Abstract
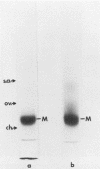
M-protein from influenza virus vaccine was purified by sodium dodecyl sulfate-gel chromatography and incorporated into liposomes by solubilization with octylglucoside and subsequent dialysis. Liposomes containing M-protein formed a distinct population with a density of 1.22 g/ml on sucrose-gradient centrifugation, regardless of the net charge on the liposomes. Treatment of the liposomes by freeze-fracture followed by electron microscopic examination showed multilamellar structures in those liposomes without M-protein; liposomes containing M-protein were mulberry-like structures which appeared unilamellar. These studies show incorporation of M-protein into the lipid bilayer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Edwards D. C., Brand C. M., Heath T. D. Formation of virosomes from influenza subunits and liposomes. Lancet. 1975 Nov 8;2(7941):899–901. doi: 10.1016/s0140-6736(75)92130-3. [DOI] [PubMed] [Google Scholar]
- Baron C., Thompson T. E. Solubilization of bacterial membrane proteins using alkyl glucosides and dioctanoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Mar 25;382(3):276–285. doi: 10.1016/0005-2736(75)90270-9. [DOI] [PubMed] [Google Scholar]
- Bucher D. J., Li S. S., Kehoe J. M., Kilbourne E. D. Chromatographic isolation of the hemagglutinin polypeptides from influenza virus vaccine and determination of their amino-terminal sequences. Proc Natl Acad Sci U S A. 1976 Jan;73(1):238–242. doi: 10.1073/pnas.73.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D. J. Purification of neuraminidase from influenza viruses by affinity chromatography. Biochim Biophys Acta. 1977 Jun 10;482(2):393–399. doi: 10.1016/0005-2744(77)90253-4. [DOI] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol. 1969 Nov;4(5):769–776. doi: 10.1128/jvi.4.5.769-776.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J. An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology. 1969 Nov;39(3):499–515. doi: 10.1016/0042-6822(69)90098-1. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. The membrane protein of influenza virus: extraction from virus and infected cell with acidic chloroform-methanol. Virology. 1973 Aug;54(2):369–383. doi: 10.1016/0042-6822(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Association of the envelope glycoproteins of influenza virus with liposomes--a model study on viral envelope assembly. Virology. 1979 Aug;97(1):212–217. doi: 10.1016/0042-6822(79)90390-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Gulik-Krzywicki T., Sardet C. Association of the membrane-penetrating polypeptide segment of the human erythrocyte MN-glycoprotein with phospholipid bilayers. I. Formation of freeze-etch intramembranous particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3294–3298. doi: 10.1073/pnas.71.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]