Approximately 50% of older hypertensive individuals have difficulties in executive function, the cognitive domain that controls complex tasks.1 Hypertensive individuals with executive dysfunction have a high rate of conversion to dementia.2 To date, no study has investigated therapeutic options for executive dysfunction. Recent evidence suggest that the renin angiotensin system plays a central role in linking hypertension to cognitive function offering new therapeutic options for cognitive protection.3 In the brain, angiotensin receptor blockers (ARB) block the type 1 but not type 2 whereas angiotensin converting enzyme inhibitors (ACEI) decrease activation of both receptors. Activating the type 2 receptor may provide cognitive protection.4 We therefore hypothesized that an ARB-based regimen would be superior to other antihypertensive regimens in cognitive protection, especially executive function, and conducted a 12-month double blind randomized clinical trial comparing candesartan, lisinopril, and HCTZ in hypertensives with early executive dysfunction.
Methods
The study design is fully described elsewhere.5 Subjects were recruited from the greater Boston area and were 60 years or older, have hypertension, and demonstrated evidence of executive dysfunction based on the executive clock draw test (CLOX1 <10). We excluded those with a Mini-Mental-State-Exam (MMSE)<20 or those with a clinical diagnosis of dementia, diabetes mellitus, stroke, or congestive heart failure. Antihypertensive medications were tapered using a standard protocol described elsewhere.5 Randomization using a computer generated random allocation sequence occurred after baseline data collection and participants were seen every 2 weeks until their blood pressure was controlled (<140/90 mm Hg). Participants were treated with escalating doses of lisinopril, candesartan, or HCTZ to achieve a blood pressure <140/90 mm Hg. Long acting nifedipine and long-acting metoprolol were added if goal blood pressure was not achieved. Cognitive assessments were repeated at 6 and 12 months and included Trail Making Test parts A and B (TMT), which assesses executive function; Hopkins Verbal Learning Test – Revised (HVLT), which assesses memory, and the Digit Span Test which assesses attention. Hebrew SeniorLife IRB approved the study a written informed consent was obtained. The study was registered in ClinicalTrials.gov (NCT00605072). An intention-to-treat analysis was performed and linear mixed models for repeated measures were used to compare the progression of cognitive outcomes in the three groups and least square means adjusted for age and baseline MMSE were computed for each visit by treatment group.
Results
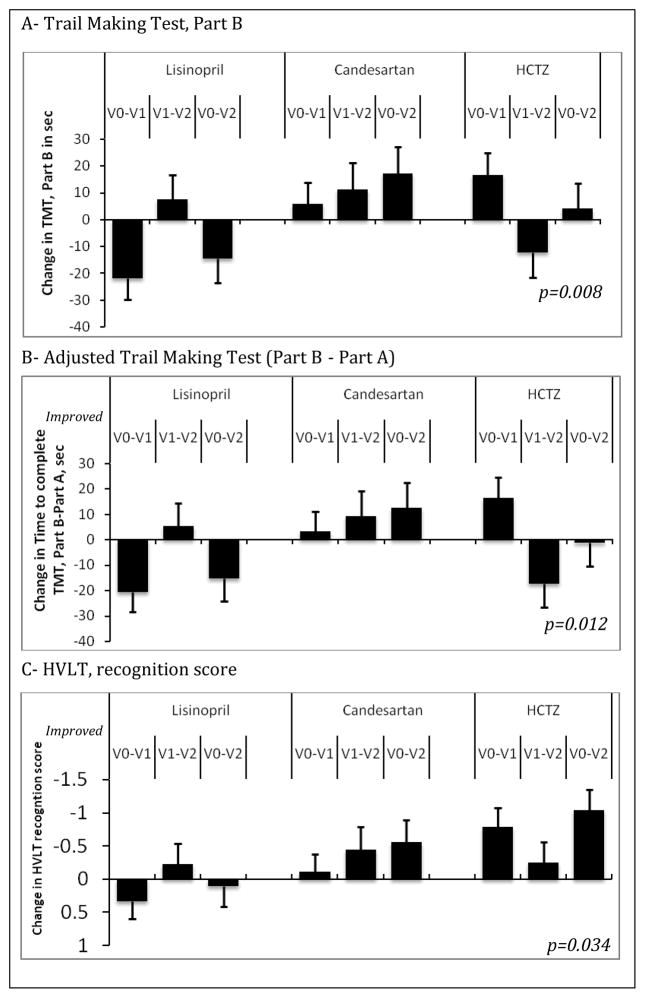
Of the 63 eligible individuals screened, 53 stopped their antihypertensives and were randomized to lisinopril (n=18), candesartan (n=20), and HCTZ (n=15), 47 completed 6 months and 31 completed 12 months. Sample description is provided in an online e-Table. Blood pressure control levels were equivalent (lisinopril 91%, candesartan 100% and HCTZ 100%, p=0.40) and systolic blood pressure reductions were equivalent in all three groups (lisinopril mean reduction ± standard error:28±5 mm Hg; candesartan:27±5 mm Hg, and HCTZ:21±5 mm Hg; p=0.75). There were no differences in the reported adverse events between the three groups. After adjusting for age and baseline MMSE, those randomized to candesartan demonstrated the greatest improvement in TMT-B (p=0.008), the adjusted TMT, B-A which adjusts the test for motor speed (p=0.012) and the recognition portion of the HVLT (p=0.034). Figure 1
Figure 1. Significant changes in the adjusted least square mean over study period in the three groups.
Least square means were adjusted for age and baseline Mini-Mental-State-Exam. p-values are obtained from the linear mixed model for the visit by group interaction parameter. V0–V1: change from baseline to 6 months; V0–V2: change from baseline to 12 months; V1–V2: change from 6 months to 12 months. TMT: Trail Making Test. HVLT: Hopkins verbal learning test. HCTZ: hydrochlorothiazide.
Discussion
This study suggests that ARBs are associated with improvement in executive function in hypertensive older adults with early executive cognitive impairment. To our knowledge, this is the first study to investigate the effect of antihypertensive therapy on executive function. Prior clinical trials that assessed cognitive outcomes of antihypertensives have excluded those with existing cognitive impairment and have used the MMSE which is not sensitive to the domains related to frontal lobe dysfunction manifesting as executive dysfunction. Our findings further support observational data showing that use of ARB was associated with lower risk of dementia and AD compared to ACEI or other antihypertensives.6 The mechanisms of the potential superior ARB cognitive effects may be related to restoring proper central endothelial function, decreasing inflammation, and preventing neuronal degeneration through the selective non-inhibition of the type 2 angiotensin receptors in the brain.4, 7, 8 If confirmed in a larger trial, ARBs may be the optimal antihypertensive treatment for elderly patients with hypertension and cognitive impairment. Future studies exploring the effects of ARB on cognitive impairment are needed.
Supplementary Material
Acknowledgments
Dr. Hajjar and the AVEC trial are supported by grant 1 K23 AG030057 from the National Institute on Aging. This work is also supported by P01-AG004390 and R37-AG025037 from the NIA to Dr. Lipsitz, the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife, and the generous donation Hinda Marcus to the Cardiovascular Research Laboratory at Hebrew SeniorLife and Harvard Medical School.
Contributor Information
Ihab Hajjar, Division of Geriatric, Hospital & General Internal Medicine, Department of Medicine, University of Southern California, Los Angeles, California.
Meaghan Hart, Institute for Aging Research, Hebrew Senior Life, Boston, Massachusetts.
Yu-Ling Chen, Alzheimer Disease Research Center and Department of Neurology, University of Southern California, Los Angeles, California.
Wendy Mack, Department of Biostatistics, University of Southern California, Los Angeles, California.
William Milberg, Department of Psychiatry, Harvard Medical School, Associate Director for Research, New England GRECC- Boston Division, VA Boston Healthcare, 150 S. Huntington Avenue, Boston, MA 02130.
Helena Chui, Department of Neurology, Raymond and Betty McCarron Chair in Neurology, Professor, University of Southern California, Los Angeles, California.
Lewis Lipsitz, Harvard School of Medicine, Co-Director, Institute for Aging Research, Hebrew SeniorLife, Chief of division of Gerontology, Beth Israel Deaconess Medical Centre, Boston, Massachusetts.
References
- 1.Grigsby J, Kaye K, Shetterly SM, Baxter J, Morgenstern NE, Hamman RF. Prevalence of disorders of executive cognitive functioning among the elderly: findings from the San Luis Valley Health and Aging Study. Neuroepidemiology. 2002 Sep-Oct;21(5):213–220. doi: 10.1159/000065638. [DOI] [PubMed] [Google Scholar]
- 2.Oveisgharan S, Hachinski V. Hypertension, executive dysfunction, and progression to dementia: the canadian study of health and aging. Archives of neurology. 2010 Feb;67(2):187–192. doi: 10.1001/archneurol.2009.312. [DOI] [PubMed] [Google Scholar]
- 3.Ciobica A, Bild W, Hritcu L, Haulica I. Brain renin-angiotensin system in cognitive function: pre-clinical findings and implications for prevention and treatment of dementia. Acta neurologica Belgica. 2009 Sep;109(3):171–180. [PubMed] [Google Scholar]
- 4.Horiuchi M, Mogi M, Iwai M. The angiotensin II type 2 receptor in the brain. Journal of the renin-angiotensin-aldosterone system: JRAAS. 2010 Mar;11(1):1–6. doi: 10.1177/1470320309347793. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar I, Hart M, Milberg W, Novak V, Lipsitz L. The rationale and design of the antihypertensives and vascular, endothelial, and cognitive function (AVEC) trial in elderly hypertensives with early cognitive impairment: role of the renin angiotensin system inhibition. BMC geriatrics. 2009;9:48. doi: 10.1186/1471-2318-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang HG, Mahoney DF, Hoenig H, et al. In situ monitoring of health in older adults: technologies and issues. J Am Geriatr Soc. 2010 Aug;58(8):1579–1586. doi: 10.1111/j.1532-5415.2010.02959.x. [DOI] [PubMed] [Google Scholar]
- 7.Rompe F, Artuc M, Hallberg A, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010 Apr;55(4):924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 8.Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000 Jan;35(1 Pt 2):501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.