Abstract
Background
Hepatocyte growth factor (HGF) is one of the major angiogenic factors being studied for the treatment of ischemic heart diseases. Our previous study demonstrated adenovirus-HGF was effective in myocardial ischemia models. The first clinical safety study showed a positive effect in patients with severe and diffused triple coronary disease.
Methods
12 Pigs were randomized (1∶1) to receive HGF, which was administered as five injections into the infarcted myocardium, or saline (control group). The injections were guided by EnSite NavX left ventricular electroanatomical mapping.
Results
The catheter-based injections caused no pericardial effusion, malignant arrhythmia or death. During mapping and injection, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, serum creatinine and creatine kinase-MB levels have no significant increase as compared to those before and after the injection in HGF group(P>0.05). HGF group has high HGF expression with Western blot, less myocardial infarct sizes by electroanatomical mapping (HGF group versus after saline group, 5.28±0.55 cm2 versus 9.06±1.06 cm2, P<0.01), better cardiac function with Gated-Single Photon Emission Computed Tomography compared with those in saline group. Histological, strongly increased lectin–positive microvessels and microvessel density were found in the myocardial ischemic regions in HGF group.
Conclusion
Intramyocardial injection guided by NavX system provides a method of feasible and safe percutaneous gene transfer to myocardial infarct regions.
Introduction
Therapeutic angiogenesis induced by vascular growth factors may represent a novel approach to the treatment of ischemic heart disease. Because of its potential angiogenic, antiapoptotic, antifibrotic and stem cell-recruiting effects, HGF has been a subject drawing more and more attention in field of cardiovascular diseases [1]. We previously constructed a replication-deficient adenovirus carrying the HGF gene (Ad-HGF) [2]. In experimental animal models, this adenovirus mediates high levels of expression of human HGF, subsequently leading to formation of neovasculature and improvement of post-infarct heart function [2], [3]. Preclinical research demonstrated that Ad-HGF was effective in both acute and chronic myocardial ischemic models and no toxicity or mutations have been observed in rat, pig or rhesus monkey [4], [5], [6]. Our phase I clinical study showed that it was safe and effective using an adenoviral gene transfer vector to deliver the human HGF to individuals with clinically significant coronary artery disease [7], [8]. Ad-HGF has been delivered either by intracoronary infusion or by direct intramyocardial injection following open chest thoracotomy [3], [9]. However, the most safe and feasible route of delivery of angiogenic factors into the myocardium remains unknown. Ideally, a catheter-based intramyocardial injection system could provide accurate intramyocardial injection of angiogenic factors (genes or peptides) without the need for surgery and general anesthesia. We developed a set of percutaneous intramyocardial injection system guided by EnSite NavX (NavX) for the gene and cell therapies in patients with coronary ischemia.
The present study describes a new electromechanical-based platform system for intramyocardial injection that may be used to achieve therapeutic angiogenesis and improvement of cardiac function. Our system includes an injecting catheter that has a 27 gauge needle for intramyocardial delivery of potentially therapeutic agents, and its intellectual property is protected in China (patent number: CN 101536902A).
The purpose of this study was to evaluate the feasibility and safety of electromechanical guidance for the catheter-based intramyocardial injection system in ischemic myocardium, and assess the accuracy of the system to locate, in real time, the sites of intramyocardial injection. Furthermore, gene transferring (GTx) into ischemic myocardium can lead to successful gene expression at high levels and improvement of cardiac function, as proven by this integrated catheter system.
Materials and Methods
Injection System
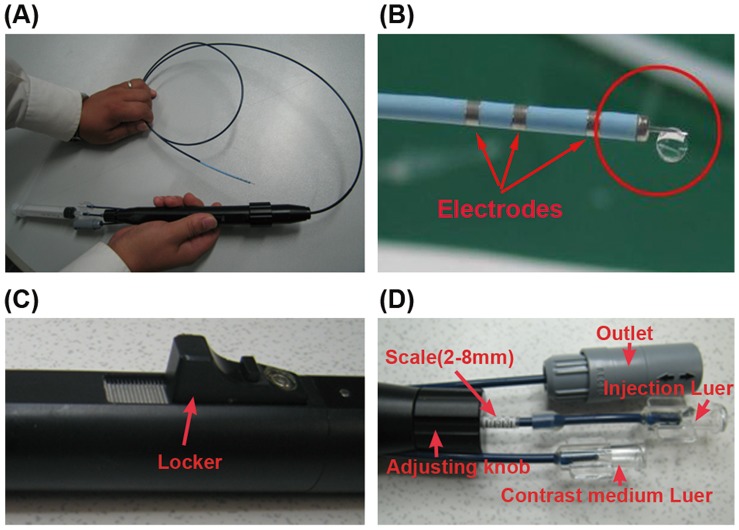
Intramyocardial injection system includes a catheter and a handle (Fig. 1A). The catheter consists of a 27 gauge needle. Importantly, the distal tip of injection catheter is fixed with a headend and a three-loop electrode to detect local signals. Once ventricular reconstruction is completed, endocardial injection could be accomplished by the injection catheter. The handle is equipped with an auto-syringe pump, adjusting knob and a locker. The pump could control injection doses (0.05–0.3 ml each time, difference≤1%), depth (3–10 mm) and speed (2–25 seconds) (Figure 1B). The needle “dead space” is approximately 0.99 cc. The needle depth into myocardium is controlled by the adjusting knob and locker. The injection handle has an injection Luer fitting for connection to a 1–4 cc syringe. Standard operation of the syringe attached to the injection handle delivers the fluid into the myocardium. The exact catheter tip location, orientation, and the injection sites are indicated in real time on the ventricular mapping, and local signals are traced to ensure catheter stability and optimal endocardial contact.
Figure 1. The intramyocardial injection system.
(A)The injection device comprises a catheter and handle. The catheter involves in mapping and injection functions. (B) The distal end of the catheter is equipped with tip and ring electrodes, a location sensor and a 27 guage needle designed for transendocardial injection. The red circle marks out a drop of saline injected through the needle tip. (C) The locker fixed the needle whose length was selected. (D) The adjusting knob controls the length of needle into myocardium. Two Luer fit for connection to an auto-syringe pump and a syringe of contrast medium, respectively.
To determine the injection speed in various doses, methylene blue dye (MB) was injected into porcine normal heart ex vivo. 3 mm, 5 mm, 10 mm into myocardium was designed for the injection depth. 0.2 ml and 0.3 ml MB was injected into myocardium at the speed of 2 s, 5 s, 10 s, 15 s, 20 s, and 25 s, respectively. A total of 180 injections were conducted in 14 porcine hearts. The MB overflow and bubbling from the site of endocardial injection were checked after the injection.
Construction of Adenoviral Vector
Adenoviral vectors constructed for previous experiments were used in this study [2]. HGF cDNA was amplified by polymerase chain reaction using human placenta cDNA library as template. Replication-deficient (E1, E3 deleted) adenoviral vectors containing the human hepatocyte growth factor (Ad-HGF) was constructed on basis of the pAdEasy-1 system (Stratagene, La Jolla, CA) following the manufacturer’s instructions. The recombinant replication defective adenovirus was purified by cesium chloride gradient ultracentrifugation. The final plaque-forming units (pfu) were determined by titration on HEK293 cells under an agarose overlay.
Animal Preparation
Eighteen mini-pigs (male, 3–4 months old, 36±3.4 kg body weight) were housed in the animal care facility of Nanjing Medical University. All animals received humane care in compliance with the Guideline for Care and Use of Laboratory Animals published by Jiangsu Province, P. R. China. The approval was granted by Nanjing Medical University ethical review board. The myocardial infarction models constructed for previous experiments were used in this study [10]. Three pigs died during the ligation of LAD. Twelve pigs were randomly divided into two groups of 6 each: Ad-HGF group (HGF group), and HEPES saline injected group (saline group). The other three animals were used to assess injection accuracy.
Left Ventricular Mapping and Injection Accuracy
The guiding NavX system utilizes a 5.68 kHz constant low-current locator signal and sensor-tipped catheters to locate the catheter position in space [11], [12]. Three-dimensional (3D) electromechanical maps of the left ventricle (LV) can be used to help differentiate between healthy and infarcted myocardium and identify most ischemic target regions without using fluoroscopy [13], [14]. Technical details of three-dimensional mapping system integrated as a part of the EnSite system have been described previously [11]. Briefly, to obtain three-dimensional left ventricular geometry, a roving mapping catheter is swept throughout the chamber, defining endocardial boundaries. Two locatable catheters (reference and mapping catheters) were introduced into the heart under fluoroscopic guidance. The location of the tip of the roving mapping catheter in left ventricular chamber was recorded according to the fixed reference catheter positioned at the coronary sinus. After the left ventricular geometry was created under the guidence of EnSite NavX (St. Jude Medicine, Inc., USA), electrograms were recorded by sampling the location of a roving catheter throughout the left ventricular endocardium. The average voltages were analyzed by diagnostic landmarking tools. Colors represent local voltage. Gray and purple respectively indicated low (≤0.5 mV) and normal voltage (≥2.0 mV). The region between gray and purple was peri-infarct zone. To analyze the voltage of scar and peri-infarct zone,over 70 points was mapped by two skilled operators in the region between gray and purple. The low voltage area was measured by diagnostic landmarking tools for detecting infarct sizes.
To estimate whether the sites of intramyocardial injection was accurately located in real time, a total of 1 ml MB was injected at the volume of 0.2 ml and speed of 20 s each time into five sites of the myocardium around the peri-infarct zone of three porcine infarcted hearts in vivo guided by NavX.
NavX Gene Transfer
Four weeks after the ligation of the LAD, the animals were randomly divided into two groups (n = 6): Ad-HGF injected group (HGF group), and HEPES saline injected group (saline group). The saline animals were used as the negative controls. An intramyocardial injection system was used to percutaneous endocardial injection of adenoviral vector. After ≥70 points had been mapped by NavX, the injection catheter was navigated into the infarcted region, and five different sites of intramyocardial injections were determined around the infarct zone (3 mm depth, 20 s, 0.2 ml each, 5 seconds interval, total volume 1.0 ml, ≥5 mm apart from each other). A total of 1×1010 pfu of viral titers in 1.0 ml of HEPES saline (pH 7.4) were used. The quantity of Ad-HGF was selected by previous studies [6]. Identical doses of saline were injected into control animals. Four weeks after gene transfer (GTx), the size of scar and average voltage were analyzed through the above method. During mapping and injection, the time and doses of radiation were measured, and then the porcine hearts were collected.
Clinical Chemistry Analysis
Safety was evaluated by animal survival, pericardial effusion, heart rhythm, blood tests. Survival were continuously monitored and recorded during the 8 weeks study period. Heart rhythm was monitored with the surface electrocardiogram and intracardiac electrical record during mapping and injection. Blood samples were collected at selected points of time. The white blood cell (WBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (Bun), creatinine (Cr) and creatine kinase-MB (CK-MB) in serum were measured at the Central Laboratory in the First Affiliated Hospital of Nanjing Medical University.
SPECT Analysis
Gated-single photon emission computed tomography (SPECT) was performed in the pigs with a commercially available system (ECAM+; Siemens, Germany). Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV) and left ventricular ejection fraction (LVEF) were determined by QGS software. SPECT analysis has been described previously [10]. In brief, the myocardial perfusion score was calculated as follows: The left ventricular cavity was divided into 17 segments for assessment of the myocardium [15]. Seven segments were assigned to the LAD distribution area. The five-point scoring used to grade each segment was as follows: 0 = absent perfusion; 1 = severe hypoperfusion; 2 = moderate hypoperfusion; 3 = mild hypoperfusion; and 4 = normal perfusion. The normal total scores of LAD areas were 28 [16].
Western Blotting Analysis
Technical details of Western blotting analysis have been described previously [10]. HGF, β-actin and the secondary goat anti-mouse IgG-AP antibody (Santa Cruz Biotechnology, USA) were used in this study.
Histological and Immunohistochemical Analysis
Transverse sections of hearts were obtained at intervals of 5 mm in the upward direction from the heart apex. Then sections were fixed in 10% formalin, embedded in paraffin and sectioned. Serial sections were made from the apex of the heart to the site of the ligation. Sections were stained with specific antibodies against lectin, Ki67 (Santa Cruz Biotechnology) antibodies. Nuclei were stained with 4′,6′-diamino-2-phenilindole (DAPI). Lectin-FITC (Santa Cruz Biotechnology) was used to visualize the blood vessels. The immunohistochemical staining was carried out according to the manufacturer’s instructions, and signals were visualized by incubating the sections with Alexa Fluor-488 or −555-labeled secondary antibody (Molecular Probes Inc., Eugene, OR, USA).
For quantification of the vessel-densities in the myocardium, four sections were randomly selected from each group, and six visual fields from each section were observed. As a surrogate for vessel counting, lectin- and Ki67-positive vessel density was determined by optical density measurements using the QWIN100 immunohistochemical imaging analyzer system (Leica Co., Germany).
Statistical Analyses
Statistical analysis was performed with SPSS 10.0 software. Student’s t-tests and one-way ANOVA testing were used to compare the differences among groups, with statistical significance considered if P<0.05. The data are presented as mean ± standard deviation (SD).
Results
Injection Speed and Depth
To determine correlation between doses and the depth and speed of injection via our catheter-based injector, MB was injected into fourteen porcine normal hearts in vitro. When 0.2 ml MB was injected into porcine myocardium at the speed of 2 s and 5 s, overflow and bubbling were significantly observed in the surface of endocardium; while at the speed of 10 s and 15 s, overflow was not significantly observed, and no bubbling occured; at 20 s and 25 s, there observed neither overflow nor bubbling in the sites of injection. While injecting 0.3 ml MB, the MB significantly flowed outward the endocardium at the speed of 2 s, 5 s, and 10 s; less MB was observed at the speed of 15 s and 20 s; the overflow was not detected in the endocardium at the speed of 25 s. Once injection time took longer than 15 s, the bubbling did not occur in the surface of endocardium. No MB staining was observed in the pericardium injected at depths of 3 mm and 5 mm. For depth of 10 mm, MB staining occurred in the pericardium of five pigs (0.2 ml MB: two pigs, 0.3 ml MB: three pigs). Overall, when injection of 0.2 ml and 0.3 ml doses took 20 s, the overflow and bubbling of MB could not be detected in the sites of injected endocardium; injection into myocardium at depths of 3 mm and 5 mm could not cause pericardium perforation (Table 1). Therefore, injection into myocardium at the speed of 20 s with the depth of 3 mm was selected in the subsequent GTx guided by NavX in vivo.
Table 1. Injection of speed and depth.
| Time/Volume/Depth | Overflow | Bubbling | MB staining |
| 2 s or 5 s/0.2 or 0.3 ml/3 or 5 mm | Yes | Yes | No |
| 10 or 15 s/0.2 or 0.3 ml/3 or 5 mm | Yes | No | No |
| 20 or 25 s/0.2 or 0.3 ml/3 or 5 mm | No | No | No |
| 2 or 5 or 10 or 15 or 20 or 25 s/0.2 or 0.3 ml/10 mm | No | No | Yes |
Yes: the overflow and bubbling were detected in the endocardium. No: the overflow and bubbling were not detected in the endocardium, or MB staining not in the pericardium.
Injection Accuracy
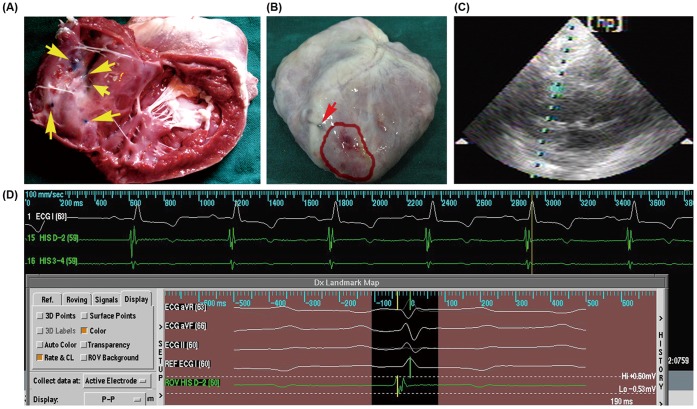
To estimate the accuracy of the intramyocardial injection system, MB was injected at the volume of 0.2 ml each time into other three porcine infarcted hearts in vivo under the guidance of NavX. The five injection points were all around myocardial infarction areas (Fig. 2A), and the MB staining was not detected in the pericardium (Fig. 2B).
Figure 2. The accuracy and safety of injection.
(A), (B) Representative images of methylene-blue injection. The yellow arrows pointed to injection sites, all of the sites were around the desired myocardial ischemic region (pale region). The red arrow pointed to the ligation site of the LAD. The myocardial ischemic region was marked with red curve. No MB staining was detected in the pericardium. (C) Representative image of the echocardiography. During the mapping and injection, the pericardial effusion was not detected in two groups. (D) Representative image of the surface electrocardiogram and intracardiac electrical recording. The operation did not cause malignant arrhythmia in real time.
Injection Safety
WBC, ALT, AST, Bun and Cr levels were not significantly changed after the injection compared to those before the injection in both groups (P>0.05, Table 2). CK-MB was significantly increased after 6, 24 hours and 3 days of LAD ligation compared to that before ligation (P<0.05). It remained predefined levels before GTx (P>0.05), and was not significantly increased after NavX-guided gene therapy (P>0.05, Table 2). During mapping and injection, no animal had pericardial effusion or malignant arrhythmia (Fig. 2C, 2D). No animal died as a result of the mapping and injection procedures. During mapping and injection, the doses and time of X-ray radiation were recorded. The average time was 14.54±4.47 min, and the average doses of radiation were 275.17±162.16 mGy.
Table 2. The analyses of clinical chemistry.
| G | T | WBC(1×109/L) | ALT(U/L) | AST(U/L) | BUN(mmol/L) | Cr(µmol/L) | CK-MB(U/L) |
| S | B | 8.36±0.99 | 29.60±5.13 | 27.80±3.70 | 6.44±0.99 | 95.60±8.85 | 23.60±1.68 |
| L1 h | 8.02±0.60 | 35.20±8.32 | 31.20±5.97 | 6.00±1.29 | 95.00±9.75 | 41.80±8.81 | |
| L6 h | 7.38±1.61 | 31.00±10.02 | 27.80±4.76 | 6.00±0.65 | 88.00±11.64 | 81.60±18.06* | |
| L24 h | 7.80±1,10 | 32.20±4.09 | 33.40±6.02 | 6.24±0.99 | 88.00±3.61 | 200.60±28.85* | |
| L3dT0 h | 8.50±0.467.50±1.59 | 32.80±8.6734.00±7.29 | 27.20±4.0934.83±4.45 | 5.52±0.895.95±0.67 | 86.40±7.0287.67±17.37 | 82.20±21.22*25.67±3.78 | |
| T24 h | 8.66±0.82 | 33.00±4.06 | 31.60±5.13 | 5.86±0.77 | 85.40±6.77 | 25.40±8.71 | |
| T3 d | 7.26±1.77 | 27.00±10.51 | 27.60±4.45 | 6.32±0.61 | 85.00±8.25 | 24.00±9.56 | |
| T4 w | 7.26±1.01 | 30.40±5.98 | 34.00±5.66 | 5.96±1.03 | 85.80±9.36 | 26.40±3.05 | |
| H | B | 7.89±2.07 | 33.16±7.68 | 34.50±7.15 | 5.85±0.96 | 88.67±17.59 | 24.83±3.87 |
| L1 h | 7.72±2.00 | 30.00±6.63 | 32.50±6.78 | 6.00±1.05 | 89.00±15.72 | 36.33±4.27 | |
| L6 h | 6.43±1.28 | 38.00±7.89 | 33.50±4.58 | 6.75±0.99 | 94.17±8.04 | 85.50±13.14* | |
| L24 h | 7.30±0.99 | 30.83±7.73 | 33.50±4.55 | 5.80±0.48 | 80.33±12.34 | 173.17±27.25* | |
| L3 dT0 h | 7.45±1.608.28±0.63 | 29.50±8.1731.00±5.81 | 34.50±6.9532.40±3.87 | 6.68±0.696.24±4.80 | 85.17±12.8395.60±8.85 | 93.83±17.81*24.60±5.32 | |
| T24 h | 7.52±1.32 | 32.00±6.42 | 33.00±6.07 | 5.70±0.91 | 83.67±10.73 | 26.17±4.88 | |
| T3 d | 7.85±0.65 | 31.50±7.39 | 31.33±6.59 | 6.05±0.94 | 90.67±10.80 | 25.17±4.62 | |
| T4 w | 6.97±1.25 | 33.50±6.22 | 33.83±4.31 | 6.30±1.12 | 91.00±9.08 | 24.83±5.53 |
G: groups; S: saline; H: HGF; T: time; B: before the LAD ligation; L1 h, L6 H, L24 h, L3 d: 1, 6, 24 hours and3 days after the LAD ligation; T0 h: before the injection; T24 h, T3 d, T4 w: 24 hours, 3 days and 4 weeks after the injection. *P<0.01 vs. before the ligation.
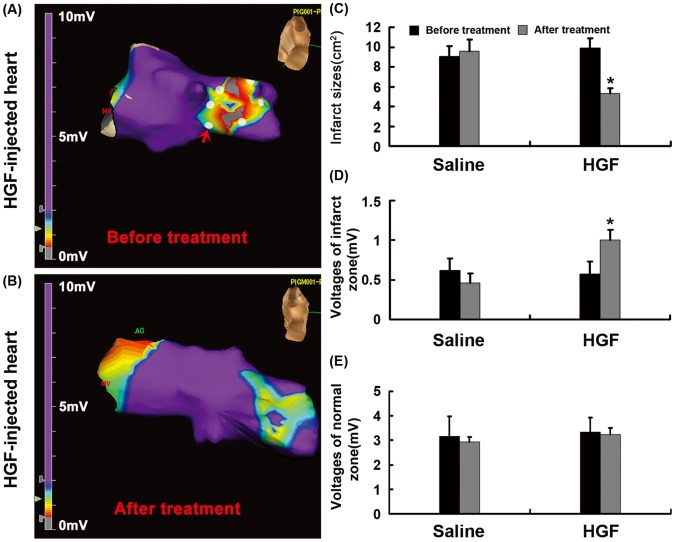
Reduction of Infarct Size and Increase of Myocardial Voltage
Local electrograms were recorded with NavX before and 4 weeks after GTx. There were significantly higher myocardial voltage levels in infarct zones of HGF-injected hearts after GTx (1.00±0.13 mV) than that before GTx (0.51±0.16 mV) (Fig. 3A, 3D, P<0.01). However, the myocardial voltage level of infarct zones have no significant difference before and after injection (0.62±0.1 versus 0.46±0.12 mV, P>0.05) in saline groups. In the zones of normal myocardium, voltage levels did not significantly change before and after the injection between two groups (Fig. 2A, 2E, P>0.05). Accordingly, infarct sizes significantly reduced in the HGF-injected hearts after GTx (5.28±0.55 cm2) than those before GTx (9.93±0.98 cm2) (P<0.01, std error: 0.39 and 0.26, 95% confidence intervals).But no significant difference was found before and after saline injection (9.06±1.06 versus 9.58±1.15 cm2) in the hearts of control group (Fig. 3A, 3B, P = 0.48, std error: 0.47 and 0.52, 95% confidence intervals). The infarct sizes significantly reduced in the HGF-injected hearts after GTx (5.28±0.55 cm2) compared to those (9.06±1.06 cm2) in the hearts of control group.
Figure 3. Infarct size and myocardial voltage.
(A), (B) the left ventricular geometry before and after the GTx. Gray and purple respectively indicates the myocardial infarct and normal zones; the region between gray and purple is transitional zone. Red arrow indicates injected site marked with white dots. (C) Bar graph showed the infarct sizes significantly reduced in the HGF-injected hearts after GTx than those before GTx. (D), (E) Bar graph explained the myocardial voltage levels in the infarct and normal zones of HGF-injected hearts. There were significantly higher myocardial voltage levels in the infarct zone of HGF-injected hearts after GTx than those before GTx. *P<0.01 vs. before GTx in HGF group.
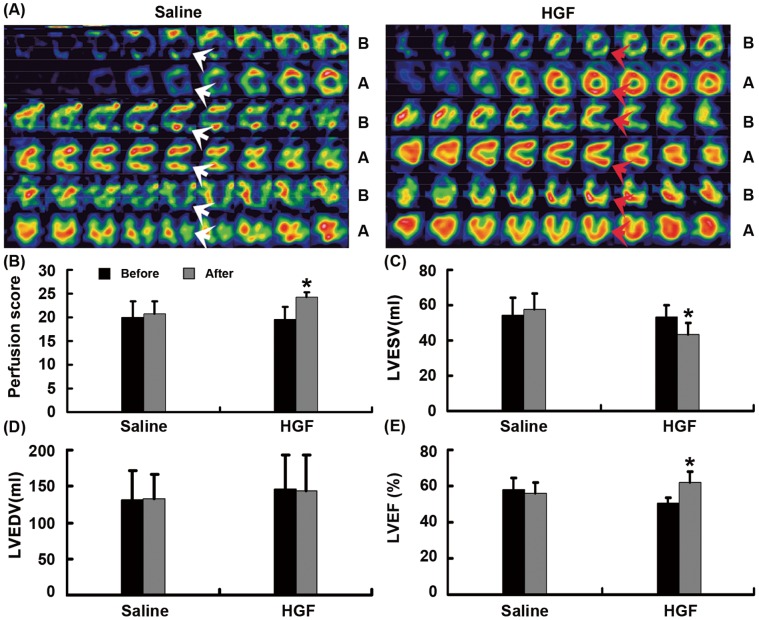
Improvement of Myocardial Perfusion and Cardiac Function
SPECT was used to detect myocardial perfusion scores and cardiac function. The myocardial perfusion scores in the HGF group were significantly improved 4 weeks after GTx compared to those before GTx (P = 0.001, Fig. 4A, 4B). The perfusion scores of control group have no significant difference before and after saline injection (P>0.05, Fig. 4A, 4B). The HGF group had greater LVEF and lower LVESV four weeks after GTx compared to that before GTx (P<0.05, Fig. 4A, 4C, 4D).
Figure 4. The myocardial perfusion and cardiac function.
(A) Representative images of SPECT of saline (left) and HGF (right) treated porcine chronic myocardial infarct heart. Red arrows indicate regions with better myocardial perfusion; white arrows indicate regions that had similar myocardial perfusion. (B) Bar graph showing myocardial perfusion score, *P = 0.001 vs. four weeks after vector injection; (C) LVESV, *P<0.01 vs. four weeks after vector injection; (D) LVEDV, there was significant difference four weeks after the injection compared to that before the injection, P>0.05. (E) LVEF, *P<0.01 vs. four weeks after vector injection; B: before GTx; A: four weeks after GTx.
HGF Expression
To investigate whether the adenovirus could mediate HGF expression in vivo, Western blots were performed 4 weeks after GTx. There was higher HGF expression in the myocardial infarct and peri-infarct zones of treatment group than that in control group (Fig. 5A, 5C, P<0.01). In the HGF group, the myocardial infarct and peri-infarct zone had higher HGF expression compared with the normal zone (P<0.01), and the normal zone was lowest in each of the three myocardial zones (Fig. 5A, 5B, P<0.01). These results indicated that the HGF gene expression could be mediated by the adenovirus vector via our injection system guided by NavX in this porcine chronic myocardial ischemia model.
Figure 5. HGF expression.
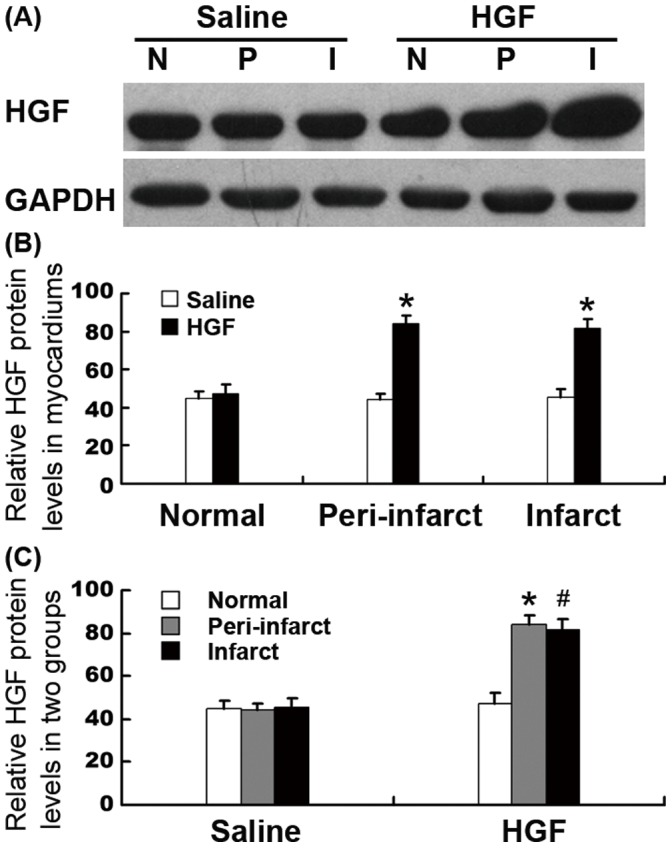
(A) Representative image of HGF expression. (B) Bar graph showed HGF protein had higher expression in the infarct and peri-infarct zone of the treatment group compared to that in saline group (*P<0.01). (C) In HGF group, the myocardial infarct and peri-infarct zone had higher HGF expression than the normal zone (*P<0.01, #P<0.01). I, infarct zone; P, peri-infarct zone; N, normal zone.
Increase of Vascular Density in Myocardial Infarct and Peri-infarct Zones
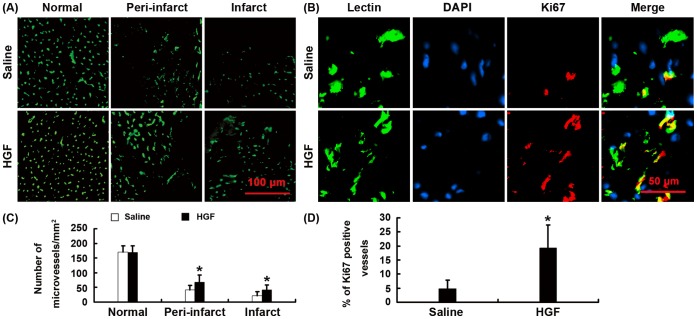
To analyze the angiogenesis mediated by expression of HGF, microvessels were counted blindly on lectin stained sections. The HGF group had significantly more capillaries in the myocardial infarct (41.10±17.52/mm2) and peri-infarct (68.20±23.14/mm2) zones than that in control group (22.20±12.56, 41.75±15.11/mm2) (Fig. 6A, 6C, P<0.01).
Figure 6. The vascular density.
(A) Representative pictures of Lectin stained sections. (B) The immunofluorescence of the Ki67-positive cells in the border zone of the infarct. (C) Bar graph showing the quantification of microvessels. (D) Bar graph showing the quantification of Ki67-positive vessels. *P<0.01 vs. saline group.
To identify newly formed blood vessels, we used an antibody specific to Ki67 to examine proliferating endothelial cells. We detected significantly more Ki67 positive endothelial cells in the HGF-injected hearts (19.25±8.17/mm2) than that in the saline-injected hearts (4.70±3.08/mm2) (Fig. 6B, 6D, P<0.01). Thus, over-expression of HGF induced active angiogenesis.
Discussion
Vector-mediated cardiac gene therapy holds tremendous promise as a translatable platform technology for treating many cardiovascular diseases, but its application has been hindered due to the lack of an acknowledged ideal delivery method in a large animal model. We report here a safe, effective, practical and relatively non-invasive protocol in the pig which results in therapeutic gene transfer mediated by adenovirus following delivery guided by NavX system. This procedure is superior to previously reported large animal cardiac gene transfer techniques because it combines the advantages of direct intramyocardial injection with those of a non-invasive percutaneous delivery system.
The phase I clinical study showed that it is safe and effective to use an adenoviral gene-transfer vector to deliver the human HGF to individuals with clinically significant coronary artery disease [7]. Although the use of a mini-thoracotomy seems to be generally well tolerated, even in patients with advanced myocardial ischemia, nevertheless, the procedure has some risk associated with the administration of general anesthesia and some morbidity associated with surgical manipulation (particularly in patients with previous bypass surgery), and it limits the feasibility of repeat administrations [17]. Our previous animal studies have proven the efficiency of activating angiogenesis and improving cardiac function by direct intramyocardial delivery of HGF into the myocardium following open chest thoracotomy [18]. However, one pig died at week 3 after the second operation, while all the pigs in the present study survived till the end of the observation period.
Currently, there are several ongoing preclinical studies and clinical trails evaluating the therapeutic efficacy of cardiac gene transfer as a novel treatment for failure heart or ischemic heart disease. The feasibility of in vivo cardiac gene transfer with the aid of percutaneous catheter-mediated intracoronary delivery has been demonstrated in many studies, and some authors believe that it is the most clinically relevant method because of the possibility of delivering vectors to the whole myocardium and because of the extensive clinical experience in coronary catheterization procedures [19], [20], [21]. However, the efficiency of adenovirus and adeno-associated virus-mediated gene delivery by this method is highly variable among studies [22]. In addition, coronary perfusion would have limited utility in ischemic heart disease, because blood flow would be limited in the targeted areas of the heart. Recently, the catheter-based injection of fluoroscopic guidance was reported in several studies [23], [24]. Although this method is relatively non-invasive, it requires contrast media added to the vector solution so that injection sites could be visualized. In addition, the injections need a long time exposed to X-ray. Radiation can cause a small increase in the risk of fatal malignancy for both the patient and electrophysiologist [25]. The intramyocardial injection strategy has been substantially improved by the introduction of the NOGA electromechanical mapping [26], [27], which offers a endocardial route to myocardial transduction as efficient as injections via thoracotomy [28]. The transendocardial injections guided by NOGA left ventricular electromechanical mapping provided an efficient, safe and practical technique, however average mapping(15±6 min) and injection time (23±6 min) guided by NOGA [29]was longer than the total time of mapping and injection (14.54±4.47 min) in our study. The reason is that two catheters were using during mapping and injection guided by NOGA system. After the procedure of mapping, the injection may be executed with the injection catheter replaced mapping catheter. Interestingly, the catheter in our study has the two characteristics of injection and signal detection, because the distal tip of injection catheter is fixed with a headend and a three-loop electrode to detect local signals. When mapping was completed, the injection may be processed at once without changing catheter. So the time of operation may be reduced compared with that based NOGA system, and the operation risk would be decreased. In summary, we believe that both coronary perfusion and endocardial gene transfer are delivery methods with potential clinical utility and that future investigation should be directed at comparing these methods in large animal model.
Moreover, our delivery method is safe and relatively non-invasive, suggesting it would be better tolerated in patients with significant heart disease than procedures requiring thoracotomy, cardiopulmonary bypass. In this study, all animals survived the procedures of mapping and injection. WBC, ALT, AST, Bun and Cr levels were not significantly changed 24 hours, 3 days and 4 weeks after vector injection compared to those before the LAD ligation. The increase of CK-MB level showed that the LAD ligation led to ischemic myocardial injury 6, 24 hours, and 3 days after the ligation. However, there was no significant increase in CK-MB 24 hours, 3 days and 4 weeks after the GTx compared to baseline values. During mapping and injection, no animal had pericardial effusion or malignant arrhythmia. These results indicated that the method of the GTx did not lead to the detectable injury of kidney, liver,myocardium, pericardial effusion or malignant arrhythmia.
Furthermore, the effective delivery guided by NavX system was identified in the present study. The myocardial infarct and peri-infarct zones had higher HGF expression compared with the normal zone, and the normal zone was the lowest among the three myocardial zones in the HGF group. The cardiac function, myocardial perfusion scores and voltage of the HGF group improved significantly, and infarction size reduced significantly four weeks after the GTx compared to those before the GTx. Our previous studies have shown that over-expression of HGF not only could induce active angiogenesis, but reduce cardiomyocyte apoptosis and proliferation in the myocardial infarct and peri-infarct zones [30]. Taniyamn Y et.al have reported that increase in local HGF expression may participate in the prevention of myocardial injury by Ang II blockade through its antifibrotic action [31]. Thus, angiogenesis, apoptosis reduction, proliferation and antifibrotic activities with the endocardial HGF transfer could lead to improvement of cardiac function in HGF-injected heart.
The limitations of this study: To investigate the efficiency and safety of the intramyocardial injection guided by NavX in the study, we need a group that should be injected with Ad-HGF via an intracoronary arterial route or surgical epicardial approach individually. However, we did not have such a group in this experiment. Thus the effect of the HGF transfer will be compared to individual transfer in the future. CK-MB is not a perfect marker of myocardial injury, and other more specific and sensitive markers such as troponin should be used for future studies. The optimal mapping technique, while preserving accuracy [32], achieves high density with short acquisition times. The number of points mapped by NavX was relatively limited due to the unavailability of Duo-Decapolar catheter. Although Ad–based gene therapy is a promising technique for the delivery of growth factors, Ads could activate the innate immune system [33], [34]. In the present study, local inflammation caused by Ads was not detected. An understanding of the innate response to Ads is important to overcome the last remaining hurdle to improve the safety and effectiveness of these agents, the immune response should be focus in future study.
To sum up, adenoviral gene transfer of HGF used in our catheter-based intramyocardial injection guided by NavX is a feasible and safe approach for the induction of angiogenesis, the increase of myocardial voltage and the reduction of infarct size that could lead to the improvement of cardiac function and myocardial perfusion. Thus, this approach may provide a safe and effective angiogenic gene therapy system for ischemic heart disease in potential clinical application.
Acknowledgments
The authors wish to thank Yingbin Ge (Nanjing Medical University), Dr. Jianlin Feng (Department of Nuclear Cardiac Imaging, the First Affiliated Hospital of Nanjing Medical University) and Dr. Guoping Yang (Centre of Animal Model, Nanjing Medical University) for their technical assistance.
Funding Statement
This work was financially supported by grants from National Nature Science Foundation of China (30971254), Supporting Program of Science and Technology of Jiangsu (Social Development, BE2009686), the Innovative Project of Clamber Scholar of Jiangsu (BK2010021), Chinese National Basic Research and Development Grants ‘973’ (2008CB517303), the National Mega-Project of Science Research of China (No.2009ZX09301-002) and Chinese High-Tech Grant‘863’(2007AA021007), and a Project Funded by the Priority Acadamic Program Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Madonna R, Cevik C, Nasser M, De Caterina R (2012) Hepatocyte growth factor: molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb Haemost 107: 656–661. [DOI] [PubMed] [Google Scholar]
- 2. Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, et al. (2003) Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther 8: 467–474. [DOI] [PubMed] [Google Scholar]
- 3. Wang W, Yang ZJ, Ma DC, Wang LS, Xu SL, et al. (2006) Induction of collateral artery growth and improvement of post-infarct heart function by hepatocyte growth factor gene transfer. Acta Pharmacol Sin 27: 555–560. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H (2000) Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest 106: 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, et al. (2000) Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther 7: 417–427. [DOI] [PubMed] [Google Scholar]
- 6. Yang ZJ, Chen B, Sheng Z, Zhang DG, Jia EZ, et al. (2010) Improvement of heart function in postinfarct heart failure swine models after hepatocyte growth factor gene transfer: comparison of low-, medium- and high-dose groups. Mol Biol Rep 37: 2075–2081. [DOI] [PubMed] [Google Scholar]
- 7. Yang ZJ, Zhang YR, Chen B, Zhang SL, Jia EZ, et al. (2009) Phase I clinical trial on intracoronary administration of Ad-hHGF treating severe coronary artery disease. Mol Biol Rep 36: 1323–1329. [DOI] [PubMed] [Google Scholar]
- 8. Yuan B, Zhao Z, Zhang YR, Wu CT, Jin WG, et al. (2008) Short-term safety and curative effect of recombinant adenovirus carrying hepatocyte growth factor gene on ischemic cardiac disease. In Vivo 22: 629–632. [PubMed] [Google Scholar]
- 9. Yang Z, Wang W, Ma D, Zhang Y, Wang L, et al. (2007) Recruitment of stem cells by hepatocyte growth factor via intracoronary gene transfection in the postinfarction heart failure. Sci China C Life Sci 50: 748–752. [DOI] [PubMed] [Google Scholar]
- 10. Tao Z, Chen B, Tan X, Zhao Y, Wang L, et al. (2011) Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci U S A 108: 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ventura R, Rostock T, Klemm HU, Lutomsky B, Demir C, et al. (2004) Catheter ablation of common-type atrial flutter guided by three-dimensional right atrial geometry reconstruction and catheter tracking using cutaneous patches: a randomized prospective study. J Cardiovasc Electrophysiol 15: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 12. Casella M, Perna F, Dello Russo A, Pelargonio G, Bartoletti S, et al. (2009) Right ventricular substrate mapping using the Ensite Navx system: Accuracy of high-density voltage map obtained by automatic point acquisition during geometry reconstruction. Heart Rhythm 6: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 13. Reithmann C, Hahnefeld A, Ulbrich M, Matis T, Steinbeck G (2009) Different forms of ventricular tachycardia involving the left anterior fascicle in nonischemic cardiomyopathy: critical sites of the reentrant circuit in low-voltage areas. J Cardiovasc Electrophysiol 20: 841–849. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen PK, Lan F, Wang Y, Wu JC (2011) Imaging: guiding the clinical translation of cardiac stem cell therapy. Circ Res 109: 962–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, et al. (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105: 539–542. [DOI] [PubMed] [Google Scholar]
- 16. Poornima IG, Miller TD, Christian TF, Hodge DO, Bailey KR, et al. (2004) Utility of myocardial perfusion imaging in patients with low-risk treadmill scores. J Am Coll Cardiol 43: 194–199. [DOI] [PubMed] [Google Scholar]
- 17. Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, et al. (2001) Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation 103: 2138–2143. [DOI] [PubMed] [Google Scholar]
- 18. Yuan B, Zhang Y, Zhao Z, Wu D, Yuan L, et al. (2008) Treatment of chronical myocardial ischemia by adenovirus-mediated hepatocyte growth factor gene transfer in minipigs. Sci China C Life Sci 51: 537–543. [DOI] [PubMed] [Google Scholar]
- 19. Chaanine AH, Kalman J, Hajjar RJ (2010) Cardiac gene therapy. Semin Thorac Cardiovasc Surg 22: 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, et al. (2008) Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther 15: 1550–1557. [DOI] [PubMed] [Google Scholar]
- 21. Kaspar BK, Roth DM, Lai NC, Drumm JD, Erickson DA, et al. (2005) Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J Gene Med 7: 316–324. [DOI] [PubMed] [Google Scholar]
- 22. Katz MG, Swain JD, White JD, Low D, Stedman H, et al. (2010) Cardiac gene therapy: optimization of gene delivery techniques in vivo. Hum Gene Ther 21: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao G, Bish LT, Sleeper MM, Mu X, Sun L, et al. (2011) Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum Gene Ther 22: 979–984. [DOI] [PubMed] [Google Scholar]
- 24. Bish LT, Sleeper MM, Brainard B, Cole S, Russell N, et al. (2008) Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther 16: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 25. Kovoor P, Ricciardello M, Collins L, Uther JB, Ross DL (1998) Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation 98: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 26. Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, et al. (2011) Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res 109: 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azuma J, Taniyama Y, Takeya Y, Iekushi K, Aoki M, et al. (2006) Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cardiomyopathy. Gene Ther 13: 1206–1213. [DOI] [PubMed] [Google Scholar]
- 28. Kornowski R, Leon MB, Fuchs S, Vodovotz Y, Flynn MA, et al. (2000) Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy. Results in normal and ischemic porcine models. J Am Coll Cardiol 35: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 29.Kornowski R, Fuchs S, Tio FO, Pierre A, Epstein SE, et al. (1999) Evaluation of the acute and chronic safety of the biosense injection catheter system in porcine hearts. Catheter Cardiovasc Interv 48: 447–453; discussion 454–445. [DOI] [PubMed]
- 30. Tao Z, Chen B, Zhao Y, Chen H, Wang L, et al. (2010) HGF percutaneous endocardial injection induces cardiomyocyte proliferation and rescues cardiac function in pigs. Journal of Biomedical Research 24: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, et al. (2000) Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation 102: 246–252. [DOI] [PubMed] [Google Scholar]
- 32. Tung R, Nakahara S, Ramirez R, Gui D, Magyar C, et al. (2011) Accuracy of combined endocardial and epicardial electroanatomic mapping of a reperfused porcine infarct model: a comparison of electrofield and magnetic systems with histopathologic correlation. Heart Rhythm 8: 439–447. [DOI] [PubMed] [Google Scholar]
- 33. Liu Q, Muruve DA (2003) Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 10: 935–940. [DOI] [PubMed] [Google Scholar]
- 34. Kiang A, Hartman ZC, Everett RS, Serra D, Jiang H, et al. (2006) Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol Ther 14: 588–598. [DOI] [PubMed] [Google Scholar]