Abstract
CaV channels are multi-subunit protein complexes that enable inward cellular Ca2+ currents in response to membrane depolarization. We recently described structure-function studies of the intracellular α1 subunit domain I-II linker, directly downstream of domain IS6. The results show the extent of the linker’s helical structure to be subfamily dependent, as dictated by highly conserved primary sequence differences. Moreover, the difference in structure confers different biophysical properties, particularly the extent and kinetics of voltage and calcium-dependent inactivation. Timothy syndrome is a human genetic disorder due to mutations in the CaV1.2 gene. Here, we explored whether perturbation of the I-II linker helical structure might provide a mechanistic explanation for a Timothy syndrome mutant’s (human CaV1.2 G406R equivalent) biophysical effects on inactivation and activation. The results are equivocal, suggesting that a full mechanistic explanation for this Timothy syndrome mutation requires further investigation.
Keywords: Timothy syndrome, voltage-dependent calcium channels, voltage-dependent inactivation, α-helix
Introduction
Our recently published studies on the structure and function of the CaV1.2 and CaV2.2 domain I-II intracellular linkers demonstrated that their structures, particularly the membrane proximal regions immediately after IS6, called the PL, conferred specific characteristics to channel biophysical properties.1 We observed distinct structural conformations for each of the channel subtype I-II linker/CaVβ complexes. Motivated by these structural observations, we tested the consequences of these structural variations on the biophysical properties of intact CaV channels. Our electrophysiological results showed divergent, channel-type PL helical structure to be highly important for various channel functional properties including VDI, CDI and the voltage dependence of activation. We also tested, shown below, the possibility that perturbations of helical structure might provide a mechanistic explanation for the biophysical properties of the Timothy syndrome mutation, G436R, the rabbit equivalent of human CaV1.2 G406R, found in the interface between IS6 and the I-II linker PL.
Results
The Timothy syndrome mutation in the PL
The CaV1.2 PL sequence includes two glycine residues. Both G436 and G449 are highly conserved in the CaV1 channel subtype. G436, located at the N-terminal end of the PL, is in fact conserved among the whole HVA VDCC family (Fig. Four in Almagor et al.1). A well-studied mutation of this glycine to almost all other amino acids is known to decelerate VDI drastically.2-7 In humans, this mutation (G406R in human CaV1.2) results in a rare multi-system disorder known as Timothy syndrome, characterized by physical abnormalities, along with developmental and neurological deficiencies.2,5
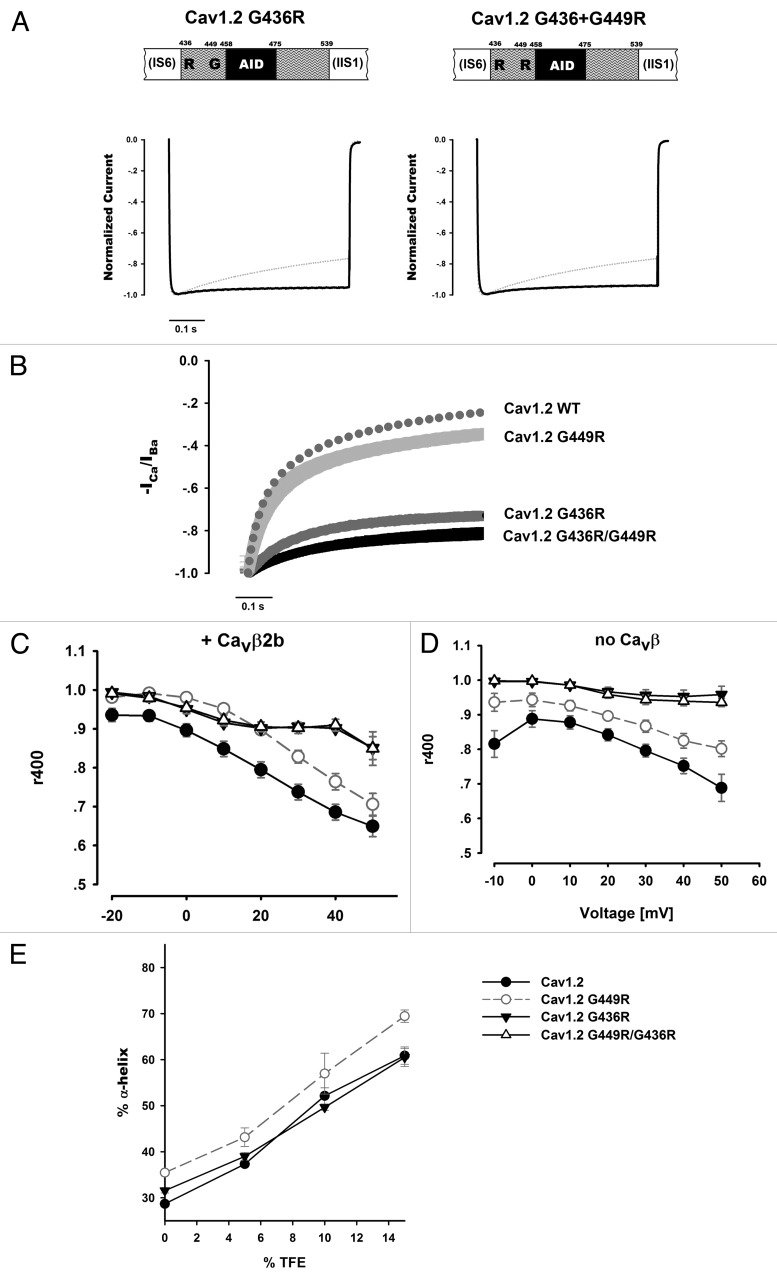
To explore whether this mutation may be mechanistically related to G449R, where we observed greater PL helicity and slowing of both VDI and CDI, we tested G436R alone and in combination with G449R electrophysiologically. Similar to CaV1.2 G449R, the CaV1.2 G436R mutant channels exhibited slow inactivation kinetics in Ba2+ (Fig. 1A and C). Unlike CaV1.2 G449R, the CaV1.2 G436R inactivation kinetics were more constant with increasing depolarization. Thus, at higher voltages, differences are more apparent. Compared at peak currents, i.e., taking into account their relative shift in voltage dependence of activation, G436R inactivation is slightly slower than that of G449R (p = 0.004, comparing r400 of G449R at +20 mV to G436R at 0 mV). In the span of 500 msec depolarization experiments, the double mutant, CaV1.2 G436R, G449R, exhibited inactivation kinetics indistinguishable from those of G436R (Fig. 1A). Apparently, the background of the Timothy syndrome G436R mutation masks the G449R mutation’s effect on inactivation kinetics, since the G436R mutant displayed almost no inactivation at all during the experiment’s time course. Our data also show that the marked effect of the G436R mutant on VDI kinetics does not involve the β subunit since it was observed either in the presence or absence of CaVβ2b (Fig. 1C and D).
Figure 1. The TS mutation. (A) Representative Ba2+ currents of CaV1.2 mutants G436R and G436R/G449R. Currents were recorded during a 0 mV depolarizing step from a holding potential of -80 mV. Traces are presented normalized to their peak value. Dotted traces denote the CaV1.2 WT current for comparison. (B) Normalized -ICa/IBa of averaged currents at +20 mV depolarization. Average values and SEM are plotted. Data were averaged from the following numbers of cells measured in Ba2+/Ca2+: CaV1.2 WT: 11/10, G449R: 14/9, G436R: 5/5, G436R/G449R: 8/6 cells.CaV1.2 G449R: p = 0.005 vs. WT, p < 0.001 vs. others; CaV1.2 G436R p < 0.001 vs. WT, p < 0.001 vs. G449R; CaV1.2 G436R/G449R: p < 0.001 vs. WT, p < 0.001 vs. G449R. (Statistical analysis based on comparing -ICa/IBa values at 400 ms from depolarization, using a One-way Anova) . (C–D) Voltage-dependence of CaV1.2 Ba2+ current inactivation kinetics with (C) or without (D) CaVβ2b. Similar to G449R, G436R (n = 5 with and without CaVβ2b) decelerates inactivation kinetics. Note the mutant's shallow voltage dependence. In the measurement range tested, G449R is masked by the G436R background effect of the double mutant (n = 8 with CaVβ2b and n = 9 without). Statistics are presented in Table 1. The symbol legend for panels C and D are found in panelE. (E) CD measurements of α-helical content for CaV1.2 Helix-PL-AID proteins with increasing TFE. G436R does not show a marked change from CaV1.2 WT while G449R increases the PL's α-helix content. All measurements were repeated at least twice.
Previous investigation of this Timothy syndrome mutant CaV1.2 channel gave divergent results regarding its effect on CDI.3,4 Our findings, shown in Figure 1B, demonstrate an extensive decrease for CaV1.2 G436R CDI. This decrease is considerably larger than that found with G449R (Fig. 5E in Almagor et al.1). Here an additive effect may be observable and the CDI of the double mutant, G436R/G449R, appeared smaller than that of both single mutants (Fig. 1B), although the sample is too small to conclude this with statistical significance.
Arginine substitutions at both G449 and G436 reduce VDI and CDI. As shown in Figure 1E, despite their generally similar functional effects, the structural consequences of these mutations differ, as assessed by CD spectroscopy. While G449R increases the CaV1.2 PL helix content, the helicity of the G436R mutant is not significantly different from the WT. This lack of structural effect for the G436R mutant, while at the same time exhibiting an even more marked electrophysiological effect than the G449R mutant on inactivation, leads us to conclude that there are other mechanistic factors beyond that of PL secondary structure modification required to explain this Timothy syndrome mutation’s effect on VDI.
PL conformational effects on the voltage-dependence of activation
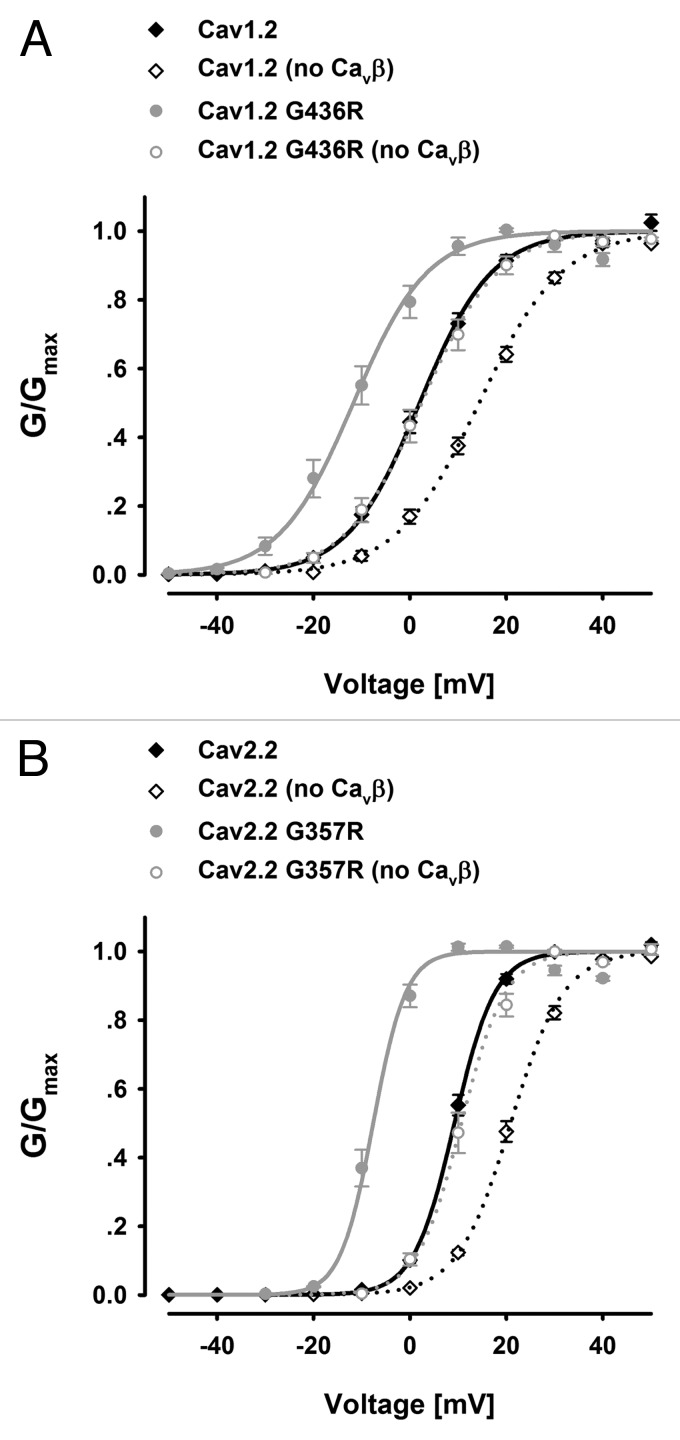
We reasoned that the PL structure may have an impact on activation also since the S6 forms a channel gate which must open for conductance and S6 is directly connected to the PL. Hence, we analyzed our data for any effects. Our results show that mutating the Timothy syndrome glycine of both CaV1.2 and CaV2.2 I-II linkers to arginine (CaV1.2 G436R and CaV2.2 G357R), resulted in appreciable hyperpolarizing shifts in activation voltage, with or without CaVβ (Fig. 2A and B). The degree of functional effect on activation largely parallels the functional changes on inactivation. The electrophysiological results are summarized in Table 1.
Figure 2. The effect of PL conformation on the voltage-dependence of activation. (A) CaV1.2 G436R (n = 5/5); (B) CaV2.2 G357R (n = 10/10) conductance vs. voltage curves compared with their respective wild types in the presence or absence of CaVβ2b.
Table 1. Ba2+ current electrophysiological properties.
| n | Ka | V0.5 | r400 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cav1.2 |
|
|
|
|
|
|
|
|
|
|
|
|
||
| Wild type, β2b |
11 |
7.5 |
± |
0.3 |
2.0 |
± |
1.1 |
0.79 |
± |
0.02 |
|
|
||
| G449R, β2b |
14 |
7.7 |
± |
0.2 |
1.5 |
± |
0.8 |
0.90 |
± |
0.01A |
|
|
||
| G436R, β2b |
5 |
7.7 |
± |
0.3 |
-11.7 |
± |
2.0 a |
0.90 |
± |
0.01A |
|
|
||
| G436R/G449R, β2b |
8 |
8.4 |
± |
0.3c |
-15.1 |
± |
2.4 a |
0.91 |
± |
0.01A |
|
|
||
| Wild type |
11 |
8.6 |
± |
0.4 |
14.4 |
± |
0.9 |
0.80 |
± |
0.02 |
|
|
||
| G449R |
5 |
9.2 |
± |
0.2 |
13.7 |
± |
1.4 |
0.87 |
± |
0.02C |
}(B) |
}(B) | ||
| G436R |
5 |
7.9 |
± |
0.2 |
2.5 |
± |
1.7 a |
0.96 |
± |
0.02A |
||||
| G436R/G449R | 9 | 8.8 | ± | 0.2 | 2.4 | ± | 0.7 a | 0.94 | ± | 0.01A | ||||
G-V relation voltage for 50% activation (V0.5) and slope factor (Ka) were calculated from voltage current relations as described in methods of Almagor et al.1 r400 for traces at +20 mV (or 30 mV when CaVβ is absent). Values are presented as mean ± SEM, the number of oocytes appears under “n.”
a p ≤ 0.001, b p ≤ 0.01, c p ≤ 0.05 compared with the corresponding WT (Unpaired t-test).
A (p ≤ 0.001), B (p ≤ 0.01), C (p ≤ 0.05) compared with the corresponding WT, (A) (p ≤ 0.001), (B) (p ≤ 0.01), (C) (p ≤ 0.05) (One-way ANOVA).
Discussion
The CaV1.2 Timothy syndrome mutation (G436R) might be expected to produce an increase in the PL α-helical content as with G449R. But, CD measurements did not detect differences from WT CaV1.2 helical content. Nevertheless, this mutation has robust effects on channel electrophysiology.2-7 TS mutant's effects occurred to a greater extent than in the other helix extending mutations, including both VDI and voltage dependence of activation (see Fig. 5D, middle column in Almagor et al.1). Additionally, in line with our suggestion for a common role of the PL in VDI and CDI, our data present a similar trend with the substantially larger effect of G436R compared with G449R on CDI (Fig. 1B).
This large functional effect, induced by a single glycine substitution at the PL N-terminus relative to the smaller effect for a similar substitution downstream (i.e., CaV1.2 G449R), implicates an additional factor other than PL α-helical content in this mutant phenotype. Interestingly, homologous mutations decelerate inactivation kinetics in both CaV2.16 and CaV2.3.3 Likewise, in our study, CaV2.2 G357R displayed extremely slow kinetics and a large hyperpolarizing shift in the voltage dependence of activation. In the case of Cav2.2, the mutation clearly produces an increase in the α-helical content of the PL (see Fig. 6A in Almagor et al.1). Hence, further stabilization of the PL α-helix may result, at least in part, in decelerated inactivation kinetics, and play a role in activation efficiency.
Materials and Methods
Molecular cloning
cDNAs used for electrophysiology and their RNA transcript preparation were as described.1 Helix-PL-AID constructs for CD experiments were prepared as previously described.1 In short, PL-AID DNA fragments, encoding CaV1.2 residues 436–475 or CaV2.2 357–396 were inserted into a pET-28a vector (Novagen) downstream to the following sequence elements, all in frame: HisTag, maltose binding protein (MBP), TEV protease site and a sequence encoding an α-helical peptide (3xAAKAAE). All constructs and mutagenesis were prepared using standard PCR methods and verified by DNA sequencing.
Electrophysiology
Xenopus maintenance and oocyte preparation were as described.8 Oocytes were injected with RNA 3–5 d before the experiment. Due to lower channel surface expression, longer incubation time was used for oocytes not injected with CaVβ (up to 10 d). In all experiments unless specified, β2b and α2δ-1 auxiliary subunits RNAs were co-injected with that of the α1 subunit in equivalent amounts to the injected α1. Whole-cell two electrode voltage-clamp recordings were performed as described.1 Data acquisition and analysis were performed using pCLAMP software (Molecular Devices). I-V relation fitting and CDI (ICa/IBa) to time relations were generated as described.1 Comparison between a single test group and the control was calculated using an unpaired t-test while comparison between several groups was done using a one-way ANOVA (Holm–Sidak) test, all with SigmaPlot (Systat Software).
Protein expression and purification
Helix-PL-AID proteins were expressed in E. coli and purified as described.1 In a final size exclusion chromatography step (Superdex-75 Hi-prep, GE Healthcare), the MBP-free Helix-PL-AID peptides were eluted in 10 mM sodium phosphate pH 8.0, 0.2 M NaCl, used also for the CD spectroscopy.
CD spectroscopy
Measurements of Helix-PL-AID proteins at increasing TFE concentrations were performed as described.1 The purity and monodispersity of all samples were checked by SDS-PAGE with Tris-Tricine gels and analytical size-exclusion chromatography. α-helical content of sampled proteins (% α-helix) was extracted from mean residue molar ellipticity values at 222 nm, as described.9
Glossary
Abbreviations:
- CaV
voltage-dependent calcium channels
- PL
proximal linker
- VDI
voltage-dependent inactivation
- CDI
calcium-dependent inactivation
- CD
circular dichroism
- TFE
trifluorethanol
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22078
References
- 1.Almagor L, Chomsky-Hecht O, Ben-Mocha A, Hendin-Barak D, Dascal N, Hirsch JA. The role of a voltage-dependent Ca2+ channel intracellular linker: a structure-function analysis. J Neurosci. 2012;32:7602–13. doi: 10.1523/JNEUROSCI.5727-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Raybaud A, Dodier Y, Bissonnette P, Simoes M, Bichet DG, Sauvé R, et al. The role of the GX9GX3G motif in the gating of high voltage-activated Ca2+ channels. J Biol Chem. 2006;281:39424–36. doi: 10.1074/jbc.M607405200. [DOI] [PubMed] [Google Scholar]
- 4.Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc Natl Acad Sci U S A. 2008;105:2157–62. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–96, discussion 8086-8. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cens T, Leyris JP, Charnet P. Introduction into Ca(v)2.1 of the homologous mutation of Ca(v)1.2 causing the Timothy syndrome questions the role of V421 in the phenotypic definition of P-type Ca(2+) channel. Pflugers Arch. 2008;457:417–30. doi: 10.1007/s00424-008-0534-1. [DOI] [PubMed] [Google Scholar]
- 7.Yarotskyy V, Gao G, Peterson BZ, Elmslie KS. The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation. J Physiol. 2009;587:551–65. doi: 10.1113/jphysiol.2008.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shistik E, Ivanina T, Blumenstein Y, Dascal N. Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J Biol Chem. 1998;273:17901–9. doi: 10.1074/jbc.273.28.17901. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield NJ, Hitchcock-DeGregori SE. Conformational intermediates in the folding of a coiled-coil model peptide of the N-terminus of tropomyosin and alpha alpha-tropomyosin. Protein Sci. 1993;2:1263–73. doi: 10.1002/pro.5560020809. [DOI] [PMC free article] [PubMed] [Google Scholar]