Abstract
Inorganic polyphosphate (polyP) is a naturally occurring polyanion made of ten to several hundred orthophosphates (Pi) linked together by phosphoanhydride bonds. PolyP is ubiquitously present in all organisms from bacteria to humans. Specific physiological roles of polyP vary dramatically depending on its size, concentration, tissue and subcellular localization. Recently we reported that mitochondria of ventricular myocytes contain significant amounts (280 ± 60 pmol/mg of protein) of polyP with an average length of 25 orthophosphates, and that polyP is involved in Ca2+-dependent activation of the mitochondrial permeability transition pore (mPTP). Here we extend our study to demonstrate the involvement of mitochondrial polyP in cardiac cell death. Furthermore, we show that polyP levels depend on the activity of the respiratory chain and are lower in myocytes from failing hearts. We conclude that polyP is a dynamically regulated macromolecule that plays an important role in mPTP-dependent cell death pathway.
Keywords: permeability transition pore, inorganic polyphosphate, mitochondria, hypercontracture, cell death, calcium, ventricular myocytes, exopolyphosphatase, DAPI
Introduction
Inorganic polyphosphate (polyP) is a polyanionic polymer consisting of tens to hundreds of orthophosphate molecules linked together by phosphoanhydride bonds like in ATP. PolyP is present in all living organisms ranging from bacteria to human. While the presence of polyP in mammals has been documented several decades ago, its physiological roles are still poorly understood. It has been proposed that in mammals (and generally in higher eukaryotes) polyP is a versatile macromolecule which can play multiple roles. Examples of the roles of polyP in mammalians include: skeletal mineralization,1 blood coagulation and inflammation,2 cell bioenergetics,3 ion channel function4 and nuclear transcription.5
At present, very little is known about the molecular details of polyP metabolism. Recently it was demonstrated that a number of enzymes possess polyP hydrolyzing activity. This includes metastasis regulator protein H-prune, which is a short-chain specific polyP hydrolase6 and a long-chain endopolyphosphatase that was purified from rat and bovine brain.7 Finally, polyP can activate the protein kinase mTOR.8 However, it remains an open question to whether polyP metabolizing activity is the central functional role of these enzymes. Furthermore, to date, no mammalian polyP producing enzymes have been identified.
One of the most intriguing and least intuitive roles of polyP is its involvement in membrane ion transport. In 1988, Reusch and Sadoff, using bilayer techniques, demonstrated that genetically competent E. coli bacteria contain an ion channel formed by a complex of polyP and poly-β-hydroxybutyrate (PHB).9 The channel formed by polyP/Ca2+/PHB interaction was selective for cations with a preference for Ca2+.9,10 Later a similar polyP/Ca2+/PHB channel was isolated from rat liver mitochondria.11 Interestingly, in addition to the cation selective conductance state, this mitochondrial complex also demonstrated a high-conductance, weakly selective, voltage-dependent state. These properties, in many ways, reflected the behavior of the mitochondrial permeability transition pore (mPTP) as seen in patch-clamp studies of native mitochondrial membranes.12,13 Interestingly, the polyP/Ca2+/PHB channel of bacterial origin also has this high-conductance state14 and the transition of the channel into a high-conductance state would most likely be deleterious for bacterial organisms, raising the question whether most of the time the bacterial channel is either closed or is in the low-conductance cationic state. The different bacterial conductance states are reminiscent of conductance states proposed for the mPTP.15–17 The parallels between bacteria and mitochondria also suggest that similar cationic channels may play a role in normal mitochondrial function. In support of such notion, the polyP/Ca2+/PHB complex has been detected in various eukaryotic organisms and cellular compartments suggesting a potential physiological role.18
Currently, the direct test whether a polyP/Ca2+/PHB complex indeed forms the pore part of the mPTP in intact mitochondria remains an experimental challenge. Nonetheless, the idea that the presence of polyP in intact mitochondria is an essential condition for mPTP opening remains an intriguing hypothesis. Indeed, it was shown that mitochondria of cultured cells with reduced levels of polyP are more resistant toward mPTP opening and Ca2+-induced cell death.19
In our recent work, we have found that in cardiac cells, polyP is a potent activator of Ca2+-induced mPTP opening.20 In the heart, mPTP activity is directly linked to tissue damage and cell death during stress conditions including ischemia-reperfusion injury. Inhibition of mPTP is considered to be one of the central strategies for protective medical intervention. Thus, the discovery of a novel critical regulator of mPTP is expected to have a significant impact on the development of specific pharmacological approaches toward disease treatments.
Here we expand our study and investigate the link between endogenous levels of polyP and cell bioenergetics in cardiac tissue. Furthermore, we experimentally demonstrate a relationship between mitochondrial polyP and stress-induced cell death in the heart.
Results and Discussion
Effect of mitochondrial metabolic activity on polyP concentration
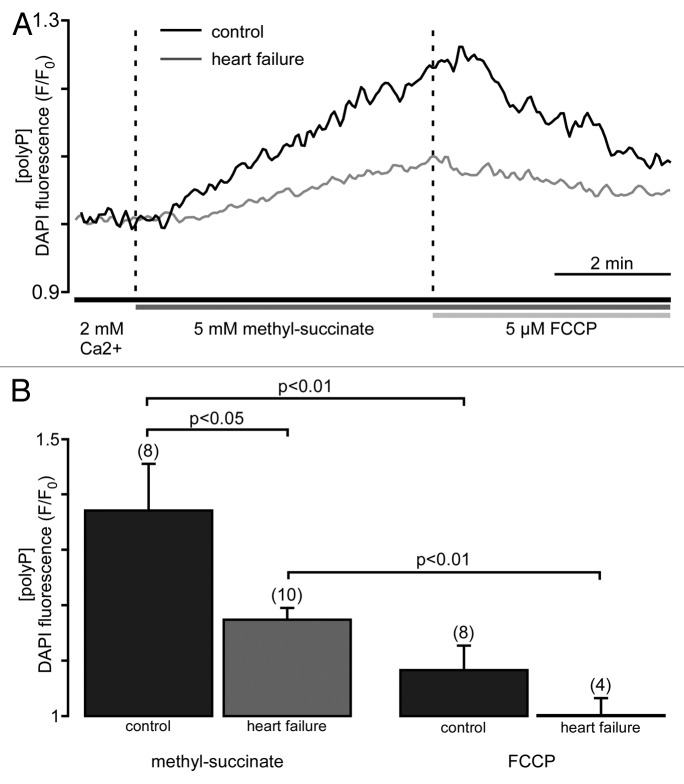
Earlier studies in mammalian cultured cells demonstrated rapid turnover of polyP. Specifically, it was found that if radioactive 32Pi was added to cultured cells it was incorporated into polyP within minutes to hours.21 Furthermore, lysis of cells resulted in a loss of polyP synthetic activity. The authors postulated that polyP synthesis is an energy-dependent process which requires participation of mitochondria.21,22 More recently, dynamics of polyP production and utilization were measured in isolated rat liver mitochondria and in intact cultured cells.3 Here we investigate the kinetics of mitochondrial polyP metabolism in cardiac cells. The relative changes in levels of polyP were measured using the fluorescent probe DAPI, with a protocol optimized for polyP detection.23 Experiments were performed in intact cells. We found that addition of membrane permeable methyl-succinate, the substrate of the complex II of the mitochondrial respiratory chain, resulted in an increase in DAPI fluorescence by 36 ± 8% (n = 8), indicating significant stimulation of the production of mitochondrial polyP (Fig. 1A and B). On the other hand, uncoupling of respiration with FCCP decreased DAPI fluorescence by 29 ± 4% (n = 8), presumably due to the stimulation of polyP hydrolysis. This indicates that polyP concentration in cardiac myocytes is variable and depends on levels of energy substrates and the degree of coupling of the mitochondrial respiratory chain. Interestingly, polyP metabolism was significantly suppressed in mitochondria of myocytes isolated from animals with heart failure (HF). Addition of methyl-succinate caused only a moderate increase in DAPI fluorescence (16 ± 2%, n = 10) (Fig. 1A and B). This observation raises the intriguing possibility that a reduction of polyP levels and diminished polyP synthesis reflect an important adaptive and protective mechanism that results from the complex remodeling processes during cardiac hypertrophy and heart failure. In HF, the propensity for mPTP opening is increased due to many different causes, including altered mitochondrial Ca2+ handling, compromised mitochondrial respiration and enhanced ROS formation.24, 25 Furthermore, in patients suffering from chronic heart failure, a 2-fold increase in blood β-hydroxybutyrate, a precursor of PHB, has been reported.26 According to the hypothesis that a polyP/Ca2+/PHB complex has channel properties and might form the mitochondrial permeability pore, increased β-hydroxybutyrate levels may further favor mPTP formation and opening in HF. On the other hand, the reduced mitochondrial polyP levels found in HF in this study can be interpreted as an adaptive and protective mechanism against enhanced vulnerability to mPTP opening in cardiac disease. Finally, respiratory chain activity is significantly impaired in HF25 and, thus, can also contribute to lower the sensitivity to mPTP opening by a mechanism which is not directly linked to polyP. Altogether, the data demonstrate the existence of a link between mitochondrial activity and polyP metabolism, and are in good agreement with previous findings that reported a strong dependence of polyP metabolism on cellular and mitochondrial energy metabolism.3
Figure 1. Mitochondrial polyP concentration is highly variable and depends on respiratory activity. (A) Original recordings of DAPI fluorescence changes in intact cardiac myocytes stimulated with 5 mM methyl-succinate followed by 5 µM FCCP from control (black) and failing myocytes (gray). DAPI fluorescence represents changes in polyP concentration. (B) Average values of maximal DAPI fluorescence after methyl-succinate (left) and minimal DAPI fluorescence during FCCP (right) addition in control (black) and heart failure (gray) cells.
Effect of polyP depletion on cell contractility and viability under working conditions
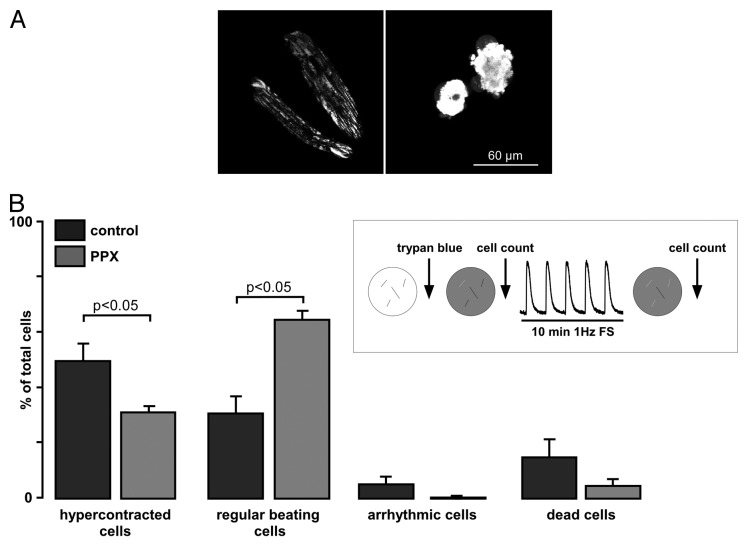
Our recent data demonstrated that depletion of polyP did not cause detectable changes in Ca2+ signaling during excitation-contraction coupling in cardiac cells under normal physiological conditions.20 However, mitochondria of polyP depleted cells were resistant to Ca2+-induced mPTP opening. Thus, we hypothesized that cells with depleted polyP will be protected against stress conditions related to Ca2+ overload. To test this hypothesis, we compared normal and polyP-depleted ventricular myocytes in respect to cell survival during extended working conditions. To deplete endogenous polyP levels, rabbit ventricular myocytes were adenovirally infected for 24 h in short-term culture to overexpress an exopolyphosphatase (PPX). Cells were subjected to an electrical pacing protocol (1 Hz field stimulation at room temperature) for 10 min. In addition, cells were stained with Trypan blue to evaluate whether continuous contractile activity lead to cell death. As shown in Figure 2, only ~30% of control cells were beating regularly at the end of the continuous stimulation period with a significant portion (~50%) of examined cells (n = 77) changing their shape and undergoing hypercontracture. In contrast, PPX-expressing cells with depleted polyP levels demonstrated an increased resistance to contractile dysfunction and cell death. From a total number of 80 PPX-expressing cells examined, only ~30% of total hypercontracted, while 64% remained regularly beating (Fig. 2B). The trypan blue exclusion test revealed that at the end of the stress test, only a small percentage of hypercontracted cells had a ruptured plasma membrane in both control and PPX cells with an increased number of dead cells observed in control (~15% of total) vs. PPX (~5% of total) group.
Figure 2. Polyphosphate depletion increases cell resistance to continuous work load and prevents hypercontracture. (A) Typical images of control GFP-expressing cells loaded with 10 nM tetramethylrhodamine methyl ester, a mitochondrial membrane potential sensitive dye, to point out the morphological changes seen during the stress test. Cells on the right represent an example of cells undergoing hypercontracture, which was defined as > 60% of shortening with respect to the initial cell length with concomitant morphological distortion of cell geometry (round-shaped morphology). (B) Summary of percentage of control and PPX-expressing cells (from the total amount of cells investigated), which underwent hypercontracture (left), remained normal function (regular beating cells, middle left), became arrhythmic (middle right) and died (right). Cells were stained with 0.01% Trypan blue for 10 min at room temperature.
In isolated cardiac myocytes, continuous contractile work load can lead to cell damage, presumably as a result of excessive Ca2+ overload. Furthermore, our observations indicate that mitochondrial polyP contributes toward the development of cell damage. The exact mechanism of polyP contribution remains to be established, however, it is likely to involve excessive mitochondrial Ca2+-uptake invoking a scenario where the formation of polyP/Ca2+/PHB complex is linked to the activation of mPTP. Nonetheless, other possibilities should be considered. It is noteworthy, that during pathological Ca2+ overload, mPTP activation does not depend on the concentration of free mitochondrial Ca2+, but likely depends on the dynamics of formation of Ca2+-phosphate precipitates inside the matrix.27,28 This raises the tantalizing possibility that the formation of Ca2+-polyP precipitates rather than Ca2+-Pi precipitates is responsible for the deleterious effect of Ca2+ overload on mitochondrial function.29
Conclusion
In conclusion, our data are consistent with a key role for polyP in activation of the mPTP and in regulation of stress-induced cell death. We speculate that endogenous levels of mitochondrial polyP reflect the ability of the cell to survive stress conditions. Mitochondrial polyP concentration is subject to remodeling processes in heart failure and the decreased polyP levels represent a protective measure against enhanced mPTP activity in cardiac disease.
Materials and Methods
Cell isolation and culture
Left ventricular myocytes were enzymatically isolated from adult New Zealand white rabbits (2.5 kg; 3–4 mo old; Myrtle’s Rabbitry) by coronary cannulation and perfusion as described previously.30 Heart failure was induced by combined left ventricular pressure and volume overload.31
Isolated cells were kept in MEM solution with 50 μM Ca2+ at room temperature (22–24°C) until they were used for experimentation or short-term (24 h) cell culture (see below).
All protocols were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by the institutional Animal Care and Use Committee.
PolyP measurements
PolyP levels were estimated in intact cells loaded with 5 μg/ml 4’,6-diamidino-2-phenylindole, dihydrochloride (DAPI) for 30 min at 37°C.23 DAPI was excited with 408-nm laser light, and emitted fluorescence was measured at 552–617 nm. Background subtracted DAPI fluorescence intensity (F) in each experiment was normalized to the level of fluorescence recorded prior to stimulation (F0). Changes in PolyP levels are expressed as F/F0.
PolyP depletion
To decrease polyP concentration, cells were infected with an exopolyphosphatase (PPX)-expressing adenovirus as described previously.20 Normal control cells were infected with an adenovirus expressing only GFP. Myocytes were cultured on laminin-covered glass coverslips in PC-1 medium. Experiments were performed 24 h after infection with GFP- or PPX-expressing adenoviruses.
Acknowledgments
We thank Dr. Jody Martin (Loyola University Chicago) for help with viral construct and Brian J. Danzer (Rush University Medical Center) for technical assistance. This work was supported by the National Institutes of Health Grants HL62231, HL80101 and HL101235, the Leducq Foundation (to L.A.B.), the American Heart Association (AHA) National Scientist Development Grant AHA 0735071N and Rush University Medical Center New Investigator Grant-in-Aid 31196 (to E.N.D.) and Heart and Stroke Foundation of Nova Scotia and Canadian Institute of Health Research (to E.P.).
Glossary
Abbreviations:
- HF
heart failure
- mPTP
mitochondrial permeability transition pore
- Pi
orthophosphate
- PHB
poly-β-hydroxybutyrate
- polyP
inorganic polyphosphate
- PPX
exopolyphosphatase
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/21939
References
- 1.Omelon S, Georgiou J, Henneman ZJ, Wise LM, Sukhu B, Hunt T, et al. Control of vertebrate skeletal mineralization by polyphosphates. PLoS One. 2009;4:e5634. doi: 10.1371/journal.pone.0005634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119:5972–9. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gómez-García MR, Abramov AY. Inorganic polyphosphate and energy metabolism in mammalian cells. J Biol Chem. 2010;285:9420–8. doi: 10.1074/jbc.M109.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez-Nunez MD, Moreno-Sanchez D, Hernandez-Ruiz L, Benitez-Rondan A, Ramos-Amaya A, Rodriguez-Bayona B, et al. Myeloma cells contain high inorganic polyphosphate levels that are associated with nucleolar transcription. Haematologica. 2012;97:1264–71. doi: 10.3324/haematol.2011.051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tammenkoski M, Koivula K, Cusanelli E, Zollo M, Steegborn C, Baykov AA, et al. Human metastasis regulator protein H-prune is a short-chain exopolyphosphatase. Biochemistry. 2008;47:9707–13. doi: 10.1021/bi8010847. [DOI] [PubMed] [Google Scholar]
- 7.Kumble KD, Kornberg A. Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J Biol Chem. 1996;271:27146–51. doi: 10.1074/jbc.271.43.27146. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Fraley CD, Faridi J, Kornberg A, Roth RA. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc Natl Acad Sci USA. 2003;100:11249–54. doi: 10.1073/pnas.1534805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reusch RN, Sadoff HL. Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci USA. 1988;85:4176–80. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reusch RN, Huang R, Bramble LL. Poly-3-hydroxybutyrate/polyphosphate complexes form voltage-activated Ca2+ channels in the plasma membranes of Escherichia coli. Biophys J. 1995;69:754–66. doi: 10.1016/S0006-3495(95)79958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlov E, Zakharian E, Bladen C, Diao CT, Grimbly C, Reusch RN, et al. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys J. 2005;88:2614–25. doi: 10.1529/biophysj.104.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabó I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991;266:3376–9. [PubMed] [Google Scholar]
- 13.Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun. 1991;176:1183–8. doi: 10.1016/0006-291X(91)90410-9. [DOI] [PubMed] [Google Scholar]
- 14.Pavlov E, Grimbly C, Diao CT, French RJ. A high-conductance mode of a poly-3-hydroxybutyrate/calcium/polyphosphate channel isolated from competent Escherichia coli cells. FEBS Lett. 2005;579:5187–92. doi: 10.1016/j.febslet.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Hüser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J. 1998;74:2129–37. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–53. doi: 10.1016/S0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 17.Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. doi: 10.1016/S0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 18.Reusch RN. Poly-beta-hydroxybutyrate/calcium polyphosphate complexes in eukaryotic membranes. Proc Soc Exp Biol Med. 1989;191:377–81. doi: 10.3181/00379727-191-42936. [DOI] [PubMed] [Google Scholar]
- 19.Abramov AY, Fraley C, Diao CT, Winkfein R, Colicos MA, Duchen MR, et al. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc Natl Acad Sci USA. 2007;104:18091–6. doi: 10.1073/pnas.0708959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidlmayer LK, Gomez-Garcia MR, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate is a potent activator of the mitochondrial permeability transition pore in cardiac myocytes. J Gen Physiol. 2012;139:321–31. doi: 10.1085/jgp.201210788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–22. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 22.Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 23.Aschar-Sobbi R, Abramov AY, Diao C, Kargacin ME, Kargacin GJ, French RJ, et al. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J Fluoresc. 2008;18:859–66. doi: 10.1007/s10895-008-0315-4. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, O’Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J Bioenerg Biomembr. 2009;41:127–32. doi: 10.1007/s10863-009-9216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidlmayer L, Blatter LA, Dedkova EN. Increased activity of mitochondrial complex II in rabbit heart failure is associated with reactive oxygen species generation and impaired excitation-contraction coupling. Heart. 2011;97:e8. doi: 10.1136/heartjnl-2011-301156.20. [DOI] [Google Scholar]
- 26.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, et al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–72. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–70. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 28.Wei AC, Liu T, Winslow RL, O’Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol. 2012;139:465–78. doi: 10.1085/jgp.201210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheu SS, Dirksen RT, Pugh EN., Jr. Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondria take center stage. J Gen Physiol. 2012;139:391–3. doi: 10.1085/jgp.201210819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dedkova EN, Blatter LA. Measuring mitochondrial function in intact cardiac myocytes. J Mol Cell Cardiol. 2012;52:48–61. doi: 10.1016/j.yjmcc.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–48. doi: 10.1161/01.CIR.92.4.1034. [DOI] [PubMed] [Google Scholar]