Abstract
We previously reported that TREK-1 gating by internal pH and pressure occurs close to or within the selectivity filter. These conclusions were based upon kinetic measurements of high-affinity block by quaternary ammonium (QA) ions that appeared to exhibit state-independent accessibility to their binding site within the pore. Intriguingly, recent crystal structures of two related K2P potassium channels were also both found to be open at the helix bundle crossing. However, this did not exclude the possibility of gating at the bundle crossing and it was suggested that side-fenestrations within these structures might allow state-independent access of QA ions to their binding site. In this addendum to our original study we demonstrate that even hydrophobic QA ions do not access the TREK-1 pore via these fenestrations. Furthermore, by using a chemically reactive QA ion immobilized within the pore via covalent cysteine modification we provide additional evidence that the QA binding site remains accessible to the cytoplasm in the closed state. These results support models of K2P channel gating which occur close to or within the selectivity filter and do not involve closure at the helix bundle crossing.
Keywords: ion channel, K2P channel, potassium channel, TREK-1, TRAAK, TWIK-1, docking, homology modeling
Introduction
TREK-1 is the prototypical member of the two-pore (K2P) family of potassium channels which are important regulators of cellular electrical excitability.1,2 However, their gating mechanism remains poorly understood.3 In other K+ channels, two structurally distinct gates have been proposed; a lower gate at the intracellular helix bundle-crossing and an upper gate which comprises the outer pore and selectivity filter. Gating at the lower bundle-crossing is thought to involve constriction of the permeation pathway by the pore-lining helices, while the conformational changes at the upper gate probably involve much smaller movements which alter permeation through the selectivity filter.4
The relative importance of these two different gating mechanisms in K2P channels remains unclear.3,5 However, several recent studies have demonstrated an important role for the selectivity filter in TREK-1 channel gating and suggest that movement of the transmembrane helices is primarily involved in transducing intracellular signals to the filter gate rather than closure of the pore at the bundle crossing.6-8 In one of these studies we used scanning mutagenesis and structural modeling of the TREK-1 pore to identify a quaternary ammonium (QA) binding site within the inner cavity of the pore, and demonstrated that tetrahexylammonium (THexA) ions exhibit state-independent accessibility to this binding site.8 This result suggested that the bundle crossing remains open even when the permeation pathway is gated shut by intracellular pH and supported a model of TREK-1 gating which occurs close to or within the selectivity filter. Subsequent to this study, crystal structures of two members of the K2P family, including the related TRAAK channel, were determined and both found to be open at the helix bundle crossing.9,10 However, these open-state structures still did not exclude the possibility of gating at the bundle crossing and it was noted that the presence of side-fenestrations within these structures might represent an alternative route for QA ions to access their binding site thereby bypassing the bundle-crossing gate.10
In this addendum to our original study, we now demonstrate that a structural model of TREK-1 based upon TRAAK provides the best fit to our experimental data and that even hydrophobic blockers such as tetrahexylammonium do not access their binding site through the side fenestrations of the channel. We also provide further direct evidence that the intracellular cavity of TREK-1 remains accessible to pore-blockers in both the open and closed states.
Results and Discussion
Homology modeling of TREK-1
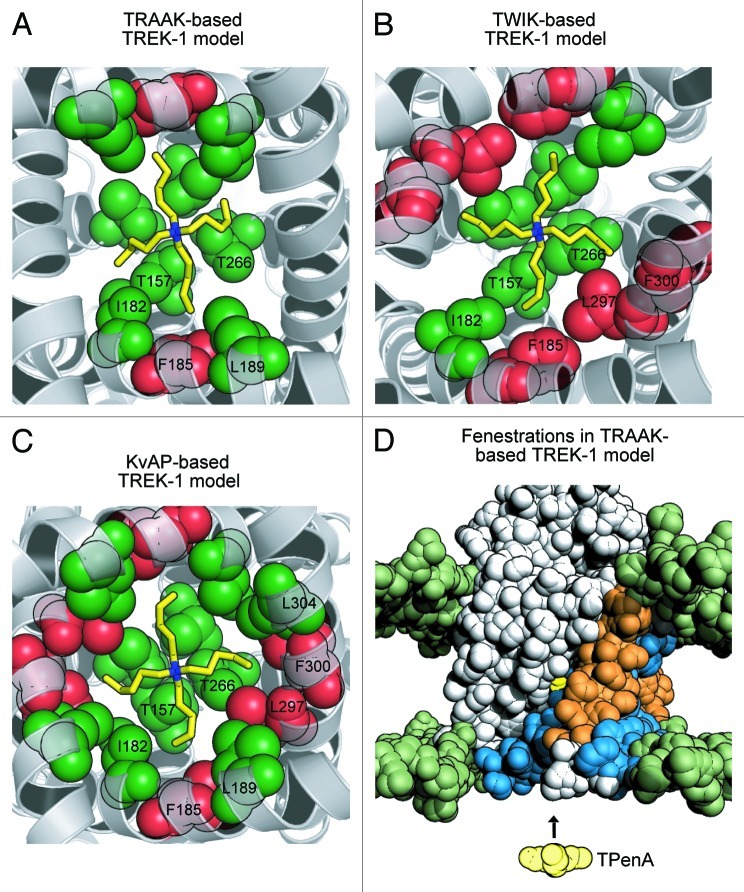
Using the recent crystal structures of two different K2P channels we have re-evaluated our previous structural model of TREK-1 which was based upon the open-state conformation of KvAP (PDB 1ORQ).8 Homology models of TREK-1 were built using TRAAK (PDB: 3UM7) and TWIK-1 (PDB: 3UKM) as structural templates, and residues within the inner cavity which contribute to the binding site for tetrapentylammonium (TPenA) were predicted by in silico docking of TPenA.11,12 In order to more accurately score these results, 100–200 individual TREK-1 models were generated per template, and those residues predicted to interact with TPenA were recorded for each model (Fig. 1A and B). For comparison to our previous model of TREK-1, the same analysis was also performed using KvAP (PDB 1ORQ) as a structural template (Fig. 1C).8
Figure 1. Refined structural models of TREK-1 with TPenA bound. (A) Bottom up view of a structural model of the TREK-1 pore with docked TPenA. The model was based upon the crystal structure of TRAAK (PDB ID: 3UM7). Those residues which interact with TPenA are highlighted as spheres and colored green if they agree with our functional scanning mutagenesis study or red if a false positive. Only residues on one protomer are numbered. (B) Similar model based upon the crystal structure of TWIK-1 (PDB ID: 3UKM). (C) Similar model based upon the crystal structure of KvAP (PDB ID: 1ORQ). (D) Solvent-accessible surface area (SASA) of a TREK-1 model, based on TRAAK, in which the side-fenestrations are still clearly visible. The SASA of the phospholipid head groups is colored green whereas the TREK-1 model is colored gray. For one protomer, the outer (M1 and M3) and inner (M2 and M4) transmembrane helices are highlighted in orange and blue, respectively. The docked TPenA is also visible through the side-fenestration (colored in yellow spheres) and QA ion access to this binding site occurs via the cytoplasmic pore of the channel (indicated by direction of the arrow).
These in silico results confirmed our previous observation that TPenA binds within the inner cavity just below the selectivity filter.8 Furthermore, when compared with the functional scanning mutagenesis data obtained in our previous study, the TRAAK-based model exhibited the least number of false positive interactions with TPenA (Fig. 1A) and is therefore likely to represent the most accurate structural model of TREK-1 currently available. This is perhaps not surprising given the high degree of homology between TREK-1 and TRAAK, especially within the transmembrane-pore region. However, our observation that a TWIK-1-based model provides no better fit to the experimental data than our original KvAP-based model highlights differences between the two pore-lining helices (M2 and M4) of TRAAK and TWIK-1.13,14
State-independent access of QA blockers to their binding via the permeation pathway
Our previous study proposed that the helix bundle-crossing gate in TREK-1 remains open even in the closed state. This key finding was based upon kinetic measurements of THexA access to its binding site which suggested that this blocking ion had access to its binding site even when the channel was gated closed by changes in intracellular pH and pressure.8 However, even though the recent crystal structures demonstrate an open bundle-crossing, there are two possible caveats to our original conclusion. First, even when gated closed at pH 8, the open probability of TREK-1 is unlikely to be zero and therefore THexA could possibly enter the pore via infrequent openings of the helix bundle crossing gate (if this is the gate) and subsequent blocker trapping might have caused the observed binding of THexA to the closed state. Second, the crystal structures of both TRAAK and TWIK-1 revealed side-fenestrations between the asymmetric subunits, and our TRAAK-based model of TREK-1 also exhibits similar fenestrations (Fig. 1D). This has led to suggestions that hydrophobic QA ions such as THexA might exhibit significant membrane solubility and access their binding site through these fenestrations instead of via the permeation pathway and helix bundle crossing.10,14
To address the latter concern we examined the effect of extracellular THexA on TREK-1 whole-cell currents expressed in Xenopus oocytes. If THexA can access the TREK-1 pore via a side fenestration pathway then it would have to do so via permeation of the membrane (Fig. 1D) and block would be independent of whether it was applied via the intracellular or extracellular surface. However, we found that THexA had no effect on TREK-1 currents when applied extracellularly, even at 50 µM for over 3 min (data not shown). By contrast, 1 µM THexA rapidly causes 50% block within 250 ms when applied intracellularly and this block is also rapidly reversible.8 This result suggests that the rapid access of THexA to its binding site is unlikely to occur via these membrane bound pathways. However, access of local anesthetics to the side fenestrations of the voltage-gated sodium channels is thought to occur via such pathways,15 but these compounds are significantly more hydrophobic than THexA.
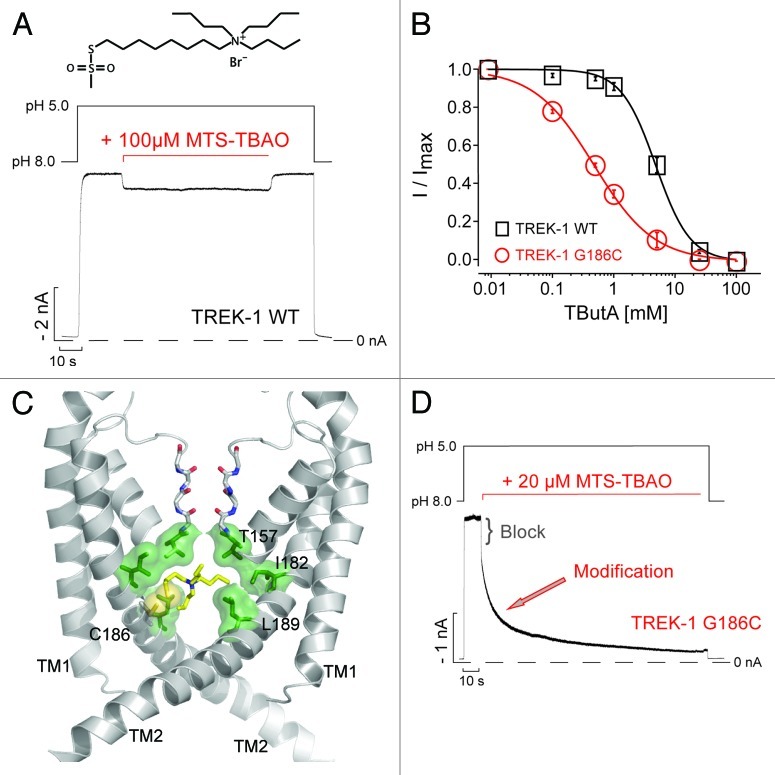
Nevertheless, even if access via a membrane permeant pathway is unlikely, this still does not exclude the possibility discussed above that brief openings of a bundle-crossing gate might allow direct access of THexA to its binding site during the closed state. An alternative approach to probe the status of this bundle-crossing gate is the use of sulfhydryl reactive MTS-reagents to probe the accessibility of cysteine residues within the inner cavity of the pore.16,17 We therefore decided to utilize a quaternary ammonium ion linked to an MTS-reagent, MTS-TBAO [8-(Tributylammonium)octyl methanethiosulfonate]. This compound has several potentially advantageous properties: First, it is considerably more hydrophilic than THexA and thus highly unlikely to access the pore via the fenestration pathway; second, it is large and will therefore only enter the pore if the bundle crossing gate is sufficiently wide open; third, the QA moiety is expected to block ion permeation; and finally, when present within the pore it will have a high effective local concentration and thus preferentially modify any accessible cysteines within the inner cavity. Importantly, our model of wild-type TREK-1 predicts that neither of the endogenous cysteines within the TM-helices (C174 and C234) will be accessible from within the inner pore, and intracellular application of 100 µM MTS-TBAO produced only a weak and reversible block (Fig. 2A) indicating the suitability of wild-type TREK-1 for cysteine modification studies.
Figure 2. MTS-TBAO block and modification of TREK-1. (A) TREK-1 channel currents expressed in Xenopus oocytes measured at -80 mV in inside-out patches, activated from pH 8 by pH 5 and exposed to MTS-TBAO for 120 sec. A small and reversible block [8.2 ± 0.5% at 100 µM; (n = 4)] by the TBAO-moiety was obtained. (B) Concentration-response curves for tetrabutylammonium (TButA) inhibition of wild-type TREK-1 and G186C channels fitted to a standard Hill function with IC50 values and Hill-coefficients of 4.8 ± 0.3 mM and 1.4 ± 0.1 (n = 5) for wt; 0.5 ± 0.1 mM and 1.1 ± 0.1 for G186 (n = 6). Data points represent mean ± SEM (C) Structural model of the P1 subunits in the TREK-1 pore, the M3 and M4 helices from the P2 subunit are removed for clarity. The MTS-TBAO was linked to the introduced cysteines at position 186 with the SH-group in yellow, which is located in close proximity to the predicted QA binding site (green). (D) Application of MTS-TBAO on TREK-1-G186C mutant channels results in an initial fast block [24.2 ± 1% at 20 µM; (n = 5)] followed by a monoexponential time course [τ = 18.6 ± 3.9 sec; (n = 5)] for the modification of this cysteine that irreversibly reduced the current by up to 90%.
In our previous study, the G186C mutation in TREK-1 exhibited a higher sensitivity to QA ion inhibition compared with wild-type TREK-1 (see also Fig. 2B).8 Consistent with this observation, our TREK-1 model suggests that a cysteine at this position would face toward the pore and be in close proximity to the predicted QA binding site. Covalent docking of MTS-TBAO to this site in our new model suggests that the G186C mutant may exhibit increased TPenA affinity via a direct, enhanced interaction with TPenA (Fig. 2C).18 Indeed, application of 20 µM MTS-TBAO on G186C TREK-1 channels previously activated by pH 5 resulted in a bi-phasic current decay. The fast phase likely represents reversible block by the TBAO-moiety, while the slower phase follows a monoexponential time course (τ = 18.6 ± 3.9 sec) and most likely represents irreversible covalent attachment of the blocker to this pore cysteine (G186C) because removal of MTS-TBAO did not result in any current recovery (Fig. 2D).
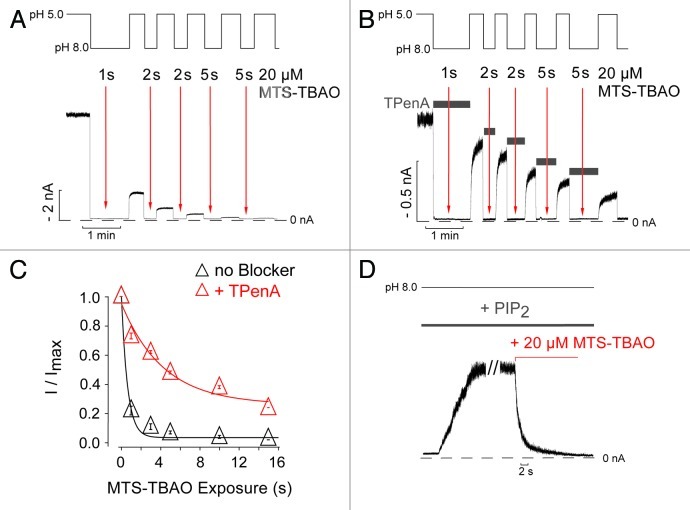
In the closed state (pH 8), application of 20 µM MTS-TBAO to G186C-TREK-1 for only 1 sec resulted in an irreversible reduction in current of > 70%. Using a cumulative reagent application protocol (Fig. 3A), the time constant for channel modification was determined to be 0.7 ± 0.1 sec (Fig. 3A and C). Furthermore, the presence of 1 mM TPenA during MTS-TBAO application to the closed channel (pH 8) profoundly slowed this rate of modification suggesting that both molecules compete for their binding site even when the channel is closed (Fig. 3B and C). The fact that channel modification appears faster at pH 8 compared with pH 5 most likely reflects the higher chemical reactivity of cysteines at alkaline pH.17 Regardless of this, the rapid modification rate observed while the channel is closed makes it unlikely that MTS-TBAO could have entered the pore via infrequent helix bundle-crossing openings. Moreover, the lack of any increase in the modification rate at pH 8 when 10 µM PtdIns(4,5)P2 application is used to produce a large increase in open probability (Fig. 3D) suggests beyond reasonable doubt that access of MTS-TBAO to its binding site within the pore is state-independent.
Figure 3. State-independent access of MTS-TBAO to the inner pore of TREK-1. (A) TREK-1 currents activated by pH 5 were exposed to repeated applications of 20 µM MTS-TBAO at pH 8 for the times indicated by the arrows in order to test the accessibility of Cys-186 in the closed state. (B) A similar experiment performed in the presence of 1 mM TPenA, which blocks TREK-1 with an IC50 of 13 ± 1 µM.8 The channels were exposed to the blocker during the time indicated by the horizontal bars that resulted in complete inhibition of the current. Twenty µM MTS-TBAO was then applied to the blocked channel at times indicated by the arrows. (C) Time course of the relative current decline from experiments such as those shown in panelsA and B as a function of cumulative reagent exposure (Modification Time x [MTS-TBAO]) resulted in a monoexponential time course of 0.7 ± 0.1 sec (n = 6) in absence and 8.2 ± 0.9 sec (n = 3) in the presence of 1 mM TPenA. Data points represent mean ± SEM. (D) Activation of TREK-1-G186C mutant channels by 10 µM PtdIns(4,5)P2 at pH 8 and subsequent application of MTS-TBAO irreversibly reduced the current. The decay of the current was fitted to a monoexponential function with a Tau (τ) of 1.2 ± 0.1 sec (n = 3).
In summary, these results support our original observations that the helix bundle-crossing in TREK-1 remains open and the inner pore cavity is accessible to QA ions even when the channel is gated closed by intracellular pH.8 Furthermore, the results are consistent with models of K2P channel gating where the primary gating mechanism is located close to or within the selectivity filter and suggest that any movement of the pore-lining helices is restricted to coupling of the cytoplasmic domains to this gating mechanism rather than closure of the channel at a lower bundle-crossing gate. Consequently, it may be difficult to determine whether the existing crystal structures of TWIK-1 and TRAAK represent fully open or closed state channels, and higher-resolution structures which address the conductive status of the selectivity filter may ultimately be necessary.
Materials and Methods
Homology models of human TREK-1 were generated as previously described,8 but in addition models were also built using the crystal structures of TWIK-1 (PDB: 3UKM, n = 100), and TRAAK (PDB: 3UM7, n = 200) as templates. In the TRAAK-based models of TREK-1, residues Tyr281–Tyr285, which are in the extracellular loop that connects the selectivity filter and M4, were modeled on TWIK-1. Symmetry restraints were imposed between the two monomers during the modeling procedure. TPenA was docked into the pore of TREK-1 and models were ranked according to their fit to our previous scanning mutagenesis data.8
Mutagenesis, cRNA synthesis, oocyte injection and electrophysiology were performed as previously described.8 MTS-TBAO (Toronto Research Chemicals) was stored as a stock solution in isopropanol at -80°C and diluted every 3h. This was stored on ice and the final dilution was made into the experimental solution immediately prior to each measurement. PtdIns(4,5)P2 (Avanti Polar Lipids) was stored as a stock solution at -80°C (1 mM in DMSO). It was then diluted to the stated final concentration in the experimental solution, sonicated for 35 min and used within 2–3 h.
Acknowledgments
This work was supported by research grants from the Deutsche Forschungsgemeinschaft, the Wellcome Trust, the Biotechnology and Biological Sciences Research Council and Pfizer Neusentis UK.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22153
References
- 1.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 2.Dedman A, Sharif-Naeini R, Folgering JH, Duprat F, Patel A, Honoré E. The mechano-gated K(2P) channel TREK-1. Eur Biophys J. 2009;38:293–303. doi: 10.1007/s00249-008-0318-8. [DOI] [PubMed] [Google Scholar]
- 3.Mathie A, Al-Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol. 2010;588:3149–56. doi: 10.1113/jphysiol.2010.192344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuello LG, Jogini V, Cortes DM, Pan AC, Gagnon DG, Dalmas O, et al. Structural basis for the coupling between activation and inactivation gates in K(+) channels. Nature. 2010;466:272–5. doi: 10.1038/nature09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen A, Ben-Abu Y, Zilberberg N. Gating the pore of potassium leak channels. Eur Biophys J. 2009;39:61–73. doi: 10.1007/s00249-009-0457-6. [DOI] [PubMed] [Google Scholar]
- 6.Bagriantsev SN, Clark KA, Minor DL., Jr. Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 2012;31:3297–308. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagriantsev SN, Peyronnet R, Clark KA, Honoré E, Minor DL., Jr. Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piechotta PL, Rapedius M, Stansfeld PJ, Bollepalli MK, Ehrlich G, Andres-Enguix I, et al. The pore structure and gating mechanism of K2P channels. EMBO J. 2011;30:3607–19. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–41. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–6. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- 11.Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J Mol Recognit. 1996;9:1–5. doi: 10.1002/(SICI)1099-1352(199601)9:1<1::AID-JMR241>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 13.Braun AP. Two-pore domain potassium channels: Variation on a structural theme. Channels (Austin) 2012;6:139–40. doi: 10.4161/chan.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulsen H, Nissen P. Structural biology. The inner workings of a dynamic duo. Science. 2012;335:416–7. doi: 10.1126/science.1217679. [DOI] [PubMed] [Google Scholar]
- 15.O’Reilly AO, Eberhardt E, Weidner C, Alzheimer C, Wallace BA, Lampert A. Bisphenol a binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One. 2012;7:e41667. doi: 10.1371/journal.pone.0041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–84. doi: 10.1016/S0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 17.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–45. doi: 10.1016/S0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 18.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]