Abstract
In South America, various species of Leishmania are endemic and cause New World tegumentary leishmaniasis (NWTL). The correct identification of these species is critical for adequate clinical management and surveillance activities. We developed a real-time polymerase chain reaction (PCR) assay and evaluated its diagnostic performance using 64 archived parasite isolates and 192 prospectively identified samples collected from individuals with suspected leishmaniasis enrolled at two reference clinics in Lima, Peru. The real-time PCR assay was able to detect a single parasite and provided unambiguous melting peaks for five Leishmania species of the Viannia subgenus that are highly prevalent in South America: L. (V.) braziliensis, L. (V.) panamensis, L. (V.) guyanensis, L. (V.) peruviana and L. (V.) lainsoni. Using kinetoplastid DNA-based PCR as a gold standard, the real-time PCR had sensitivity and specificity values of 92% and 77%, respectively, which were significantly higher than those of conventional tests such as microscopy, culture and the leishmanin skin test (LST). In addition, the real-time PCR identified 147 different clinical samples at the species level, providing an overall agreement of 100% when compared to multilocus sequence typing (MLST) data performed on a subset of these samples. Furthermore, the real-time PCR was three times faster and five times less expensive when compared to PCR - MLST for species identification from clinical specimens. In summary, this new assay represents a cost-effective and reliable alternative for the identification of the main species causing NWTL in South America.
Author Summary
Leishmaniasis is a neglected disease with more than two million new human infections annually worldwide. Tegumentary leishmaniasis, cutaneous and mucocutaneous, is mainly caused by five Leishmania species of the Viannia complex in South America. Different species can cause disease with similar symptoms but have dissimilar prognoses and may need different therapeutic regimens. Identification of Leishmania species traditionally relies on the multilocus enzyme electrophoresis (MLEE) assay, but it can only be applied to culture-positive samples and takes at least six weeks of intense laboratory work. A reliable and rapid assay for species identification can be a valuable tool. Molecular assays are the fastest and most accurate way to identify the etiological agents causing leishmaniasis. This paper describes a novel real-time PCR assay for identification of the five main species that cause tegumentary leishmaniasis in the New World. The assay correctly identified each of these five species of Leishmania directly from clinical samples. Because of its reliability, speed and simplicity, this assay could be used for species identification in routine laboratory diagnosis of leishmaniasis in endemic regions.
Introduction
The leishmaniases are a globally widespread group of vector-borne diseases that are endemic to 88 countries and affect an estimated 12 million people, with approximately 350 million people at risk worldwide [1]. Depending on the parasite species and host genetic background, infection can range from self-healing cutaneous ulcers to disfiguring mucocutaneous forms and lethal visceral disease [2]. In South America, New World tegumentary leishmaniasis (NWTL) is mainly caused by species of the Viannia complex. The most prevalent species are L. (V.) braziliensis, L. (V.) panamensis, L. (V.) peruviana, L.(V.) guyanensis and L. (V.) lainsoni [1]–[2]. In Peru, 70% of the country's region is affected with leishmaniasis, 96% of which are caused by three species: L. (V.) braziliensis, L. (V.) peruviana and L. (V.) guyanensis [2]–[3].
Diagnosis of leishmaniasis is based on criteria that consider epidemiological data, clinical features and laboratory test results [2]. Most laboratory methods are based on findings of the etiological agent microscopically or by culture; however, they have relatively low sensitivity and they do not identify the infecting species [2]. Serological tests and the leishmanin skin test (LST) have also been used as diagnostic tools but they do not distinguish between present and past infections, and their specificity is low in endemic areas [2]. A variety of molecular approaches have been developed. For instance, PCR methods based on the detection of kinetoplastid DNA (kDNA PCR) are highly sensitive due to the presence of thousands of copies of these sequences in the parasite. Thus, correct diagnosis of leishmaniasis should be based on clinical suspicion of leishmaniasis with a confirmed laboratory diagnosis, where the physician must take into account the reliability of the given laboratory result.
The correct diagnosis and characterization of the infecting parasite may be important for directing appropriate treatment and evolution of the disease [2]. For instance, patients infected with L. (V.) braziliensis have a higher risk of developing the disfiguring mucosal manifestations [4]. In addition, different species show varying response rates to therapeutic drugs [5]. Therefore, early identification of the etiological species may lead to improved patient management.
The identification of Leishmania species has been traditionally performed by multilocus enzyme electrophoresis (MLEE), for which mannose phosphate isomerase (MPI) is the only reliable marker for discrimination between the closely related species L. (V.) braziliensis and L. (V.) peruviana [6]. However, this technique presents several drawbacks: a) it can only be applied to culture-positive cases; b) it requires the isolation and mass growth of the parasite; and c) it is time-consuming, taking up to six weeks for definitive parasite identification. These limitations underscore the pressing need for improved identification methods that are fast, cost-effective and more reliable for the diagnosis and characterization of leishmaniasis cases [7].
Several real-time PCR and melting curve analysis using SYBR Green or fluorescence resonance energy transfer (FRET) probes in combination with kDNA or 18S rDNA amplification have recently been reported for the detection and identification of Old and New World Leishmania species. L. (V.) braziliensis has been the only New World Leishmania species primarily included in these assays [8]–[13]. Another study revealed that a SYBR Green-based real-time PCR assay targeting the conserved region of kDNA mini-circles was able to differentiate between L. (Leishmania) and L. (Viannia) at the complex level [14], although this assay did not distinguish species within each complex. Other molecular approaches such as PCR followed by restriction fragment length polymorphism (PCR-RFLP) [15]–[16], multilocus sequence typing (MLST) [17]–[18] or multiplex PCR [19] have been used to discriminate species within the Viannia complex. However, these approaches present a number of limitations, including laborious procedures, complex data interpretation and long processing times for species identification [8]–[10], [13]–[14], [17], [19].
We recently identified new species-specific genetic polymorphisms in the genes that confer the phenotypic variations in the MLEE assay [18]. A combination of sequencing of the MPI and 6-phosphogluconate dehydrogenase (6PGD) genes was sufficient to differentiate among seven closely related species causing New
World leishmaniasis [18]. In this study, we took advantage of these polymorphisms in order to devise a new real-time PCR assay based on FRET technology and melting curve analysis. The assay was highly sensitive and correctly identified each of the five species of Leishmania being evaluated. Because of its reliability, short turnaround time and simplicity, this assay could be used for species identification in routine laboratory diagnosis of leishmaniasis in endemic regions, thus allowing better management of patients affected with NWTL.
Materials and Methods
Source of Specimens and Ethics Statement
Leishmania reference strains were obtained from the World Health Organization (WHO) by Lucas et al. [3]. Leishmania isolates were previously obtained from patients enrolled using a written informed consent [3] and anonymized for this study. Isolates from the Instituto de Medicina Tropical “Alexander von Humboldt” (IMTAvH) samples were obtained from clinical cases seen in 2008 and sent to the U.S. Naval Medical Research Unit No. 6 (NAMRU-6) for diagnosis confirmation and species identification. Samples from the Hospital Militar Central (HMC) were collected by the Peruvian Army during an investigation of an outbreak (February-April 2010), and sent to NAMRU-6 for diagnosis confirmation and species identification. The analysis of both sets of samples was approved by the Institutional Review Board (IRB) of NAMRU-6 in compliance with all applicable federal regulations governing the protection of human subjects. All samples were anonymized before being sent to NAMRU-6.
Leishmania Reference Strains and Isolates
Five Leishmania reference strains from the WHO and 59 well-characterized Leishmania strains were assessed to determine the melting curves for each MPI and 6PGD locus. Strains and isolates belonged to species of the subgenus Viannia: L. (V.) braziliensis, L. (V.) peruviana, L. (V.) guyanensis, L. (V.) panamensis, and L. (V.) lainsoni. Reference strains for L. (Leishmania) amazonensis, L. (L.) mexicana and L. infantum (syn. L. chagasi) [20]–[21] were included as well. Species identification of all reference strains and isolates was previously performed by MLEE [3] and confirmed by MLST [18].
Patients, Clinical Samples and Diagnosis
Clinical samples from 192 patients with leishmaniasis-like lesions were prospectively collected from the IMTAvH (n = 117) and the HMC (n = 75) in Lima, Peru. These clinics are the National Reference Centers for leishmaniasis diagnosis and treatment within the Peruvian Ministry of Health and Ministry of Defense, respectively. In total, lancet scrapings (n = 14) or punch biopsy samples (n = 178) were collected from both centers and processed for direct microscopic observation after Giemsa staining. For some patients, aspirate samples were collected from skin lesions using sterile technique and inoculated into Senekji's blood-agar medium as previously described [3]. In a subgroup of patients, the LST was performed as previously published [22]. Briefly, leishmanin antigen (0.1 ml) from the L. (V.) guyanensis strain LP52 (IPRN/PE/87/Lp52) was injected intradermally into the forearm, and the extent of induration and erythema measured after 48 h. The LST result was considered positive if the diameter of the induration was 5 millimeters or more. All specimens were processed for kDNA PCR, which is specific for the Leishmania Viannia complex [23], and the FRET-based real-time PCR assay. Overall, all Leishmania strains in this study (isolates and clinical samples) are considered geographically diverse since they were isolated from cases occurring across the Peruvian coast (Lima, Piura, La Libertad and Lambayeque), the highlands (Ancash, Apurimac, Cerro de Pasco, Cajamarca, Cuzco and Junín) and the Amazon rainforest (Amazonas, Huánuco, Loreto, Madre de Dios, San Martin and Ucayali). Furthermore, we also analyzed 13 L. (V.) panamensis strains (4 isolated from Ecuador and 9 from Colombia), but these results are not included in this manuscript.
DNA Isolation
DNA was isolated from parasite culture or clinical samples using the QIAamp DNA mini kit (QIAGEN) following the manufacturer's instructions. DNA pellets were resuspended in Tris-EDTA buffer and quantified using a NanoDrop 1000 Spectrophotometer.
kDNA PCR Conditions
Conventional PCRs for kDNA targeting mini-circles DNA were carried out to diagnose all specimens as a gold standard. This kDNA PCR can detect all Leishmania species from the Viannia complex [23] and has shown high sensitivity and specificity on different type of samples [24]. The PCRs were performed using a Gene Amp PCR System 9700 thermocycler (Applied Biosystems, Foster City, CA) in a total volume of 20 µl containing 4 µl DNA, 1X PCR buffer (Invitrogen), 0.5 µM of each primer (MP1-L: 5′-TAC TCC CCG ACA TGC CTC TG-3′ and MP3-H: 5′-GAA CGG GGT TTC TGT ATG C-3′), 1 U Taq DNA Polymerase (Invitrogen, Grand Island, NY), 1.5 mM MgCl2, and 125 µM of each dNTP. Initial denaturation at 94°C for 5 min was followed by 35 cycles of denaturation at 94°C for 45 sec, annealing at 58°C for 45 sec, and extension at 72°C for 60 sec; and a final extension at 72°C for 5 min. Amplified products were analyzed on 2% agarose gels; the expected product size is 70 bp.
Real-time PCR Conditions
Conventional PCRs for MPI and 6PGD genes were carried out prior to the nested real-time PCR amplifications in order to increase sensitivity of the assays. The initial PCRs were performed using a Gene Amp PCR System 9700 thermocycler (Applied Biosystems) in a total volume of 50 µl containing 5 µl DNA, 1X PCR buffer (Invitrogen), 1 µM of each primer ( Table 1 ), 1.5 U Platinum Taq DNA Polymerase (Invitrogen), 1.5 mM MgCl2, and 200 µM of each dNTP. Initial denaturation at 94°C for 5 min was followed by 35 cycles of denaturation at 94°C for 45 sec, annealing at 57°C (for MPI) or 62°C (for 6PGD) for 45 sec, and extension at 72°C for 90 sec; and a final extension at 72°C for 7 min for MPI or 5 min for 6PGD. The subsequent PCR products were then used to perform the nested real-time PCR assays without further processing. Independent real-time reactions for MPI and 6PGD genes were performed in a LightCycler 480 Instrument (Roche Applied Science). Reactions were carried out in a 20 µl total volume containing 1X LightCycler 480 Genotyping Master (Roche), 1.25 µM of forward primer, 0.25 µM of reverse primer, 0.18 µM of anchor probe, 0.18 µM of sensor probe ( Table 2 ), and 5 µL of DNA (for reference strains used as positive controls) or PCR products. The amplification setting consisted of an initial denaturation at 95°C for 5 min followed by 45 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 20 sec (a single acquisition step) and extension at 72°C for 20 sec. After amplification, a melting curve analysis was performed by heating the real-time PCR products at 95°C for 10 sec, cooling at 50°C for 59 sec and then increasing the temperature to 80°C while continuously monitoring the fluorescence (one acquisition step per °C). Melting curves were analyzed using the LightCycler 480 Software Version 1.0 release 1.1.0.0520 (Roche Molecular System, Penzberg, Germany) to determine the species-specific melting temperatures (Tm). To calculate and enhance the visualization of the Tm values, melting peaks were derived from the initial melting curves (fluorescence [F] versus temperature [T] by plotting the negative derivative of fluorescence over temperature –dF/dT versus dT) [12]. A 483–670 nm filter combination was used for MPI while a 483–610 nm combination was used for 6PGD. We followed all appropriate recommendations to avoid cross-contamination, including physical separation of PCR reaction and amplification products, use of UV light to eliminate DNA traces on work surface, aliquoting reagents and master mixes, etc [25]. For conventional PCR assays, L. (V.) braziliensis DNA (1 ng) was used as a positive control and nuclease-free water was used as a negative control. For real-time PCR assays, DNA (1 ng) from L. (V.) braziliensis, L. (V.) peruviana, L. (V.) guyanensis, L. (V.) panamensis, L. (V.) lainsoni, L. (L.) amazonensis, and L. (L.) mexicana strains were used as both references and positive controls and nuclease-free water was used as a negative control.
Table 1. Primers used in the conventional PCR assays for MPI and 6PGD genes carried out prior to the nested real-time PCR assay.
Table 2. Primers and labeled-probes used in the nested real-time PCR assays for MPI and 6PGD genes.
| Gene | Primer and probe name | Sequence (5′ – 3′) |
| MPI | MPI.1082.F | ACG CCC AAG TGG AAG GAT G |
| MPI.1082.R | ACA CCA CTG TAC CGT TCA CC | |
| MPI.1082.anchor | TTC CAG ACA GAA GCC CAG CCC AAT CGT CGG – fluorescein | |
| MPI.1082.sensor | red670 – GTC ACG GAG GTC GTC CCG CTT CCA G | |
| 6PGD | 6PGD.1262.F | CAA GGA GAT GAA GGA GGG TC |
| 6PGD.1262.R | CTT GTC AAC ACG TTC GTA GC | |
| 6PGD.1262.anchor | GCC AGG GAG GCA GTC ATC ACC G – fluorescein | |
| 6PGD.1262.sensor | red610 – AAC GAT ACA GCC GTG CTC GC |
MLST
Seventy-two clinical samples were assessed by MLST as described previously [8]. Briefly, MPI and 6PGD specific primers were used to amplify each product individually. Direct sequencing was performed using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) and analyzed on an ABI Prism 3100×l automated DNA sequencer (Applied Biosystems). Sequence analysis was performed using Sequencher v4.8 (Gene Codes Corporation, Ann Arbor, MI).
Analytical Sensitivity Evaluation
PCR products of the MPI and 6PGD genes of L. (V.) braziliensis, L. (V.) peruviana, L. (V.) guyanensis, and L. (V.) panamensis were purified and cloned into pGEM-T Easy Vector System (Promega). Serial dilutions from 106 to 1 plasmid copy were evaluated in single and multiplexed reactions. The detection limit was defined as the minimum number of plasmids that could be amplified and correctly identified by melting curve analysis.
Cost Analysis
Consumables, laboratory reagents and labor were considered in the cost estimations. Total costs per type of assay were calculated for a batch of 10 samples plus positive and negative controls and divided by 10 to estimate costs per individual sample. MPI and 6PGD assays were considered as a combined analysis for cost analysis. Fixed costs such as facility space, electricity, air conditioning, etc., as well as costs and labor associated with DNA isolation were considered equivalent between the two methods and were not included for this analysis. All costs were obtained directly from U.S.-based manufacturers and shipping costs were not taken into consideration.
Statistical Analysis
Data were analyzed using Stata v11.0 for Windows (Stata Corporation, College Station, TX). Descriptive statistics (mean, standard deviation, median, range) were calculated for continuous variables. Sensitivity, specificity and predictive values including 95% confidence intervals (95% CI) were estimated for all diagnostic tests using kDNA PCR as the gold standard. Statistical differences between these values were estimated using the exact McNemar test for matched observations. Categorical variables were assessed by proportions, and differences among the groups were compared using Fisher's exact chi-square analysis. p-values less than 0.05 were considered statistically significant.
Results
Development of the Real-Time PCR Assays
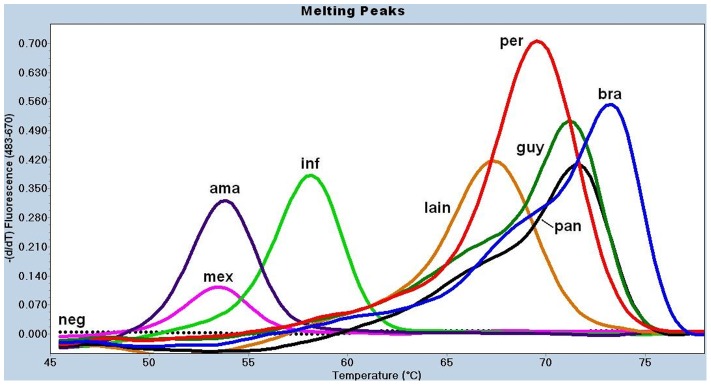
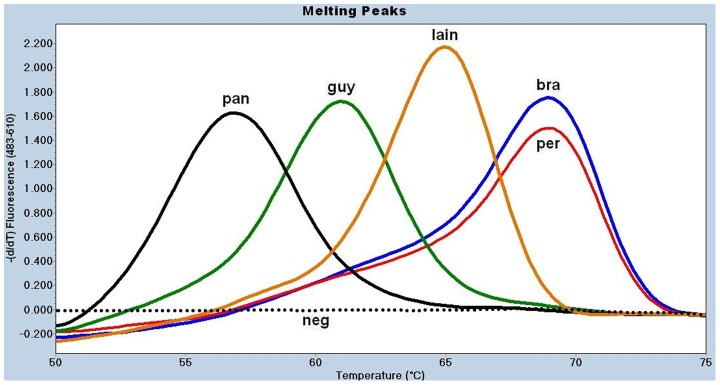
We designed two sets of primers and hybridization probes capable of distinguishing previously identified single nucleotide polymorphisms (SNPs) in the MPI and 6PGD loci [18]. Hybridization probes for the MPI gene were designed to detect the C1082G mutation, which differentiates between L. (V.) braziliensis and L. (V.) peruviana [19]. Several SNPs present in the region spanned by the anchor and sensor probes were found to differentiate L. (V.) lainsoni and L. infantum (syn L. chagasi). The probes for the 6PGD gene were designed around the C1263G SNP, which differentiates between L. (V.) guyanensis and L. (V.) panamensis [18] ( Table 2 ). Next, we performed melting curve analysis of the amplification products for both loci in order to assess whether intraspecific genetic variability affected correct species discrimination. For this purpose, we used five reference strains and 59 well-characterized Leishmania isolates that were previously differentiated by MLEE and MLST [3], [18]. The MPI-based assay yielded non-overlapping Tm values calculated for the melting peaks of L. (V.) braziliensis (74.0±0.1°C, mean ± standard deviation), L. (V.) peruviana (70.1±0.3°C), L. (V.) lainsoni (67.8±0.2°C), and L. infantum (58.7°C), allowing for a method of discrimination among these species ( Table 3 and Figure 1 ). However, this assay did not discriminate between L. (V.) guyanensis and L. (V.) panamensis or L. (L.) amazonensis and L. (L.) mexicana because their Tms overlapped ( Table 3 and Figure 1 ). When the 6PGD-based real-time PCR assay was used, we obtained clearly different Tms for L. (V.) guyanensis (60.2±0.1°C), L. (V.) panamensis (56.2±0.7°C), and L. (V.) lainsoni (65±0.2°C), thus allowing identification of these species ( Table 3 and Figure 2 ). In addition to being able to differentiate five Leishmania species of the Viannia complex, the MPI-based real-time PCR gives distinct Tm for L. infantum, which belongs to the Leishmania donovani complex. However, this assay cannot distinguish between L. (L.) amazonensis and L. (L.) mexicana, two species of the Leishmania mexicana complex ( Figure 1 ). Overall, the combined use of both MPI- and 6PGD-based real-time PCR assays allows the correct identification of five New World Leishmania species of the Viannia complex and one species of Leishmania donovani complex ( Table 3 and Figures 1 and 2 ). Melting peaks were not observed for negative controls, nuclease-free water or non-Leishmania DNA, indicating the absence of primer-dimers formation ( Figures 1 and 2 ). Thus, Tm values could not be calculated.
Table 3. Melting temperature (°C ± SD) calculated for the melting peaks detected in 65 Leishmania strain isolates.
| Species | n | MPI | 6PGD |
| L. (V.) braziliensis | 19 | 74.0±0.1 | 68.2±0.2 |
| L. (V.) peruviana | 14 | 70.1±0.3 | 68.0±0.3 |
| L. (V.) guyanensis | 8 | 71.7±0.1 | 60.2±0.1 |
| L. (V.) panamensis | 10 | 71.6±0.1 | 56.2±0.7 |
| L. (V.) lainsoni | 6 | 67.8±0.2 | 65.0±0.2 |
| L. infantum | 1 | 58.7 | ND* |
| L. (L.) amazonensis | 6 | 53.5±0.1 | ND |
| L. (L.) mexicana | 1 | 53.6 | ND |
ND = non detected.
Figure 1. Melting curve analysis for the MPI-based real-time PCR assay on Leishmania reference strains.
Five nanograms of DNA (references) or 5 ul of nested-PCR products (samples) were used as template to run the assay. The species designation is given based on the Tm calculated for each melting peak (point in the x axis for the peak of the curve). FRET probes on L. (L.) amazonensis (ama) or L. (L.) mexicana (mex) DNA are melted at lower temperature (53°C) while on L. (V.) braziliensis (bra) DNA are melted at the highest temperature (74°C). This assay could not differentiate between L. (V.) guyanensis (guy) and L. (V.) panamensis (pan) due to their overlapping Tm (72°C). All assessed Leishmania species yielded a melting curve. A straight dotted line corresponds to the negative controls (neg), DNA free or parasite-free human DNA. inf = L. infantum; lain = L. (V.) lainsoni; per = L. (V.) peruviana.
Figure 2. Melting curve analysis for the 6PGD-based real-time PCR assay on Leishmania reference strains.
Five nanograms of DNA (references) or 5 ul of nested-PCR products (samples) were used as template to run the assay. The species designation is given based on the Tm calculated for each melting peak (point in the x axis for the peak of the curve). FRET probes on L. (V.) panamensis (pan) DNA are melted at the lowest temperature (56°C) while on L. (V.) braziliensis (bra) or L. (V.) peruviana (per) DNA are melted at the highest temperature (68°C). Due to their overlapping Tm, this assay could not differentiate these two species. L. (L.) amazonensis, L. (L.) mexicana and L. infantum did not yield a melting curve. A straight dotted line corresponds to the negative controls (neg), DNA free or parasite-free human DNA. guy = L. (V.) guyanensis; lain = L. (V.) lainsoni.
Analytical Sensitivity of the Real-Time PCR Assays
Following optimization of the real-time PCR conditions, we determined the analytical sensitivity of the assays. The MPI- and 6PGD-based real-time PCR assays detected as few as one copy of plasmid when carried out in separate reaction tubes (data not shown). When the MPI and 6PGD assays were multiplexed into a single reaction, the detection limit was 10–100 copies (data not shown).
Cross-Reactivity of the Real-Time PCR Assays
No cross-reaction was observed when the two real-time PCR assays were used to test DNA from Homo sapiens (20 ng DNA), Trypanosoma cruzi (ATCC 30013 strain, American Type Culture Collection, 1–2 ng DNA), Trypanosoma cruzi Tulahuen strain (1–2 ng DNA), Plasmodium falciparum D6 strain (1–2 ng DNA), and P. vivax OBT9140 isolate (20 ng DNA).
Sensitivity and Specificity with Clinical Specimens
A total of 192 specimens belonging to the same number of individuals with leishmaniasis-like lesions were evaluated (178 biopsy and 14 lesion scraping samples). Of these, 178 samples (92.7%) showed a positive result by at least one of the four diagnostic methods (microscopy, culture, LST and kDNA PCR). One hundred sixty-six samples (86.5%) were positive by kDNA PCR. When kDNA-PCR was used as the gold standard for leishmaniasis diagnosis, the real-time PCR had a sensitivity and specificity of 92% and 77%, respectively, and the positive and negative predictive values were 97% and 59%, respectively ( Table 4 ). The sensitivity of the real-time PCR among kDNA PCR positive samples was superior to that of each of the other diagnostic tests, including microscopy (93% vs. 59%, p<0.001), culture (93% vs. 31%, p<0.001) and LST (93% vs. 68%, p<0.001) ( Table 4 ). Stratification of the analysis by site revealed that the sensitivity of the real-time PCR tended to be higher among samples collected from IMTAvH compared to those collected at HMC ( Table 5 ). The analysis of the discordant samples showed that fourteen samples were positive by kDNA PCR but negative by real-time PCR (false negative), and six samples were negative by kDNA PCR but positive by real-time PCR (false positive). Of the fourteen samples classified as false negatives, one was positive by microscopy and thirteen other samples were consistently negative by both microscopy and culture, likely indicative of low levels of parasites in these specimens (Table S1). The presence of PCR inhibitors was ruled out in these fourteen samples since DNA preparations in a 10-fold dilution were tested by PCR assays targeting two human housekeeping genes: β-globin and Ribonuclease P. While both undiluted and diluted samples were positive for β-globin and Ribonuclease P, all remained negative by the MPI and 6PGD real-time PCR assays. Additionally, of the six specimens classified as false positive, four tested positive by microscopy or LST (Table S2), indicating that these four samples (two only detected by 6PGD real-time PCR) likely were truly positive but not detected by kDNA PCR.
Table 4. Sensitivity, specificity and predictive values for all tested methods using kDNA PCR as the gold standard.
| kDNA PCR | % Sensitivity (95% CI) | % Specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) | ||
| No. Positive | No. negative | |||||
| Real-time PCR | ||||||
| Positive | 152 | 6 | 92 (86–95) | 77 (58–93) | 96 (92–98) | 59 (42–74) |
| Negative | 14 | 20 | ||||
| Microscopy | ||||||
| Positive | 89 | 5 | 59* (51–67) | 78 (58–93) | 95 (88–98) | 23 (15–32) |
| Negative | 62 | 18 | ||||
| Culture | ||||||
| Positive | 36 | 2 | 31* (23–39) | 89 (69–97) | 95 (83–99) | 17 (11–26) |
| Negative | 82 | 17 | ||||
| LST | ||||||
| Positive | 50 | 10 | 68* (57–77) | 52 (32–72) | 83 (72–91) | 31 (19–48) |
| Negative | 24 | 11 | ||||
Statistically significant (p<0.001) when compared to real-time PCR by the exact McNemar's test. For this analysis, we considered only those samples that were processed by both assays being compared.
All samples were not assessed with all methods.
Table 5. Sensitivity and specificity of the real-time PCR assay compared to kDNA PCR stratified by site, type of sample and type of lesion.
| Variable | Samples assessed | kDNA positives | n/N, % Sensitivity (95% CI) | n/N, % Specificity (95% CI) |
| Site | ||||
| IMTAvH | 117 | 92 | 91/92, 99*(94–100) | 19/25, 76(55–91) |
| HMC | 75 | 74 | 61/74, 82(72–90) | 1/1, 100(3–100) |
| Type of sample | ||||
| Biopsy | 178 | 156 | 142/156, 91(85–95) | 16/22, 73(50–89) |
| Scraping | 14 | 10 | 10/10, 100(69–100) | 4/4, 100(40–100) |
| Form of lesion | ||||
| Cutaneous | 163 | 144 | 130/144, 90(84–95) | 14/19, 74(49–91) |
| Mucocutaneous | 29 | 22 | 22/22, 100(85–100) | 6/7, 86(42–100) |
Statistically significant (p<0.001) by Chi-square test.
Leishmania Species Identification
Among the 158 clinical samples that tested positive by real-time PCR, we were able to correctly identify 147 (93%) specimens to the species level. Four Leishmania species were identified among the IMTAvH samples: L. (V.) peruviana (43%), L. (V.) braziliensis (30%), L. (V.) guyanensis (12%), and L. (V.) lainsoni (3%). Two species were identified among the HMC samples: L. (V.) braziliensis (97%) and L. (V.) guyanensis (3%) ( Table 6 ). In 11 IMTAvH clinical samples (11%), the MPI real-time PCR failed to amplify but 6PGD real-time PCR did amplify. The 6PGD real-time PCR results suggested infection by either L. (V.) peruviana or L. (V.) braziliensis. However, in the absence of MPI results, we could not discriminate between these Leishmania species ( Table 6 ). In a subset of 72 clinical samples, regions of the MPI and 6PGD genes were sequenced as previously described [18]. The concordance between the real-time PCR and the MLST analysis for species identification was 100% (Table S3). In summary, the real-time PCR assays reliably discriminated among New World Leishmania species that are highly prevalent in South America.
Table 6. Leishmania species identified by real-time PCR from individuals with suspected leishmaniasis from IMTAvH and HMC samples.
| Species | IMTAvH | % | HMC | % |
| L. (V.) braziliensis | 29 | 29.9 | 59 | 96.7 |
| L. (V.) peruviana | 42 | 43.3 | – | – |
| L. (V.) guyanensis | 12 | 12.4 | 2 | 3.3 |
| L. (V.) lainsoni | 3 | 3.1 | – | – |
| BRA/PER* | 11 | 11.3 | – | – |
| Total | 97 | 100 | 61 | 100 |
No amplification for the MPI real-time PCR assay. 6PGD real-time PCR results suggested infection by either L. (V.) peruviana or L. (V.) braziliensis but in the absence of MPI results, we could not discriminate between these two Leishmania species.
Processing Time and Cost Analysis
We estimated the turnaround time and assay costs between the real-time PCR and the combination of kDNA PCR plus MLST for the specific identification of Leishmania species. The real-time PCR assay had lower costs and required less processing time compared to kDNA PCR plus MLST for the correct identification of Leishmania species ( Table 7 ).
Table 7. Turnaround time and cost analysis per sample for the nested real-time PCR and MLST assays for the identification of Leishmania species from clinical specimens†.
| Procedures\assays | kDNA-PCR/MLST (USD) | Nested real-time PCR (USD) |
| kDNA-PCR | 1.3 | NAa |
| PCRb | 2.2 | 2.2 |
| Bi-directional sequencingb | 30.6 | NA |
| real-time PCRb | NA | 4.3 |
| Total cost (US dollars) | 34.1 | 6.5 |
| Turnaround time (hours) | 20 | 6 |
Costs represent U.S. dollar amount per individual sample based on batch analysis of 10 samples plus one positive and one negative control.
NA, not applicable.
The cost includes the analysis of both MPI and 6PGD genes per each processed sample.
Discussion
In this report, we describe the development and evaluation of a real-time PCR assay for the diagnosis and characterization of NWTL species from tissue samples. This assay has several advantages: 1) It is highly sensitive, detecting as few as one copy of DNA, i.e. half of a diploid parasite genome, 2) It has higher sensitivity and specificity for the diagnosis of NWTLs when compared to conventional diagnostic tests, 3) It can reliably identify the infecting Leishmania species, including L. (V.) braziliensis, L. (V.) panamensis L. (V.) peruviana, L. (V.) guyanensis, and L. (V.) lainsoni (the most prevalent species causing NWTL in South America) [1]–[2], and 4) It is three times faster and five times less expensive compared to MLST for species identification. Because of these features, this real-time PCR assay can be a valuable contribution to the diagnosis and management of leishmaniasis in resource-limited settings.
In recent years, PCR has been established as the preferred method of Leishmania diagnosis and species identification due to its higher sensitivity and short turnaround time compared to traditional diagnostic tests [26]–[28]. We recently reported the presence of polymorphisms in various MLEE markers that could discriminate New World species of the Viannia complex [18]. Based on these mutations, we designed a real-time PCR assay to identify Leishmania species of the Viannia complex by virtue of their unique melting profiles. Melting curve analysis of the MPI and 6PGD genes showed non-overlapping curves for five different Leishmania species among 59 isolates, suggesting that intraspecific genetic polymorphisms are not likely to affect correct species identification using this method. Since the strains belonged to patients from diverse regions of Peru (and a few from Ecuador and Colombia), we believe that intraspecific genetic variability will not affect correct species identification by the real-time PCR assay, thus allowing identification of geographically diverse strains of Leishmania species. This is further supported by sequencing analysis showing that the SNPs in the MPI and 6PGD housekeeping genes are extremely well conserved even among genetically diverse strains [18].
The real-time PCR technique performed considerably better when compared to conventional diagnostic techniques such as microscopy, culture and LST in prospectively evaluated individuals with suspected leishmaniasis (n = 192). Additionally, our study confirmed the low sensitivity of microscopy, culture and LST, which ranged from 50 to 80% among various studies [29]–[31]. Given the high sensitivity values reported for kDNA PCR, we used this assay as a gold standard to assess the performance of the real-time PCR to diagnose NWTL [23], [29], [31]–[32], producing sensitivity and positive predictive values of 92% and 96%, respectively. Fourteen samples gave a positive result for kDNA PCR but were negative by the real-time PCR assay and were, therefore, classified as false negative. We ruled out the presence of PCR inhibitors in DNA preparations. The decreased sensitivity of the real-time PCR may be explained by larger amplicon size amplified for first-round PCR (∼1600 bp for the MPI locus versus ∼70 bp for the kDNA PCR), since partially-degraded DNA is more likely to amplify shorter amplicons compared to larger amplicons [33]. In keeping with this hypothesis, by shortening the first-round PCR of the MPI real-time PCR to 721 bp, we were able to detect 4 out of 10 initially-negative real-time PCR samples (data not shown). We are currently refining the assay using other strategies such as the use of alternative primers, shorter first-round PCR products and increasing the concentration of magnesium of the first PCR. The mini-circles of kDNA of Leishmania are also present as thousands of copies per parasite, whereas the MPI and 6PGD genes are present as a single copy in the parasite's genome (Leishmania braziliensis GeneDB) [19], which could explain the higher sensitivity of the kDNA PCR assay over the nested PCR approach used in the real-time PCR assay [19], [34].
The specificity and negative predictive values of the real-time PCR were 77% and 59% respectively, when compared to kDNA PCR. However, 4 out of the 6 false positive samples had a positive result by either microscopy or LST. As a positive smear alone is not sufficient as criterion for a positive diagnosis of leishmaniasis, these results together with the available clinical diagnosis suggests that at least three of these six cases could be true positives that were missed by the kDNA PCR (Table S2). When these 3 samples were considered true positives, the adjusted specificity of the real-time PCR was 87%. These results highlight the importance of defining the right gold standard criteria for the diagnosis of leishmaniasis [24], [35] and support the hypothesis that the seemingly suboptimal specificity may be due to misclassification by the kDNA PCR rather than low specificity of the real-time PCR assay. The negative predictive value remained low (63%) after correcting for misclassification. However, this may be a reflection of the very high prevalence of leishmaniasis in the studied group (overall disease prevalence was ∼85%), composed mainly of suspected cases at reference centers. Further studies with larger sample size including subpopulations with lower prevalence of leishmaniasis are needed to confirm our findings and better estimate the sensitivity and specificity of the real-time PCR assay.
Several methods for diagnosis and species identification have been developed but most of these procedures only discriminate a few species within the Viannia complex [9], [13]–[17], [19]. While the real-time PCR assay we developed has proven to be highly sensitive for the diagnosis of NWTL, its main advantage resides in its ability to simultaneously identify up to five members of the Viannia subgenus. This assay reliably identified 64 archived parasite isolates and 147 prospectively collected skin samples at the species level with 100% concordance with MLST when compared in parallel in a subset of these samples. Besides the potential impact of this assay in the clinical setting, we successfully applied this real-time PCR assay for the identification and characterization of Leishmania species in field-collected sand fly specimens [36], underscoring the potential broad applicability of this assay.
One limitation of this study was the small number of negative samples included given the high prevalence of leishmaniasis in the study settings. This may have resulted in a less precise estimation of the predictive values since only few negative subjects were included in the analysis. An additional study is being planned to provide more accurate estimates of the specificity and the negative predictive value. A second limitation was that we were unable to apply all diagnostic tests simultaneously to all study participants. As a consequence, comparisons across diagnostic tests had to be done in subsets of subjects, which could limit the comparability of the tests. Finally, although we carried out this study at two major reference clinics for leishmaniasis, it remains possible that the strains included in this study do not represent all strains in Peru. Future studies in more diverse groups, including a larger pool of negative samples and belonging to wider geographic areas, are warranted to confirm the results of this study.
In summary, our real-time PCR assay can simultaneously diagnose New World leishmaniasis and identify the five causative Leishmania species most prevalent in South America, highlighting its potential regional applicability in all these countries. Thus, given its diagnostic performance, short turnaround time, scalability and relatively low costs, this assay could have great utility in the clinical setting and help to improve case management and direct appropriate therapy for patients with cutaneous and mucocutaneous leishmaniasis in resource-limited countries of South America.
Supporting Information
Results of conventional diagnostic tests for 14 clinical samples reported as false negatives. Conventional diagnostic tests and real-time PCR were negatives but the kDNA PCR assay was positive. Since melting peaks were not observed, Leishmania species could not be identified for these 14 samples.
(DOC)
Results of conventional diagnostic tests for 6 clinical samples reported as false positives. At least one conventional diagnostic test and one real-time PCR assay were positives but the kDNA PCR assay was negative. Two samples yield melting peaks for both MPI and 6PDG real-time PCR assays and the involved Leishmania species were identified. Four samples yield a melting peak for 6PGD real-time PCR assay only and the involved Leishmania species were reported as BRA/PER.
(DOC)
Seventy two clinical samples comparing Leishmania speciation by MLST and by our novel real-time PCR assay. A 100% concordance between MLST and real-time PCR assay was obtained when this subset of samples were compared in parallel. The shaded rows correspond to reference strains (sequenced in [18]) that were used as comparison. SNPs in MPI and 6PGD that are detected by the real-time PCR probes are shown in bold. Dots indicate missing data. Dashes indicate that no sequencing was performed.
(DOC)
Acknowledgments
We would like to thank Hugo Valdivia for support with the DNA sequencing and analysis for our work. In addition, we would like to thank Ashkan Zand, Ellen Jones and Wendy K. Leung for their critical review of this manuscript. Also, we would like to thank Dr. José Aguilar for supplying the Trypanosoma cruzi Tulahuen strain and Carmen Lucas for supplying DNA from Homo sapiens, Plasmodium falciparum D6 strain, and P. vivax, OBT9140 isolate.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Funding Statement
This study was funded by grants CO497_11_L1 and CO466_11_L1 of the Global Emerging Infections Surveillance and Response System (AFHSC/GEIS) of the U.S. Department of Defense. Additionally, this work was supported by the training grant 2D43 TW007393 awarded to the U.S. Naval Medical Research Unit No. 6 (NAMRU-6) by NIH/FIC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305–318. [DOI] [PubMed] [Google Scholar]
- 2. Goto H, Lindoso JA (2010) Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther 8: 419–433. [DOI] [PubMed] [Google Scholar]
- 3. Lucas CM, Franke ED, Cachay MI, Tejada A, Cruz ME, et al. (1998) Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg 59: 312–317. [DOI] [PubMed] [Google Scholar]
- 4. Ameen M (2007) Cutaneous leishmaniasis: therapeutic strategies and future directions. Expert Opin Pharmacother 8: 2689–2699. [DOI] [PubMed] [Google Scholar]
- 5. Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, et al. (2007) Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis 195: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 6. Arana M, Evans DA, Zolessi A, Cuentas AL, Arevalo J (1990) Biochemical characterization of Leishmania (Viannia) braziliensis and Leishmania (Viannia) peruviana by isoenzyme electrophoresis. Trans R Soc Trop Med Hyg 84: 526–529. [DOI] [PubMed] [Google Scholar]
- 7. Schonian G, Kuhls K, Mauricio IL (2011) Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania . Parasitology 138: 405–425. [DOI] [PubMed] [Google Scholar]
- 8. Wortmann G, Hochberg L, Houng HH, Sweeney C, Zapor M, et al. (2005) Rapid identification of Leishmania complexes by a real-time PCR assay. Am J Trop Med Hyg 73: 999–1004. [PubMed] [Google Scholar]
- 9. Schulz A, Mellenthin K, Schonian G, Fleischer B, Drosten C (2003) Detection, differentiation, and quantitation of pathogenic Leishmania organisms by a fluorescence resonance energy transfer-based real-time PCR assay. J Clin Microbiol 41: 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicolas L, Prina E, Lang T, Milon G (2002) Real-time PCR for detection and quantitation of Leishmania in mouse tissues. J Clin Microbiol 40: 1666–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Monbrison F, Mihoubi I, Picot S (2007) Real-time PCR assay for the identification of cutaneous Leishmania parasite species in Constantine region of Algeria. Acta Trop 102: 79–83. [DOI] [PubMed] [Google Scholar]
- 12. Nicolas L, Milon G, Prina E (2002) Rapid differentiation of Old World Leishmania species by LightCycler polymerase chain reaction and melting curve analysis. J Microbiol Methods 51: 295–299. [DOI] [PubMed] [Google Scholar]
- 13. Weirather JL, Jeronimo SM, Gautam S, Sundar S, Kang M, et al. (2011) Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples. J Clin Microbiol 49: 3892–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pita-Pereira D, Lins R, Oliveira MP, Lima RB, Pereira BA, et al. (2012) SYBR Green-based Real-Time PCR targeting kinetoplast DNA can be used to discriminate between the main etiologic agents of Brazilian cutaneous and visceral leishmaniases. Parasit Vectors 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montalvo AM, Fraga J, Monzote L, Montano I, De Doncker S, et al. (2010) Heat-shock protein 70 PCR-RFLP: a universal simple tool for Leishmania species discrimination in the New and Old World. Parasitology 137: 1159–1168. [DOI] [PubMed] [Google Scholar]
- 16. Volpini AC, Passos VM, Oliveira GC, Romanha AJ (2004) PCR-RFLP to identify Leishmania (Viannia) braziliensis and L. (Leishmania) amazonensis causing American cutaneous leishmaniasis. Acta Trop 90: 31–37. [DOI] [PubMed] [Google Scholar]
- 17. de Almeida ME, Steurer FJ, Koru O, Herwaldt BL, Pieniazek NJ, et al. (2011) Identification of Leishmania spp. by molecular amplification and DNA sequencing analysis of a fragment of rRNA internal transcribed spacer 2. J Clin Microbiol 49: 3143–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsukayama P, Lucas C, Bacon DJ (2009) Typing of four genetic loci discriminates among closely related species of New World Leishmania . Int J Parasitol 39: 355–362. [DOI] [PubMed] [Google Scholar]
- 19. Zhang WW, Miranda-Verastegui C, Arevalo J, Ndao M, Ward B, et al. (2006) Development of a genetic assay to distinguish between Leishmania viannia species on the basis of isoenzyme differences. Clin Infect Dis 42: 801–809. [DOI] [PubMed] [Google Scholar]
- 20. Kuhls K, Alam MZ, Cupolillo E, Ferreira GE, Mauricio IL, et al. (2011) Comparative microsatellite typing of new world Leishmania infantum reveals low heterogeneity among populations and its recent old world origin. PLoS Negl Trop Dis 5: e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leblois R, Kuhls K, Francois O, Schonian G, Wirth T (2011) Guns, germs and dogs: On the origin of Leishmania chagasi . Infect Genet Evol 11: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 22. Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, et al. (2008) Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis 46: 223–231. [DOI] [PubMed] [Google Scholar]
- 23. Lopez M, Inga R, Cangalaya M, Echevarria J, Llanos-Cuentas A, et al. (1993) Diagnosis of Leishmania using the polymerase chain reaction: a simplified procedure for field work. Am J Trop Med Hyg 49: 348–356. [DOI] [PubMed] [Google Scholar]
- 24. Boggild AK, Ramos AP, Valencia BM, Veland N, Calderon F, et al. (2011) Diagnostic performance of filter paper lesion impression PCR for secondarily infected ulcers and nonulcerative lesions caused by cutaneous leishmaniasis. J Clin Microbiol 49: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwok S, Higuchi R (1989) Avoiding false positives with PCR. Nature 339: 237–238. [DOI] [PubMed] [Google Scholar]
- 26. Disch J, Pedras MJ, Orsini M, Pirmez C, de Oliveira MC, et al. (2005) Leishmania (Viannia) subgenus kDNA amplification for the diagnosis of mucosal leishmaniasis. Diagn Microbiol Infect Dis 51: 185–190. [DOI] [PubMed] [Google Scholar]
- 27. Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL (2006) Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol 44: 1435–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aviles H, Belli A, Armijos R, Monroy FP, Harris E (1999) PCR detection and identification of Leishmania parasites in clinical specimens in Ecuador: a comparison with classical diagnostic methods. J Parasitol 85: 181–187. [PubMed] [Google Scholar]
- 29. Boggild AK, Miranda-Verastegui C, Espinosa D, Arevalo J, Adaui V, et al. (2007) Evaluation of a microculture method for isolation of Leishmania parasites from cutaneous lesions of patients in Peru. J Clin Microbiol 45: 3680–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boggild AK, Miranda-Verastegui C, Espinosa D, Arevalo J, Martinez-Medina D, et al. (2008) Optimization of microculture and evaluation of miniculture for the isolation of Leishmania parasites from cutaneous lesions in Peru. Am J Trop Med Hyg 79: 847–852. [PubMed] [Google Scholar]
- 31. Ameen M (2010) Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics. Clin Exp Dermatol 35: 699–705. [DOI] [PubMed] [Google Scholar]
- 32. Martins L, Alexandrino A, Guimaraes G (2010) Detection of Leishmania braziliensis DNA in American tegumentary leishmaniasis patients. Rev Saude Publica 44: 571–574. [DOI] [PubMed] [Google Scholar]
- 33. Ryan P, Bennett MW, Aarons S, Lee G, Collins JK, et al. (2002) PCR detection of Mycobacterium paratuberculosis in Crohn's disease granulomas isolated by laser capture microdissection. Gut 51: 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shlomai J (2004) The structure and replication of kinetoplast DNA. Curr Mol Med 4: 623–647. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez-Cortes A, Ojeda A, Francino O, Lopez-Fuertes L, Timon M, et al. (2010) Leishmania infection: laboratory diagnosing in the absence of a “gold standard”. Am J Trop Med Hyg 82: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valdivia HO, De Los Santos MB, Fernandez R, Baldeviano GC, Zorrilla VO, et al. (2012) Natural Leishmania Infection of Lutzomyia (Trichophoromyia) auraensis in Madre de Dios, Peru, Detected by a Fluorescence Resonance Energy Transfer-Based Real-Time Polymerase Chain Reaction. Am J Trop Med Hyg 87 (3) 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of conventional diagnostic tests for 14 clinical samples reported as false negatives. Conventional diagnostic tests and real-time PCR were negatives but the kDNA PCR assay was positive. Since melting peaks were not observed, Leishmania species could not be identified for these 14 samples.
(DOC)
Results of conventional diagnostic tests for 6 clinical samples reported as false positives. At least one conventional diagnostic test and one real-time PCR assay were positives but the kDNA PCR assay was negative. Two samples yield melting peaks for both MPI and 6PDG real-time PCR assays and the involved Leishmania species were identified. Four samples yield a melting peak for 6PGD real-time PCR assay only and the involved Leishmania species were reported as BRA/PER.
(DOC)
Seventy two clinical samples comparing Leishmania speciation by MLST and by our novel real-time PCR assay. A 100% concordance between MLST and real-time PCR assay was obtained when this subset of samples were compared in parallel. The shaded rows correspond to reference strains (sequenced in [18]) that were used as comparison. SNPs in MPI and 6PGD that are detected by the real-time PCR probes are shown in bold. Dots indicate missing data. Dashes indicate that no sequencing was performed.
(DOC)