 52766 and P30DK084567).
Glossary
Abbreviations:
- AP
action potential
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22017
References
- 1.Wilde AA, Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ Res. 2011;108:884–97. doi: 10.1161/CIRCRESAHA.110.238469. [DOI] [PubMed] [Google Scholar]
- 2.Bennett PB, Yazawa K, Makita N, George AL., Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–5. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 3.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J Physiol. 2010;588:4969–85. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris CE, Juranka PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–33. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyder A, Sachs F. Electromechanical coupling in the membranes of Shaker-transfected HEK cells. Proc Natl Acad Sci U S A. 2009;106:6626–31. doi: 10.1073/pnas.0808045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu CX, Juranka PF, Morris CE. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage-gated K+ channel. Biophys J. 2001;80:2678–93. doi: 10.1016/S0006-3495(01)76237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin W, Laitko U, Juranka PF, Morris CE. Dual stretch responses of mHCN2 pacemaker channels: accelerated activation, accelerated deactivation. Biophys J. 2007;92:1559–72. doi: 10.1529/biophysj.106.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, et al. alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol. 2002;283:C1001–8. doi: 10.1152/ajpcell.00140.2002. [DOI] [PubMed] [Google Scholar]
- 9.Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, et al. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol. 2003;284:C60–6. doi: 10.1152/ajpcell.00532.2001. [DOI] [PubMed] [Google Scholar]
- 10.Beyder A, Strege PR, Bernard C, Farrugia G. Membrane permeable local anesthetics modulate NaV 1.5 mechanosensitivity. Channels (Austin) 2012;6:6. doi: 10.4161/chan.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyder A, Strege PR, Reyes S, Bernard CE, Terzic A, Makielski J, et al. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel Na(v)1.5: a novel mechanism of drug action. Circulation. 2012;125:2698–706. doi: 10.1161/CIRCULATIONAHA.112.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershberger RE, Parks SB, Kushner JD, Li D, Ludwigsen S, Jakobs P, et al. Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with familial or idiopathic dilated cardiomyopathy. Clin Transl Sci. 2008;1:21–6. doi: 10.1111/j.1752-8062.2008.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, et al. A mutation in telethonin alters Nav1.5 function. J Biol Chem. 2008;283:16537–44. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig X, Dysek S, Kimbacher S, Mike AK, Cervenka R, Lukacs P, et al. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS One. 2011;6:e20300. doi: 10.1371/journal.pone.0020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou Y, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, et al. Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J Biol Chem. 2003;278:1915–23. doi: 10.1074/jbc.M209938200. [DOI] [PubMed] [Google Scholar]
- 16.Maier LS. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: inhibition of late I(Na) using ranolazine. J Cardiovasc Pharmacol. 2009;54:279–86. doi: 10.1097/FJC.0b013e3181a1b9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–71. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 18.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, et al. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–21. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 19.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–8. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–26. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirakawa R, El-Bizri N, Shryock JC, Belardinelli L, Rajamani S. Block of Na+ currents and suppression of action potentials in embryonic rat dorsal root ganglion neurons by ranolazine. Neuropharmacology. 2012;62:2251–60. doi: 10.1016/j.neuropharm.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Kong CR, Bursac N, Tung L. Mechanoelectrical excitation by fluid jets in monolayers of cultured cardiac myocytes. J Appl Physiol 2005; 98:2328-36; discussion 0. [DOI] [PubMed]
- 23.Lorenzen-Schmidt I, Schmid-Schönbein GW, Giles WR, McCulloch AD, Chien S, Omens JH. Chronotropic response of cultured neonatal rat ventricular myocytes to short-term fluid shear. Cell Biochem Biophys. 2006;46:113–22. doi: 10.1385/CBB:46:2:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claycomb WC, Lanson NA, Jr., Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajamani S, El-Bizri N, Shryock JC, Makielski JC, Belardinelli L. Use-dependent block of cardiac late Na(+) current by ranolazine. Heart Rhythm. 2009;6:1625–31. doi: 10.1016/j.hrthm.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szél T, Koncz I, Jost N, Baczkó I, Husti Z, Virág L, et al. Class I/B antiarrhythmic property of ranolazine, a novel antianginal agent, in dog and human cardiac preparations. Eur J Pharmacol. 2011;662:31–9. doi: 10.1016/j.ejphar.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 28.Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–90. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H, Sachs F. Mechanically activated currents in chick heart cells. J Membr Biol. 1996;154:205–16. doi: 10.1007/s002329900145. [DOI] [PubMed] [Google Scholar]