Abstract
Electrothermal supercharging of protein ions formed by electrospray ionization from buffered aqueous solutions results in significant increases to both the maximum and average charge states compared to native mass spectrometry in which ions are formed from the same solutions but with lower spray potentials. For eight of the nine proteins investigated, the maximum charge states of protonated ions formed from native solutions with electrothermal supercharging is greater than those obtained from conventional denaturing solutions consisting of water/methanol/acid, although the average charging is slightly lower owing to contributions of small populations of more folded low charge-state structures. Under these conditions, electrothermal supercharging is slightly less effective for anions than for cations. Equivalent sequence coverage (80%) is obtained with electron transfer dissociation of the same high charge-state ion of cytochrome c formed by electrothermal supercharging from native solutions and from denaturing solutions. Electrothermal supercharging should be advantageous for combining structural studies of proteins in native environments with mass spectrometers that have limited high m/z capabilities and for significantly improving tandem mass spectrometry performance for protein ions formed from solutions in which the molecules have native structures and activities.
Introduction
Mass spectrometry (MS) is an important tool in structural biology owing to its high sensitivity, speed, and information content obtainable from complex samples. Accurate measurements of molecular mass require ionization of intact molecules, which can readily be achieved with many methods, such as the commonly used matrix-assisted laser desorption/ionization1,2 and electrospray ionization (ESI).3,4 Protein identification and determinations of posttranslational modifications can be accomplished with various forms of tandem mass spectrometry.5,6 For these measurements, the highly charged ions formed by electrospray ionization are advantageous owing to their ability to be more readily dissociated7–10 and also more efficiently detected in instruments, such as orbitrap and Fourier transform ion cyclotron resonance (FT/ICR) mass spectrometers, where charge-sensitive detection is used. Solutions in which the protein is denatured are commonly used for these measurements to increase the charge states formed by ESI and to enhance stable ion formation at high solution flow rates. However, information about protein activity, conformation, protein-protein binding, complex assembly and stoichiometry is lost in solutions where proteins do not have native structures.
The advent of nanoelectrospray has greatly increased the application of “native” mass spectrometry11,12 by enhancing stable ion formation from buffered aqueous solutions in which proteins have native structures, even from solutions where high levels of essential salts that can adversely affect electrospray performance are required.13 Experiments aimed at encoding information about protein conformation, protein-substrate binding sites, allosteric regulation, and biomolecular complex structure can be done in solutions in which the protein or biomolecule complex has native structure, and mass spectrometry can be used to read out the encoded information. For example, footprinting strategies, such as oxidative footprinting,14–16 hydrogen/deuterium exchange (HDX),16–20 or limited proteolysis21–23 can be used to study changes in protein structure in solution. Non-native conditions are often subsequently used to produce the high charge states that are advantageous in order to read out the encoded information with MS or tandem MS. If sufficiently high charge states are produced from solutions in which the protein has native structure and activity, tandem MS analysis can be done directly via top-down fragmentation.19,24
Charge states formed in ESI depend on a number of factors, including solvent surface tension,25,26 relative gas-phase basicities of sample solution components,26–29 protein conformation,30–32 and various instrument parameters.33,34 One method to increase charging in native mass spectrometry is with supercharging reagents.19,35–45 When added to solutions at low concentrations, these supercharging reagents do not affect protein structure,19,43,45 but can cause chemical and/or thermal destabilization of the protein in the ESI droplet as the concentration of these low vapor pressure reagents increases. Destabilization can lead to a change in the protein structure, including partial unfolding or denaturation, which leads to higher charging,19,41–45 although other effects, such as changes in surface tension, also play a role.42 The higher charge-state ions produced by supercharging from native solutions often have larger collisional cross sections measured by ion mobility,19,39,43 but this is not always the case.39,44 For concanavalin A tetramer, there is a threshold of enhanced charging below which there is not an increase in cross sections, but above which cross sections increase significantly.44 For the SAP (serum amyloid P) 5-mer, no increase in cross section was observed with supercharging, which led the authors to conclude that no significant structural changes occurred with supercharging.39 However, differences in ion conformation are not always reflected by a difference in collision cross section.46,47 For example, different conformers of ubiquitin that have the same charge state and collision cross section can have distinctly different rates of HDX.46,47 Protein ions that are initially more elongated can also fold to more compact conformations in the gas phase.7,48,49
Although higher charge states are normally associated with more unfolded structures, recent results with the octameric form of the Bacillus anthracis toxin complex showed that higher charge states are formed when a specific pH induced conformational change takes place in solution, which makes the rotationally averaged size of the complex more compact both in solution and in the gas phase.44 An extended β-barrel domain in the structure that results in a lower rotationally averaged size of the entire complex appears to be able to accommodate more charge.
High charge-state ions can also be produced from unbuffered aqueous solutions by introducing acid or base vapor into drying gas used in an ESI interface.50–52 Protein denaturation that can occur when the pH of the ESI droplet changes can result in the formation of high charge-state ions. The use of supercharging reagents has the advantage that they are effective even when heavily buffered solutions that are often necessary to preserve native structure and function are used.19,41–45
A new electrothermal supercharging method to produce high charge-state ions from buffered aqueous solution was recently introduced.53 In this method, typical low charge-state distributions of proteins and intact complexes in buffered aqueous solutions are obtained using low ESI spray potentials, but high charge-state ions are produced from the same solutions simply by increasing the ESI spray potential. The charge-state distributions obtained with the electrothermal supercharging method can resemble those obtained from standard denaturing solutions even though the protein has a native folded structure in the original ESI solution prior to droplet formation. These results indicate that the increased charging is due to protein unfolding that occurs in the ESI droplet as a result of activation that occurs at high electric field.53
Here, the effectiveness of electrothermal supercharging at producing high charge-state ions from native solutions is compared to charging obtained from denaturing solutions for a variety of proteins. This method can overcome instrumental m/z limits in native mass spectrometry and can be used to greatly increase the sequence information obtainable from tandem MS of large protein ions formed from solutions in which the protein has native structure and activity.
Experimental
All proteins were purchased as lyophilized solids from Sigma (St. Louis, MO, USA) with the exception of truncated edema factor (EFN) and full length protective antigen (PA) proteins, which were a kind gift of Professor Bryan Krantz of the University of California, Berkeley, and were prepared as previously published,54 and barstar, which was expressed in E.coli and purified as described previously.55 Solutions consisting of 10 μM protein in either “native” conditions (aqueous 100 mM ammonium bicarbonate, pH 7) or denaturing conditions (48/48/4 by volume methanol/water/acetic acid or methanol/water/ammonium hydroxide for positive and negative ions, respectively) were prepared. About 5 μL of each solution was loaded into a borosilicate capillary (1.0 mm o.d./0.78 mm i.d., Sutter Instruments, Novato, CA, USA) pulled to a tip i.d. of ~1 μm with a Flaming/Brown micropipette puller (Model P-87, Sutter Instruments, Novato, CA, USA). Nanospray was initiated by applying about ±1 kV to a platinum wire (0.127 mm diameter, Sigma, St. Louis, MO, USA) inserted into the sample solution with the nanospray emitter positioned ~3 mm from the entrance to the mass spectrometer. To obtain native mass spectra, the spray potential was about ±0.7 kV. For electrothermal supercharging, the spray potential was increased to achieve the maximum extent of electrothermal supercharging while maintaining stable ion formation. A spray potential of ±0.8 kV was used for all denaturing solutions.
Mass spectra were acquired in triplicate with three different ESI emitters per sample using a Thermo Linear Trap Quadrupole (LTQ™) (Thermo Fischer Scientific, Waltham, MA, USA) with a heated entrance capillary temperature of 275 °C. The glass windows on this instrument’s source assembly were removed for all experiments to maintain a low temperature in the air space around the nanoESI emitters to prevent protein unfolding in the emitter prior to electrospray. Mass spectra of bovine serum albumin and a mixture of PA and the dendrimer DAB-Am-16 were obtained on this same instrument, but with an entrance capillary temperature of 220 °C and 275 °C, respectively. Mass spectra from the latter samples were also acquired using a Waters Quadrupole-Time-of-Flight (Q-TOF) Premier™ (Waters, Milford, MA, USA). Electron transfer dissociation (ETD) experiments were done using a Thermo LTQ-Orbitrap™ (Thermo Fischer Scientific, Waltham, MA, USA) with a 20 ms reaction time with fluoranthene anion, which depleted >80% of the precursor ion abundance.
Results and Discussion
Electrothermal supercharging of positive ions
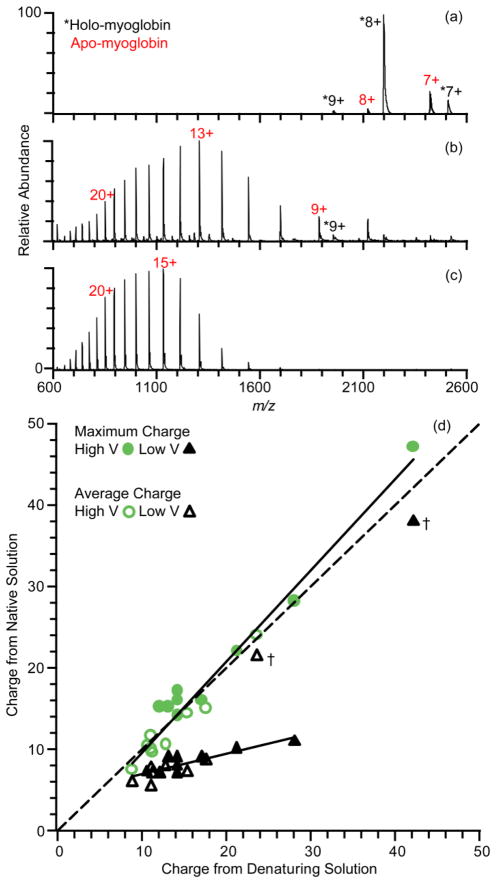
The extent to which electrothermal supercharging can increase the charge states of protein ions formed by nanoESI from native solutions (100 mM aqueous ammonium bicarbonate, pH 7.0) is illustrated for the protein myoglobin in Figure 1. An ESI mass spectrum obtained at a spray potential of +0.7 kV and a heated entrance capillary temperature of 275 °C is shown in Figure 1a. There are charge-state distributions corresponding to both holo- and apo-myoglobin that are centered around 8+ under these conditions, with an average charge of 8.1 ± 0.1+ and 7.6 ± 0.1+, respectively. Narrow, low charge-state distributions such as these are typically observed for protein ions formed from buffered aqueous solutions in which the protein has native or native-like structure.30 Low charge states of apo-myoglobin (23% of the total ion abundance) indicates that there is some activation of the protein from the relatively high temperature source conditions that results in a slight conformational change of the protein with loss of the non-covalently bound heme group. In solution, the heme binding pocket remains until extensive unfolding of the protein occurs.56 The absence of high charge state apo-myoglobin ions suggests that loss of the heme occurs as a result of activation in the gas phase.
Figure 1.
Positive ion nanoelectrospray mass spectra of 10 μM myoglobin in 100 mM ammonium bicarbonate, pH 7, at electrospray potentials of (a) +0.70 kV (native) and (b) +1.4 kV (electrothermal supercharging), and in (c) methanol/water/acetic acid (48/48/4, v/v) denaturing solution at a spray potential of +0.80 kV. (d) Correlation plot of the maximum and average charge state for nine proteins obtained from denaturing solution and from native and electrothermal supercharging from buffered aqueous solution. Data marked by “†” are discussed in the text.
With the spray potential at +1.4 kV and all other parameters the same, apo-myoglobin ions are predominantly formed (94% of the total ion abundance) with a charge-state distribution that is significantly broader and shifted to higher charge (Figure 1b). Charge states up to 28+ are formed, with 13+ the most abundant. The overall average charge of both forms of myoglobin is 14.8 ± 0.5+, which is nearly twice that obtained at the lower spray potential. By comparison, the charge-state distribution of myoglobin formed from a typical denaturing solution (48/48/4 by volume water/methanol/acetic acid; +0.8 kV spray potential) under the same conditions is monomodal, and corresponds to just apo-myoglobin with an average charge of 17.46 ± 0.02+. Although the relative abundances of the higher charge states are somewhat greater from the denaturing solution (Figure 1c), the maximum charge state of 28+ formed from the denaturing solution is the same as that obtained by electrothermal supercharging from the native solution (Figure 1b). In contrast to results from the native solution, increasing the spray potential of the denaturing solution from +0.8 kV to +1.4 kV results in only a minimal increase in the average charge (17.5+ to 18.1+).
The presence of apo-myoglobin ions with high charge and holo-myoglobin with low charge obtained by electrothermal supercharging under these conditions indicates the presence of populations of both folded (low charge-state distribution) and unfolded (high charge-state distribution) proteins molecules in the ESI droplet prior to ion formation. The temperature of the tip of the ESI capillary is less than 40 °C with these source conditions.53 Thermal unfolding of myglobin at neutral pH occurs around 76.5 °C (in 100 mM sodium phosphate buffer, pH 7.0),57 so there should be an insignificant population of unfolded myoglobin in the original solution prior to droplet formation. Electrothermal supercharging also occurs for ubiquitin and cytochrome c, which also have significantly higher thermal melting temperatures than the temperature of the nanoESI tip.53 These results are consistent with protein unfolding occurring in the ESI droplet prior to ion formation under these electrothermal supercharging conditions.53
A comparison between the charging obtained with electrothermal supercharging of protein ions formed from native solutions and charging obtained for the same protein ion formed from denaturing solutions for nine different proteins ranging in size from 8.6 kDa to 30.0 kDa is given in Table 1 and plotted in Figure 1d. The dashed line corresponds to a direct correlation between maximum charge or average charge of ions formed from denaturing solutions (+0.8 kV spray potential) and native solutions at either the highest nanoESI potential (high V, electrothermal supercharging), or the lowest potential (low V, native MS) at which stable electrospray could be maintained. The maximum and average charge with native MS is generally much lower than that obtained from denaturing solution (on average 41% and 36% lower than from denaturing solution, respectively), and there is a greater difference with increasing protein molecular weight, consistent with previous reports.58,59 In striking contrast, the maximum charge state for eight of the nine protein ions formed with electrothermal supercharging is equal to or higher than that obtained for the same protein ions formed from denaturing solution. The average charge with electrothermal supercharging is only slightly lower than that obtained from the denaturing solution owing to the broader distribution of charges formed as a result of contributions from both unfolded and folded structures. Electrothermal supercharging also produces high charge-state ions comparable to denaturing conditions for proteins that have multiple disulfide bonds, such as phospholipase A2 and lysozyme, which have six and four disulfide bonds, respectively.
Table 1.
Charge states and average charge of positive and negative ions formed in nanoESI by electrothermal supercharging from native solution and from denaturing solution.
| Protein | MW (kDa) | Charge States (+) | Average Charge (+) | Charge States (−) | Average Charge (−) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Supercharged | Denatured | Supercharged | Denatured | Supercharged | Denatured | Supercharged | Denatured | ||
| Ubiquitin | 8.6 | 17 – 4+ | 14 – 4+ | 9.8 ± 0.1 | 11.0 ± 0.1 | 9 – 3− | 11 – 3− | 3.99 ± 0.03 | 6.0 ± 0.2 |
|
| |||||||||
| Barstar | 10.2 | 15 – 5+ | 12 – 5+ | 7.5 ± 0.3 | 8.8 ± 0.1 | 13 – 3− | 13 – 4− | 7.9 ± 0.3 | 9.20 ± 0.04 |
|
| |||||||||
| Cytochrome c | 12.3 | 22 – 6+ | 21 – 7+ | 14.4 ± 0.1 | 15.2 ± 0.1 | 17 – 4− | 14 – 4− | 6.6 ± 0.1 | 9.46 ± 0.02 |
|
| |||||||||
| RNase A | 13.7 | 14 – 5+ | 14 – 7+ | 9.6 ± 0.1 | 11.2 ± 0.1 | 6 – 4− | 11 – 4− | 4.98 ± 0.01 | 7.0 ± 0.1 |
|
| |||||||||
| α-Lactalbumin | 14.2 | 15 – 5+ | 13 – 6+ | 10.26 ± 0.04 | 10.38 ± 0.04 | 11 – 4− | 13 – 4− | 5.4 ± 0.1 | 8.2 ± 0.1 |
|
| |||||||||
| Lysozyme | 14.7 | 16 – 6+ | 14 – 7+ | 11.5 ± 0.5 | 10.93 ± 0.04 | 6 – 4− | 7 – 4− | 4.94 ± 0.04 | 5.47 ± 0.03 |
|
| |||||||||
| Phospholipase A2 | 15.2 | 16 – 7+ | 17 – 9+ | 10.4 ± 0.3 | 12.6 ± 0.1 | 6 – 5− | 7 – 5− | 5.4 ± 0.1 | 5.73 ± 0.03 |
|
| |||||||||
| Myoglobin | 17.6 | 28 – 7+ | 28 – 8+ | 14.9 ± 0.5 | 17.46 ± 0.02 | 20 – 5− | 20 – 5− | 12.3 ± 0.7 | 13.1 ± 0.2 |
|
| |||||||||
| EFN | 30.0 | 47 – 10+ | 42 – 10+ | 24.0 ± 0.5 | 23.5 ± 0.4 | 37 – 8− | 35 – 8− | 21.0 ± 0.2 | 22.9 ± 0.2 |
Unusually high charge states of EFN, a truncation mutant of anthrax edema factor, are formed under native (low V) conditions (marked by † in Figure 1d; spectra shown in Figure S-1a). The maximum and average charge are 38+ and 21.3 ± 1.0+, respectively, values which are very similar to those from denaturing conditions (42+ and 23.5 ± 0.4+, respectively) and from electrothermal supercharging conditions (47+ and 24.0 ± 0.5+, respectively). Even under very gentle conditions used with a Waters Q-TOF mass spectrometer, a bimodal charge-state distribution is observed (Figure S-1b; 80 °C inlet temperature), but a low charge-state distribution centered around 10+ predominates. A nearly identical spectrum is obtained with this instrument when 100 mM ammonium acetate, pH 7, is used as the native buffer solution (Supplementary Figure 1c). These data suggest that both folded and unfolded forms of EFN may be present in solution. The molten globule state of this truncation mutant EFN is only 1 kcal/mol higher in Gibbs free energy than the native form.60 Edema factor is an enzyme translocated by anthrax toxin into the cytosol of a host’s cell via a β-barrel channel the toxin forms through the cell membrane.61 This β-barrel is too narrow for folded EFN to pass, so EFN must unfold in order to enter the cell.60,62 The ease with which this protein unfolds may account for the high charge-state distribution formed in native MS.
Electrothermal supercharging of negative ions
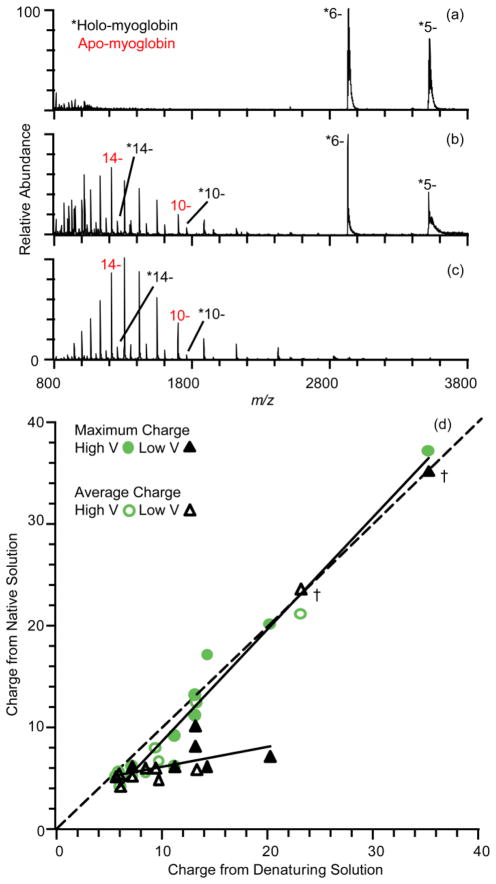
Results for the same nine proteins formed as anions under the same experimental conditions as the cations (Figure 1) are shown in Figure 2. With a spray potential of −0.7 kV, only holo-myoglobin ions are formed with an average charge of 5.7 ± 0.1−. The absence of apo-myoglobin under the same conditions as the positive ion data (Figure 1a) suggests that less activation occurs with the anions. This also appears to be the case at high spray potential (−1.4 kV) (Figure 2b), where there is a bimodal distribution with both a holo-myoglobin distribution centered around the 6− charge state and a largely apo-myoglobin distribution centered around the 14− charge state. 57 ± 5% of the total ion abundance consists of apo-myoglobin, significantly less than the 93 ± 1% under the same electrothermal supercharging conditions with positive ions (Figure 1b). Even with denaturing conditions of 48/48/4 water/methanol/ammonium hydroxide (−0.8 kV spray potential), 13 ± 2% of the ion signal corresponds to holo-myoglobin (Figure 2c). The maximum and average charge of holo-myoglobin under electrothermal supercharging are 18− and 9.9 ± 1.7−, respectively, and these values for apo-myoglobin are 20− and 14.1 ± 0.2− (Figure 2b). Electrothermal supercharging and denaturing conditions both result in a maximum charge of 20−, but electrothermal supercharging results in an average charge of 12.3 ± 0.7−, which is slightly lower than that obtained from denaturing solution (13.1 ± 0.2−) as a result of the significant population of holo-myoglobin with low charge.
Figure 2.
Negative ion nanoelectrospray mass spectra of 10 μM myoglobin in 100 mM ammonium bicarbonate, pH 7, at electrospray potentials of (a) −0.70 kV (native) and (b) −1.4 kV (electrothermal supercharging), and in (c) methanol/water/ammonium hydroxide (48/48/4, v/v) denaturing solution at a spray potential of −0.80 kV. (d) Correlation plot of the maximum and average charge state for nine proteins obtained from denaturing solution and from native and eletrothermal supercharging from buffered aqueous solution. Data marked by “†” are discussed in the text.
The maximum charge state and average charge from electrothermal supercharging for all nine proteins are compared to those from denaturing solution in Figure 2d. At low electrospray potentials, the maximum and average charge are much lower than those formed from denaturing solution (on average 34% and 27% lower than from denaturing solution, respectively), except for phospholipase A2, lysozyme and EFN. Lysozyme has a maximum and average charge of 7− and 5.5 ± 0.1− under denaturing conditions, which is not much higher than the values of 6− and 4.94 ± 0.04− obtained under native conditions, consistent with previous reports of low charge states centered around 6− for unreduced lysozyme formed from solutions in which the protein is non-native.63 The average charge of EFN under native conditions (23.4 ± 0.6−) is about equal to that from denaturing solutions (22.9 ± 0.2−) (Figure S-2a). As was the case for EFN cations, there is a distribution of high charge anions indicative of more unfolded conformers of the protein even when very soft source conditions in a Q-TOF mass spectrometer are used (Figure S-2b).
The increase in average charge of ions from electrothermal supercharging compared to ions from native MS is 78% of that from denaturing solution for positive ions, and 40% for negative ions. Similarly, the fraction of unfolded ions (the abundances of high charge distributions divided by the total protein ion abundance) from electrothermal supercharging is 81% of that from denaturing solution for positive ions and 33% for negative ions. The overall effectiveness of electrothermal supercharging at producing high charge states and unfolding proteins during ESI is lower for negative ions than for positive ions for eight of the nine proteins under the conditions used. There is no apparent trend with protein pI (pI = 4.5–11), indicating that electrothermal supercharging is more effective for positive than negative ions for acidic and basic proteins alike. The net negative charge in native MS is lower than the net positive charge for all proteins except barstar, for which the charging is nearly the same. Only for this protein is electrothermal supercharging slightly more effective for negative compared to positive ions. This suggests that the difference in efficiency of electrothermal supercharging for positive versus negative ions may be due to the difference in the charge on the protein. Lower charge state ions have less Coulombic repulsion and may require more energetic conditions to cause unfolding. The effectiveness of electrothermal supercharging of positive ions has been shown previously to depend on the source capillary temperature.53 To determine if supercharging of negative ions may be increased at higher source capillary temperatures, spectra for cytochrome c were obtained at source capillary temperatures ranging from 150 °C to 300 °C with spray potentials between ±0.8 kV and ±1.6 kV (Figure S-3). No electrothermal supercharging is observed for anions at either 150 °C or 175 °C, but the extent of electrothermal supercharging increases significantly between 200 °C and 300 °C, where ~58% of the ions are unfolded (average charge 8.1 ± 1.6−) at the highest temperature and spray potential. This value is still significantly less than for positive ions, where ~84% of cytochrome c ions are unfolded (average charge 13.8 ± 0.5+) at the same source temperature, but it shows that the effectiveness of electrothermal supercharging can be optimized for anions, as it can for cations, by adjusting source temperatures. In contrast, the source capillary temperature has little effect on the average charge state of ions formed from denaturing solution.
Instrument m/z Limits and Native MS
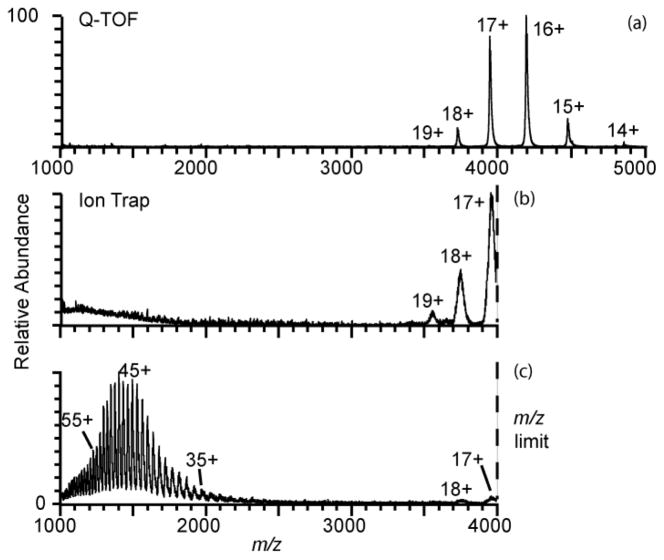
Charging of a protein or protein complex in native mass spectrometry generally increases as R3/2, where R is the radius of the protein approximated as a sphere, or as M1/2, where M is the protein molecular weight.58,59,64–66 Thus, instruments with high m/z capabilities are typically used for measuring mass spectra of large proteins and protein complexes from buffered aqueous solutions in which these molecules have native structures. For example, an ESI mass spectrum of bovine serum albumin (583 residues, 67 kDa; aqueous 100 mM ammonium bicarbonate, pH 7) acquired using a Q-TOF mass spectrometer is shown in Figure 3a. Molecular ions with charges states ranging from 14+ to 19+ are formed. The 16+ at m/z = 4176 is the most abundant ion and the average charge is 16.4+. A mass spectrum acquired with this same solution using a Thermo LTQ mass spectrometer (+0.8 kV spray potential) has similar relative abundances of the 17+ to 19+ charge states, but the most abundant ion measured with the Q-TOF (16+) is outside the m/z range (≤ 4000) of this LTQ instrument (Figure 3 b). With electrothermal supercharging with the LTQ mass spectrometer (+1.6 kV spray potential, all other conditions identical), predominantly high charge-state ions ranging from 29+ to 65+ are formed, and the average charge is 48+. There is a low abundance of 17+ and 18+ charge states, indicating that some fraction of the population remains folded under these conditions. Electrothermal supercharging shifts the charge-state distribution from the upper edge of the m/z range of the LTQ instrument to one that is almost entirely within the range of this instrument even though the protein is folded in the original solution from which these ions are formed.
Figure 3.
Positive ion nanoelectrospray mass spectra of 10 μM bovine serum albumin (67 kDa) in 100 mM ammonium bicarbonate, pH 7, obtained (a) using a Q-TOF mass spectrometer with high m/z capabilities, and using a Thermo LTQ™ mass spectrometer with an upper m/z limit of 4,000 at (b) +0.80 kV (native) and (c) +1.6 kV (electrothermal supercharging) spray potentials.
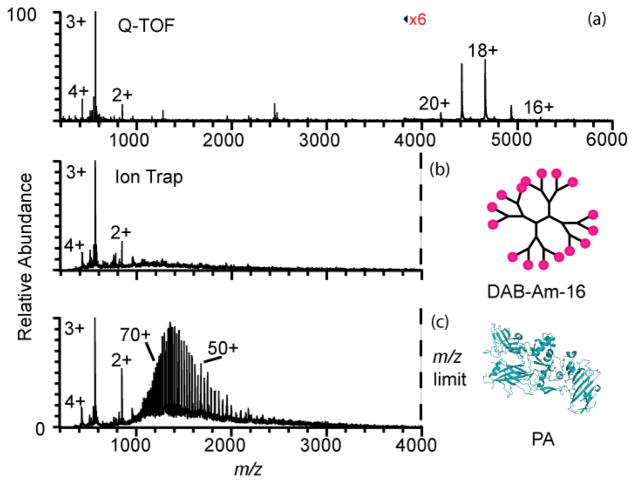
The charge states of molecular ions of even larger proteins formed in native MS can be entirely outside the m/z range of many types of mass spectrometers. A mass spectrum of an equimolar mixture of the 83 kDa protective antigen (PA) protein from Bacillus anthracis and a 1.7 kDa dendrimer poly(propyleneimine) hexadecaamine generation 3.0, DAB-Am-16, measured using a Q-TOF mass spectrometer is shown in Figure 4a. PA ions with charge states ranging from 15+ to 20+ are formed with an average charge state of 18.3+. DAB-Am-16 2+ to 4+ ions are formed with an average charge of 3.0+. Even the most highly charged molecular ion of PA (20+) formed from this buffered aqueous solution has a m/z = 4192 that is outside the range of the LTQ instrument. No PA ions are observed in a mass spectrum obtained from this same solution using a LTQ instrument (Figure 4b) under native conditions (+0.8 kV spray potential). The observation of a charge-state distribution for DAB-Am-16 with an average charge state of 2.9+ (Figure 4b) indicates that that absence of PA ions in this spectrum is due to formation of molecular ion charge states that are outside the m/z range of the mass spectrometer rather than an inability to form ions from this solution under these conditions. The data in Figure 4a show that the presence of the dendrimer does not suppress PA ion formation from this solution. With electrothermal supercharging with the LTQ instrument (+1.6 kV spray potential, all other conditions the same as those used for Figure 4b), a charge-state distribution of PA ions ranging from 36+ to 86+ is observed with an average charge of 60.2+. Thus, electrothermal supercharging produces more than a 3-fold gain in the number of charges for this protein. The charge-state distribution with electrothermal supercharging is centered around m/z = 1400, well within the m/z range of the mass spectrometer, despite these ions being formed from a buffered aqueous solution in which the structure of the protein was originally native prior to ESI droplet formation.
Figure 4.
Positive ion nanoelectrospray mass spectra of a mixture of 10 μM protective antigen (83 kDa) (PDB ID: 1ACC)73 and 10 μM DAB-Am-16 dendrimer (1.7 kDa) in 100 mM ammonium bicarbonate, pH 7, obtained (a) using a Q-TOF mass spectrometer with high m/z capabilities, and using a Thermo LTQ™ with an upper m/z limit of 4,000 at (b) +0.80 kV (native) and (c) +1.6 kV (electrothermal supercharging) spray potentials.
Interestingly, the charge-state distribution for DAB-Am-16 is slightly higher under native conditions (Figure 4b) than under electrothermal supercharging conditions (Figure 4c), with an average charge of 2.9+ and 2.8+, respectively. A similar result was observed for a mixture of ubiquitin and DAB-Am-16 (100 mM aqueous ammonium bicarbonate, data not shown) where a change in the spray potential from 0.7 kV to 1.7 kV resulted in a 30% increase in the average charge of ubiquitin compared to a 13% decrease in the average charge of DAB-Am-16. DAB-Am-16 has 16 primary amine termini that can potentially be protonated in these experiments, and 5+ ions can be readily formed under some instrumental conditions from these ammonium bicarbonate solutions. In contrast to both ubiquitin and PA, DAB-Am-16 cannot undergo large structural changes, i.e., DAB-Am-16 maintains a compact, spherical structure without significant changes in solvent exposed surface area in aqueous and organic solvents67 and cannot unfold into a more extended structure like these globular proteins can do when denatured. Thus, the absence of a charge enhancement for DAB-Am-16 with electrothermal supercharging is consistent with the inability to cause unfolding of this molecule in the ESI droplet as opposed to the inability for this molecule to be able to carry additional charge. The slightly lower charge on DAB-Am-16 obtained with electrothermal supercharging is consistent with the higher spray potential causing more activation to the ions prior to their reaching the inlet capillary. Energetic collisions can drive endothermic proton transfer reactions68 between multiply protonated DAB-Am-16 and water, which is the solvent in these experiments, and result in a lowering of the net charge state of DAB-Am-16.
Improving Dissociation of Ions Formed from Native MS
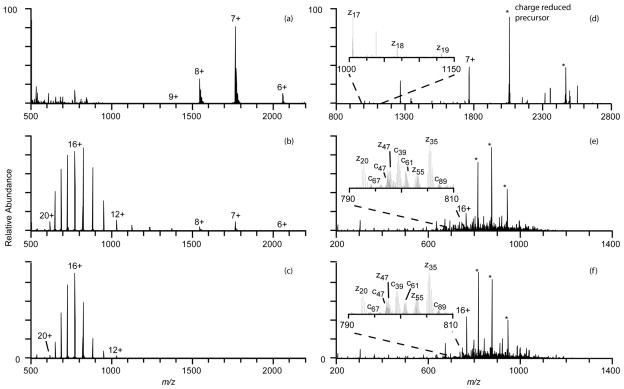
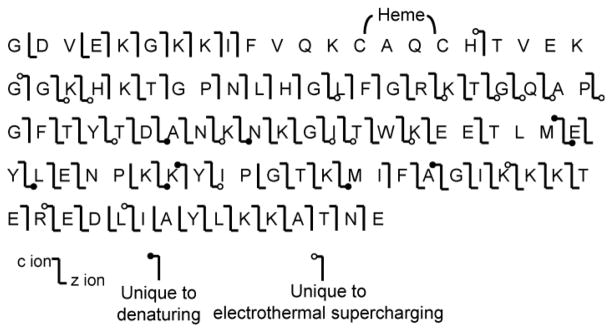
Dissociation of low charge states of protein ions formed in native MS generally results in limited fragmentation and low sequence coverage compared to the high charge-state ions formed from denaturing solutions. For example, the most abundant cytochrome c ion formed from a native solution (+0.75 kV; Figure 5a) is 7+, less than half the number of charges as the most abundant ion formed for this protein from denaturing solutions (16+; Figure 5c). ETD (electron transfer dissociation) of the 7+ ion formed under native conditions results in mostly charge-reduced precursor ions (85% product ion abundance) and few sequence specific ions. Fragment ions z17 - z19 and z35 are formed, resulting in only 4% sequence coverage under these conditions. These results are consistent with previous ETD and ECD (electron capture dissociation) studies of low charge states of cytochrome c formed from native conditions that report little to no sequence coverage for charge states up to the 8+ and 9+ precursor ions without thermal or collisional activation prior to or subsequent to fragmentation by ETD or ECD.69–71 Reduced precursors are also formed in ETD of the 16+ ion formed from denaturing solution (11% product ion abundance), but extensive c and z fragment ions are also formed as a result of cleavages at 79 of the 99 possible backbone cleavage sites, excluding sites N-terminal to cytochrome c’s proline residues as possible cleavage sites, resulting in 80% sequence coverage. Abundant 16+ charge-state ions are also formed with electrothermal supercharging from the native solution (Figure 5b), and ETD of this ion also results in cleavage at 79 cleavage sites (80% sequence coverage) that are mostly the same as those from ETD of the 16+ ions from denaturing solution (Figure 6), suggesting the more open gas-phase structures or locations of the charges of the 16+ ions formed from these two different solutions are similar, but not identical.72 No fragmentation occurs between residues 12 and 22 as a result of covalent bonds to the heme moiety at residues 14 and 17, consistent with previous ECD results for this protein.69–71 These results show that essentially the same sequence coverage can be obtained from the high charge-state ions formed by electrothermal supercharging of proteins from native solutions as that obtained from the same high charge states formed from denaturing solutions.
Figure 5.
Positive ion nanoelectrospray mass spectra of cytochrome c formed from 100 mM ammonium bicarbonate, pH7, at (a) +0.75 kV spray potential (native) and (b) +1.3 kV spray potential (electrothermal supercharging), and from (c) a methanol/water/acetic acid solution (denaturing) at a spray potential of +0.80 kV. ETD of (d) the 7+ ion from native solution, (e) the 16+ ion from electrothermal supercharging from the same native solution, and (f) the 16+ ion from denaturing solution resulted in sequence coverages of 4%, 80%, and 80%, respectively.
Figure 6.
Sequence coverage map showing the cleavage sites resulting from ETD of the 16+ cytochrome c ion produced by nanoESI from native solution with electrothermal supercharging and by nanoESI from denaturing solution.
Conclusions
Electrothermal supercharging with ammonium bicarbonate can effectively produce high charge-state ions of proteins from buffered aqueous solutions in which the protein has a native or native-like structure. The extent of supercharging from these buffered aqueous solutions is comparable to that obtained from denaturing solutions in which no supercharging reagents are used. Electrothermal supercharging makes it possible to combine native MS of large molecules with instruments that have limited upper m/z limits for molecular weight measurements. This method also enables greatly increased sequence information from tandem MS of ions formed from native solutions. This method could be particularly advantageous for more routinely combining structural studies of proteins in native environments with MS. For example, this method could be used in continuous H/D exchange studies by replacing acid denaturation20 or conventional supercharging reagents19 to produce high charge-state ions for tandem MS determination of where solution-phase exchange has occurred. The short lifetime of the ESI droplet essentially eliminates the opportunity for back-exchange to occur in these experiments. Information about protein complex stoichiometries can be obtained from the mass of intact complexes measured at low spray potential, and from the same solutions, information about the molecular sequence can be obtained from tandem MS experiments of high charge-state ions formed by electrothermal supercharging simply by increasing the spray potential. The extent of electrothermal supercharging depends on the protein, and is more effective for positive ions than for negative ions for eight of the nine proteins investigated here. The effectiveness of electrothermal supercharging depends on a number of factors, including the source temperature, physical properties of the protein, and the nature of the buffer. It is possible that other buffers or salts could be identified that would improve the charge enhancement for negative ions.
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health (Grant No. R01GM096097) and the National Science Foundation (Graduate Research Fellowship for CAC; Grant No. DGE1106400) for financial support, and Professor Bryan A. Krantz and Dr. Geoffrey K. Feld for their generous donation of protective antigen and edema factor samples.
References
- 1.Berkenkamp S, Kirpekar F, Hillenkamp F. Science. 1998;281:260–262. doi: 10.1126/science.281.5374.260. [DOI] [PubMed] [Google Scholar]
- 2.Karas M, Krüger R. Chem Rev. 2003;103:427–440. doi: 10.1021/cr010376a. [DOI] [PubMed] [Google Scholar]
- 3.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 4.Kebarle P, Verkerk UH. Mass Spectrom Rev. 2009;28:898–917. doi: 10.1002/mas.20247. [DOI] [PubMed] [Google Scholar]
- 5.Aebersold R, Mann M. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 6.Siuti N, Kelleher NL. Nat Meth. 2007;4:817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breuker K, Oh HB, Horn DM, Cerda BA, McLafferty FW. J Am Chem Soc. 2002;124:6407–6420. doi: 10.1021/ja012267j. [DOI] [PubMed] [Google Scholar]
- 8.Iavarone AT, Williams ER. Anal Chem. 2003;75:4525–4533. doi: 10.1021/ac034144i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen JA, Brodbelt JS. J Am Soc Mass Spectrom. 2009;20:349–358. doi: 10.1016/j.jasms.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Kjeldsen F, Giessing AMB, Ingrell CR, Jensen ON. Anal Chem. 2007;79:9243–9252. doi: 10.1021/ac701700g. [DOI] [PubMed] [Google Scholar]
- 11.Heck AJR. Nat Meth. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 12.Benesch JLP, Robinson CV. Curr Opin Struct Biol. 2006;16:245–251. doi: 10.1016/j.sbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Sterling HJ, Batchelor JD, Wemmer DE, Williams ER. J Am Soc Mass Spectrom. 2010;21:1045–1049. doi: 10.1016/j.jasms.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gau BC, Sharp JS, Rempel DL, Gross ML. Anal Chem. 2009;81:6563–6571. doi: 10.1021/ac901054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smedley JG, Sharp JS, Kuhn JF, Tomer KB. Biochemistry. 2008;47:10694–10704. doi: 10.1021/bi800533t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Y, Piyadasa H, O’Neil JD, Konermann L. J Mol Biol. 2012;416:400–413. doi: 10.1016/j.jmb.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Abzalimov RR, Kaplan DA, Easterling ML, Kaltashov IA. J Am Soc Mass Spectrom. 2009;20:1514–1517. doi: 10.1016/j.jasms.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wales TE, Engen JR. Mass Spectrom Rev. 2006;25:158–170. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 19.Sterling HJ, Williams ER. Anal Chem. 2010;82:9050–9057. doi: 10.1021/ac101957x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan J, Han J, Borchers CH, Konermann L. J Am Chem Soc. 2008;130:11574–11575. doi: 10.1021/ja802871c. [DOI] [PubMed] [Google Scholar]
- 21.de Laureto PP, Tosatto L, Frare E, Marin O, Uversky VN, Fontana A. Biochemistry. 2006;45:11523–11531. doi: 10.1021/bi052614s. [DOI] [PubMed] [Google Scholar]
- 22.Monti MC, Riccio R, Casapullo A. Bioorg Chem. 2009;37:6–10. doi: 10.1016/j.bioorg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Sawaya MR, Eisenberg D. Nat Struct Mol Biol. 2011;18:49–55. doi: 10.1038/nsmb.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin S, Loo JA. Int J Mass Spectrom. 2011;300:118–122. doi: 10.1016/j.ijms.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iavarone AT, Williams ER. J Am Chem Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iavarone AT, Jurchen JC, Williams ER. J Am Soc Mass Spectrom. 2000;11:976–985. doi: 10.1016/S1044-0305(00)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirza UA, Chait BT. Anal Chem. 1994;66:2898–2904. doi: 10.1021/ac00090a017. [DOI] [PubMed] [Google Scholar]
- 28.Schnier PD, Gross DS, Williams ER. J Am Soc Mass Spectrom. 1995;6:1086–1097. doi: 10.1016/1044-0305(95)00532-3. [DOI] [PubMed] [Google Scholar]
- 29.Wang GD, Cole RB. Org Mass Spectrom. 1994;29:419–427. [Google Scholar]
- 30.Chowdhury SK, Katta V, Chait BT. J Am Chem Soc. 1990;112:9012–9013. [Google Scholar]
- 31.Konermann L, Douglas DJ. Biochemistry. 1997;36:12296–12302. doi: 10.1021/bi971266u. [DOI] [PubMed] [Google Scholar]
- 32.Frimpong AK, Abzalimov RR, Eyles SJ, Kaltashov IA. Anal Chem. 2007;79:4154–4161. doi: 10.1021/ac0704098. [DOI] [PubMed] [Google Scholar]
- 33.Thomson BA. J Am Soc Mass Spectrom. 1997;8:1053–1058. [Google Scholar]
- 34.Loo JA, Udseth HR, Smith RD, Futrell JH. Rapid Commun Mass Spectrom. 1988;2:207–210. [Google Scholar]
- 35.Lomeli SH, Yin S, Loo RRO, Loo JA. J Am Soc Mass Spectrom. 2009;20:593–596. doi: 10.1016/j.jasms.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lomeli SH, Peng IX, Yin S, Loo RRO, Loo JA. J Am Soc Mass Spectrom. 2010;21:127–131. doi: 10.1016/j.jasms.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan CJ, Loo RRO, Loo JA, de la Moraa JF. Phys Chem Chem Phys. 2010;12:13476–13483. doi: 10.1039/c0cp01208d. [DOI] [PubMed] [Google Scholar]
- 38.Erba EB, Ruotolo BT, Barsky D, Robinson CV. Anal Chem. 2010;82:9702–9710. doi: 10.1021/ac101778e. [DOI] [PubMed] [Google Scholar]
- 39.Hall Z, Robinson CV. J Am Soc Mass Spectrom. 2012;23:1161–1168. doi: 10.1007/s13361-012-0393-z. [DOI] [PubMed] [Google Scholar]
- 40.Enyenihi AA, Yang HQ, Ytterberg AJ, Lyutvinskiy Y, Zubarev RA. J Am Soc Mass Spectrom. 2011;22:1763–1770. doi: 10.1007/s13361-011-0182-0. [DOI] [PubMed] [Google Scholar]
- 41.Sterling HJ, Williams ER. J Am Soc Mass Spectrom. 2009;20:1933–1943. doi: 10.1016/j.jasms.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterling HJ, Cassou CA, Trnka MJ, Burlingame AL, Krantz BA, Williams ER. Phys Chem Chem Phys. 2011;13:18288–18296. doi: 10.1039/c1cp20277d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterling HJ, Daly MP, Feld GK, Thoren KL, Kintzer AF, Krantz BA, Williams ER. J Am Soc Mass Spectrom. 2010;21:1762–1774. doi: 10.1016/j.jasms.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterling HJ, Kintzer AF, Feld GK, Cassou CA, Krantz BA, Williams ER. J Am Soc Mass Spectrom. 2012;23:191–200. doi: 10.1007/s13361-011-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sterling HJ, Prell JS, Cassou CA, Williams ER. J Am Soc Mass Spectrom. 2011;22:1178–1186. doi: 10.1007/s13361-011-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson EW, Williams ER. J Am Soc Mass Spectrom. 2005;16:1427–1437. doi: 10.1016/j.jasms.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merenbloom SI. Ph D Thesis. Indiana University; Bloomington, IN: 2009. [Google Scholar]
- 48.Gross DS, Schnier PD, Rodriguez-Cruz SE, Fagerquist CK, Williams ER. Proc Natl Acad Sci U S A. 1996;93:3143–3148. doi: 10.1073/pnas.93.7.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badman ER, Myung S, Clemmer DE. J Am Soc Mass Spectrom. 2005;16:1493–1497. doi: 10.1016/j.jasms.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Kharlamova A, Prentice BM, Huang T-Y, McLuckey SA. Anal Chem. 2010;82:7422–7429. doi: 10.1021/ac101578q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kharlamova A, McLuckey SA. Anal Chem. 2010;83:431–437. doi: 10.1021/ac1027319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kharlamova A, DeMuth JC, McLuckey SA. J Am Soc Mass Spectrom. 2012;23:88–101. doi: 10.1007/s13361-011-0258-x. [DOI] [PubMed] [Google Scholar]
- 53.Sterling HJ, Cassou CA, Susa AC, Williams ER. Anal Chem. 2012;84:3795–3801. doi: 10.1021/ac300468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kintzer AF, Thoren KL, Sterling HJ, Dong KC, Feld GK, Tang II, Zhang TT, Williams ER, Berger JM, Krantz BA. J Mol Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnaswamy SR, Williams ER, Kirsch JF. Protein Sci. 2006;15:1465–1475. doi: 10.1110/ps.062083406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stocks BB, Konermann L. Anal Chem. 2008;81:20–27. doi: 10.1021/ac801888h. [DOI] [PubMed] [Google Scholar]
- 57.Wan LL, Twitchett MB, Eltis LD, Mauk AG, Smith M. Proc Natl Acad Sci U S A. 1998;95:12825–12831. doi: 10.1073/pnas.95.22.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de la Mora JF. Anal Chim Acta. 2000;406:93–104. [Google Scholar]
- 59.Loo JA, Udseth HR, Smith RD. Anal Biochem. 1989;179:404–412. doi: 10.1016/0003-2697(89)90153-x. [DOI] [PubMed] [Google Scholar]
- 60.Krantz BA, Trivedi AD, Cunningham K, Christensen KA, Collier RJ. J Mol Biol. 2004;344:739–756. doi: 10.1016/j.jmb.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 61.Krantz BA, Finkelstein A, Collier RJ. J Mol Biol. 2006;355:968–979. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Blaustein RO, Finkelstein A. J Gen Physiol. 1990;96:905–919. doi: 10.1085/jgp.96.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konermann L, Douglas DJ. J Am Soc Mass Spectrom. 1998;9:1248–1254. doi: 10.1016/S1044-0305(98)00103-2. [DOI] [PubMed] [Google Scholar]
- 64.Kaltashov IA, Mohimen A. Anal Chem. 2005;77:5370–5379. doi: 10.1021/ac050511+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heck AJR, van den Heuvel RHH. Mass Spectrom Rev. 2004;23:368–389. doi: 10.1002/mas.10081. [DOI] [PubMed] [Google Scholar]
- 66.Nesatyy VJ, Suter MJF. J Mass Spectrom. 2004;39:93–97. doi: 10.1002/jms.522. [DOI] [PubMed] [Google Scholar]
- 67.Scherrenberg R, Coussens B, van Vliet P, Edouard G, Brackman J, de Brabander E, Mortensen K. Macromolecules. 1998;31:456–461. [Google Scholar]
- 68.Williams ER. J Mass Spectrom. 1996;31:831–842. doi: 10.1002/(SICI)1096-9888(199608)31:8<831::AID-JMS392>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breuker K, McLafferty FW. Angew Chem Int Ed. 2005;44:4911–4914. doi: 10.1002/anie.200500668. [DOI] [PubMed] [Google Scholar]
- 70.Horn DM, Breuker K, Frank AJ, McLafferty FW. J Am Chem Soc. 2001;123:9792–9799. doi: 10.1021/ja003143u. [DOI] [PubMed] [Google Scholar]
- 71.Rožman M, Gaskell SJ. Rapid Commun Mass Spectrom. 2012;26:282–286. doi: 10.1002/rcm.5330. [DOI] [PubMed] [Google Scholar]
- 72.Robinson EW, Leib RD, Williams ER. J Am Soc Mass Spectrom. 2006;17:1470–1479. doi: 10.1016/j.jasms.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.