Abstract
TRPM3 has been reported to play an important role in Ca2+ homeostasis, but its gating mechanisms and regulation via Ca2+ are unknown. Ca2+ binding proteins such as calmodulin (CaM) could be probable modulators of this ion channel. We have shown that this protein binds to two independent domains, A35-K124 and H291-G382 on the TRPM3 N-terminus, which contain conserved hydrophobic as well as positively charged residues in specific positions, and that these residues have a crucial impact on its binding. We also showed that another Ca2+ binding protein, S100A1, is able to bind to these regions and that CaM and S100A1 compete for these binding sites on the TRPM3 N-terminus. Moreover, our results suggest that another very important TRP channel activity modulator, PtdIns(4,5)P2, interacts with the CaM/S100A1 binding sites on the TRPM3 N-terminus with high affinity.
Keywords: TRPM3, calmodulin, S100A1, PIP2, surface Plasmon resonance, fluorescence anisotropy
Introduction
TRPM3 (transient receptor potential melastatin 3 channel) is a member of the melastatin TRP subfamily. It is closely related to the founding member of this subfamily, TRPM1.1 Its physiological role remains poorly understood and interest in this channel is steadily growing. The channel was proposed to mediate a concentration-dependent Ca2+ entry pathway into kidney cells and is thought to play an important role in renal Ca2+ homeostasis.2,3 TRPM3 was recently also suggested to behave as a thermosensor able to detect noxious heat.4
As the function of TRPM3 was proposed to be connected with Ca2+ homeostasis, we investigated the binding of two different Ca2+ binding proteins to this channel, calmodulin (CaM) and S100A1.
CaM is a small soluble cytoplasmic protein that is highly conserved in all eukaryotic organisms.5 It was found to regulate the function of numerous TRP channels via binding to their intracellular termini. Multiple binding sites were reported on either the N- or C-terminus of various TRPC, TRPV and TRPM ion channel family members.5-8 These binding sites contain one or more consensus CaM recognition motifs, such as 1–5-10, 1–8-14, etc. with hydrophobic residues being present at these positions.
S100A1 belongs to the family of S100 proteins found only in vertebrates. These proteins contain two EF hand motifs and most of them can bind calcium and undergo a conformational change allowing them to interact with the target proteins. They are able to act in a similar way to CaM and some of them were shown to bind to the same or similar binding motifs.9 In contrast to CaM, the first EF hand motif binds calcium with a lower affinity than the second one. Until now there has been no evidence of direct modulation of any of the TRP channels by S100 proteins. The trafficking of TRPV5 and TRPV6 to the plasma membrane has been reported to be regulated by a protein complex of calcium-insensitive S100A10 and annexin A2 proteins,10 but a direct interaction has not been shown.
Some of the CaM binding sites in various TRP channel family members have also been reported to be associated with the binding of phosphatidylinositols (PIPs).11,12 Of those, phosphatidylinositol-4,5-bisphophate [PtdIns(4,5)P2] and phosphatidylinositol-3,4,5-triphosphate [PtdIns(3,4,5)P3] seem to play the most important roles. Under physiological conditions the PIPs are negatively charged, which allows them to electrostaticaly interact with positively charged residues on other proteins.11 CaM binding domains often contain such residues and regions binding both CaM and PtdIns(4,5)P2 has been discovered on the C-termini of TRPC613 and the regulation of CaM binding to the TRPV1 C-terminus by phosphoinositides has been suggested.13 According to the mutagenesis experiments on these channels, CaM and PIP2 bind to overlapping but not identical binding sites, because different amino acids were indicated as important for CaM or PtdIns(4,5)P2 binding.13
CaM and S100A1 bind to two different domains on TRPM3 N-terminus
We recently investigated two CaM binding domains, A35-K124 (TRPM335–124) and H291-G382 (TRPM3291-382) on the N-terminus of TRPM3. We used steady-state fluorescence anisotropy and surface Plasmon resonance (SPR) measurement as two independent types of binding assays to test the binding of the TRPM3 protein constructs to CaM. Both assays confirmed that CaM is able to bind to these proteins with high affinity. In the next step we used another Ca2+ binding protein, S100A1, which is known to form dimers and contains 4 EF hand Ca2+ binding motifs in its structure resembling CaM. This protein has never been shown to regulate the activity of any TRP channel, but as was suggested earlier, CaM is not the only Ca2+ binding protein modulating the activity of TRP channels.5 We proved by both binding assays that S100A1 binds to the two regions on the TRPM3 N-terminus with a high affinity similar to that of CaM, suggesting that these Ca2+ binding proteins might be involved in the modulation of the TRPM3 receptor.
In addition we found out that the two ligands CaM and S100A1 compete for the same binding sites on the TRPM3 N-terminus. The surface Plasmon resonance experiment was used as a competition assay. CaM and S100A1 proteins were bound covalently onto the chip into separate lines. The protein complexes TRPM335–124/CaM, TRPM335–124/S100A1, TRPM3291–382/CaM and TRPM3291–382/S100A1 were prepared by mixing the appropriate protein solutions and performing gel chromatography. The complexes were then washed over the chip. The complexes were neither observed to bind to CaM nor S100A1 during the experiment, suggesting that the proteins bind to the same or overlapping binding sites.
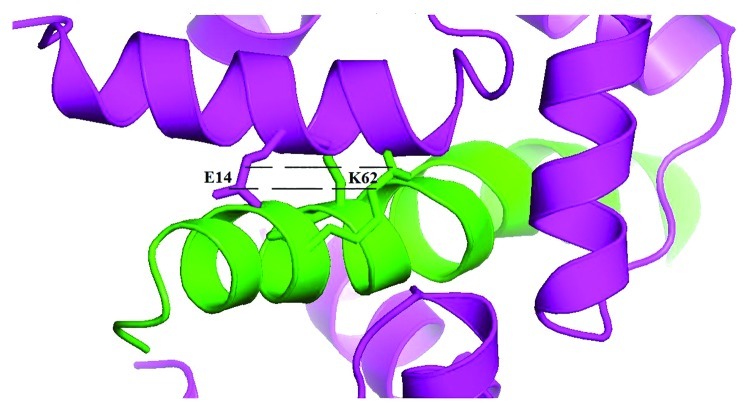
Moreover, we found that if some of the positively charged residues within the CaM/S100A1 binding sequences of the TRPM3 N-terminus are mutated to neutral alanine, the binding of CaM and S100A1 is reduced or does not occur at all. These findings suggest that the residues could be involved in the binding and this could be due to their polar interactions with negatively charged glutamates and aspartates on CaM and S100A1 (Fig. 1).
Figure 1. Example of predicted ionic interactions between positively charged residues on TRPM335–124 (K62, in green) and negatively charged amino acids on CaM (E14, in magenta); the figure was generated from a computer homology model of the 41–70 N-terminal sequence of human TRPM3 based on the structure of the voltage-gated calcium channel CaV1.2 IQ-domain-Ca2+-CaM complex (PDB code 2be6).
The fluorescence anisotropy measurements were also performed in solutions lacking calcium, to check if the binding of the proteins is dependent on calcium concentration. There was no increase in anisotropy values when calcium was lacking, but immediately after adding calcium into the solution, the anisotropy values rose significantly. These results suggest that the binding of CaM and S100A1 to TRPM335–124 and TRPM3291–382 is calcium-dependent, as was also shown for other TRP family members.
PtdIns(4,5)P2 binds to CaM binding domains on TRPM3 N-terminus
The analysis of the primary structure of the CaM binding sites within the TRPM3 N-terminus revealed possible conserved PIP2 binding motifs, which typically contain a number of positively charged residues in specific positions. This feature is often shared with CaM binding domains.12 We though tested whether the CaM/S100A1 binding regions within the TRPM3 N-terminus are able to bind PtdIns(4,5)P2.
Steady-state fluorescence anisotropy measurement was used as a binding assay to assess the possible binding of TRPM335–124 and TRPM3291–382 to fluorescently labeled PtdIns(4,5)P2. Increasing amounts of the proteins were titrated into the Bodipy-PtdIns(4,5)P2 solution and the fluorescence anisotropy was recorded for each protein concentration.
The resulting binding isotherms (Fig. 2A and B) show a significant increase in fluorescence anisotropy for both protein constructs A35-K124 and H291-G382 and the estimated equilibrium dissociation constants for TRPM335–124 0.191 ± 0.033 μM and for TRPM3291–382 0.309 ± 0.054 μM respectively confirm a high affinity of the CaM binding domains on the TRPM3 N-terminus for Bodipy-PtdIns(4,5)P2.
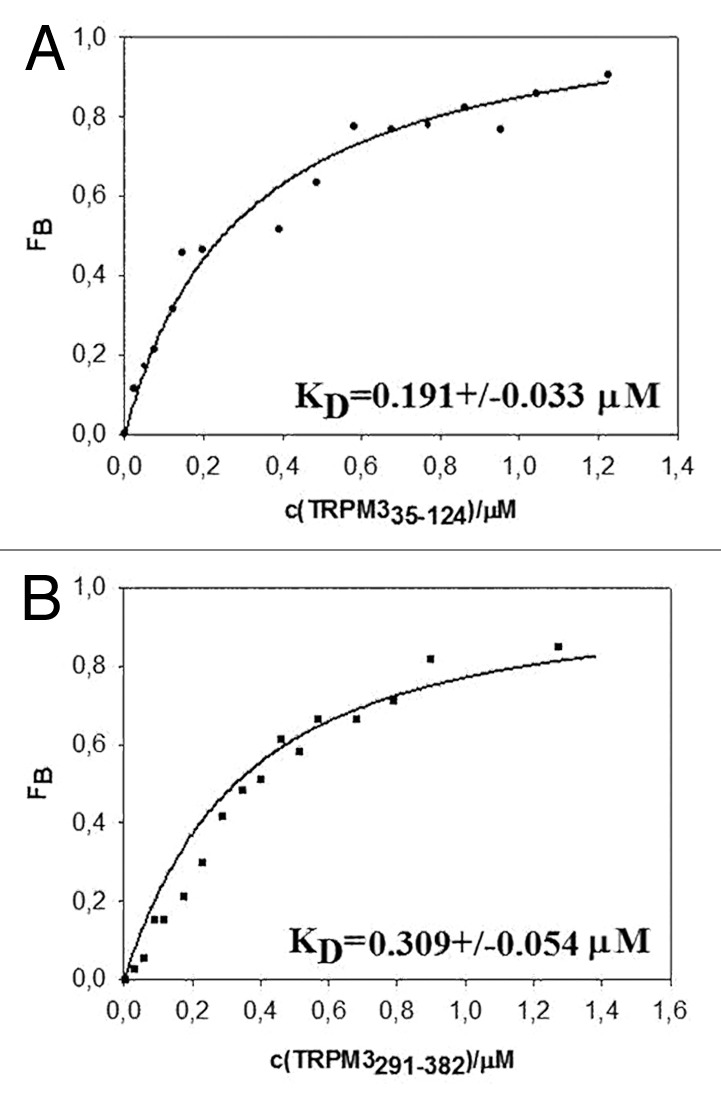
Figure 2. Binding of TRPM335–124 (A) and TRPM3291–382 (B) to Bodipy-PtdIns(4,5)P2 estimated by steady-state fluorescence anisotropy measurement.
TRPM335–124 binds to liposomes containing PtdIns(4,5)P2
SPR experiment with liposomes was used as another type of binding assay to assess the dissociation constant for creation of the PtdIns(4,5)P2 complexes with TRPM3 N-terminal constructs and also to show possible different behavior of PtdIns(4,5)P2 in solution and in bound state. We investigated whether liposomes containing PtdIns(4,5)P2 are able to bind to TRPM3 N-terminus. Liposomes containing 80% phosphatidylcholine (PC) and 20% PtdIns(4,5)P2 were prepared and bound covalently to an NLC chip. The TRPM3 A35-K124 protein construct was washed over the chip at five different concentrations (Fig. 3A) and the dissociation constant was estimated to be 0.253 ± 0.152 μM (Fig. 3B). As these data show, the TRPM335–124 binds to liposomes containing PtdIns(4,5)P2 with high affinity and the results are in very good agreement with the data obtained by fluorescence anisotropy measurement, confirming that PtdIns(4,5)P2 binds to the distal region of the TRPM3 N-terminus.
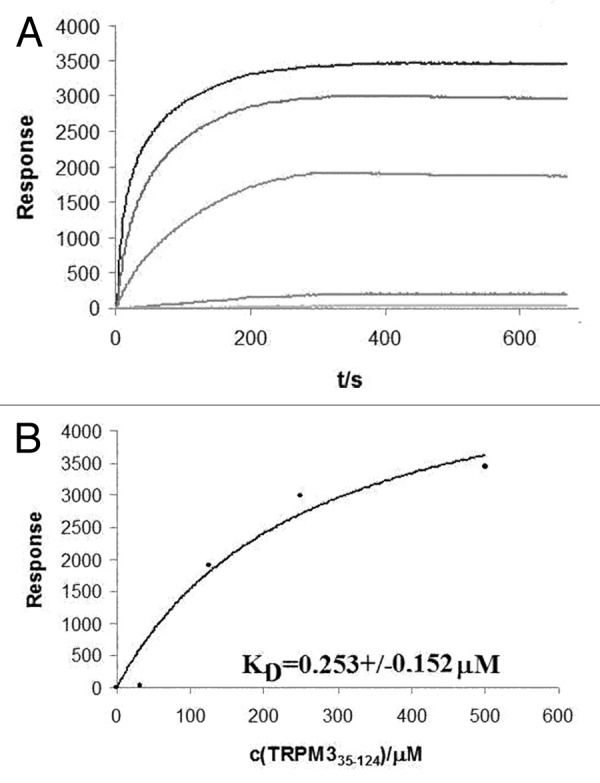
Figure 3. Surface Plasmon resonance experiment with PtdIns(4,5)P2-containing liposomes;(A) association phase of TRPM3 protein at five different concentrations; (B) Assessment of dissociation constant at equilibrium.
PIPs/CaM binding sites on TRPM3 channel
PIPs and CaM are without doubt crucial regulators of the various TRP channels, resulting in responses on the whole-cell level as a result of changing external conditions. However, the exact mechanisms of how this is achieved remain unknown or poorly understood and they remain a subject of intensive research.
As has been reported,12,13 PIPs and CaM can bind to overlapping regions on TRPV1 and TRPC6, but different amino acid residues were indicated to be important for the binding of these compounds, suggesting that their binding mechanisms are similar but not the same.
According to our results, PtdIns(4,5)P2 is able to bind to CaM binding sites on the TRPM3 N-terminus. Whether the same or different residues are important for the binding of PtdIns(4,5)P2 is being further investigated. It will also be interesting to determine whether PtdIns(4,5)P2 affects the ion channel as a soluble molecule or as an integrated compound in the plasma membrane and also whether other PIPs bind and influence the activity of the channel.
Materials and Methods
Steady-state fluorescence anisotropy measurement with Bodipy-PtdIns(4,5)P2
The fluorescently labeled PtdIns(4,5)P2 probe [Bodipy®-FL-PtdIns(4,5)P2] was obtained from Echelon Bioscience, USA. The probe was diluted in water to a concentration of 11 nM and used for the steady-state anisotropy measurement. The experiment was performed on an ISS PC1Photon™ Counting Spectrofluorimeter at room temperature. Increasing amounts of TRPM335–124 and TRPM3291–382 protein constructs were titrated into the Bodipy-PtdIns(4,5)P2 solution and at each concentration step fluorescence anisotropy was recorded. The data were processed according to previously described procedure6 and the equilibrium dissociation constants were obtained for Bodipy-PIP2/ TRPM335–124 and Bodipy-PIP2/ TRPM3291–382 protein complexes.
Surface Plasmon resonance experiment with liposomes containing PtdIns(4,5)P2
The lipids L-α-phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2], and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (PC) were obtained from Avanti Polar Lipis, Inc. A stock solution of PC was prepared in chloroform, PtdIns(4,5)P2 stock solution was prepared in a chloroform: methanol (2:1) mixture. Liposomes containing 80% of PC and 20% of PtdIns(4,5)P2 were prepared by mixing appropriate volumes of the stock solutions. After being dried under an N2 stream, lipid films were hydrated with 1x HBSS buffer containing 8 µM oligonucleotide 5′-TATTTCTGATGTCCACCCCC-3′, modified at the 3′ end with cholesterol (Generi-Biotech)- followed by extrusion 20 times through a polycarbonate membrane with a 100 nm pore diameter (Avestin Europe). Finally 8µM complementary oligonucleotide Biotin conjugate 5′-TGGACATCAGAAATACCCCC-3′ (Generi-Biotech) was added to the liposome mixture. A 20 min incubation was followed by centrifugation and washing steps to remove the unbound oligonucleotide-Biotin conjugate. All SPR measurements were performed at 25°C using a liposome-coated NLC chip in aProteOn XPR36 Protein Interaction Array System (Bio-rad). The vesicles were diluted to a final concentration of 1 mg/ml in HBSS and immobilized on the streptavidin-coated NLC chip (Bio-rad) surface at a flow rate of 25 µl/min for 10 min to give ~800–1,500 resonance units (RU). All SPR measurements were performed in HBSS at a flow rate of 30 µl/min for both the association and dissociation phase of the sensograms. For determining equilibrium binding, the association phases of five different concentrations of the protein were brought to near-equilibrium values (Req). Surfaces were typically regenerated with 50 μl of 1 M NaCl and 20 mM glycin buffer, pH 3.5. The sensograms were corrected for sensor background by interspot referencing (the sites within the 6 × 6 array which are not exposed to ligand immobilization but are exposed to analyte flow), and double referenced by subtraction of the analyte using a “blank” injection. Assuming a Langmuir-type binding between the protein (P) and protein binding sites (S) on vesicles (i.e., P + S ↔ PS), Req values were then plotted vs. protein concentration (P0), and the Kd value was determined by nonlinear least-squares analysis of the binding isotherm using the equation Req = Rmax / (1+Kd/P0).
Acknowledgments
This project was supported by grants GACR 301/10/1159, GACR P205/10/P308 and Research project No. AV0Z50110509.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/22177
References
- 1.Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:307–14. doi: 10.1007/s00210-005-1034-x. [DOI] [PubMed] [Google Scholar]
- 2.Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, et al. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3) J Biol Chem. 2003;278:20890–7. doi: 10.1074/jbc.M211232200. [DOI] [PubMed] [Google Scholar]
- 3.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- 4.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–94. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Zhu MX. Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–15. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]
- 6.Holakovska B, Grycova L, Bily J, Teisinger J. Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails. Amino Acids. 2011;40:741–8. doi: 10.1007/s00726-010-0712-2. [DOI] [PubMed] [Google Scholar]
- 7.Friedlova E, Grycova L, Holakovska B, Silhan J, Janouskova H, Sulc M, et al. The interactions of the C-terminal region of the TRPC6 channel with calmodulin. Neurochem Int. 2010;56:363–6. doi: 10.1016/j.neuint.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Grycova L, Lansky Z, Friedlova E, Obsilova V, Janouskova H, Obsil T, et al. Ionic interactions are essential for TRPV1 C-terminus binding to calmodulin. Biochem Biophys Res Commun. 2008;375:680–3. doi: 10.1016/j.bbrc.2008.08.094. [DOI] [PubMed] [Google Scholar]
- 9.Rezvanpour A, Shaw GS. Unique S100 target protein interactions. Gen Physiol Biophys. 2009;28 Spec No Focus:F39–46. [PubMed] [Google Scholar]
- 10.van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U, et al. Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003;22:1478–87. doi: 10.1093/emboj/cdg162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–8. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 12.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J. 2008;27:2809–16. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]