Abstract
Non-neoplastic tumor-like lesions in the pancreas are uncommon. Here, we present a case of multiple solid pancreatic hamartomas in a 78-year-old Japanese woman. Her computed tomography revealed a pancreatic mass, measuring 1.8 cm in maximum diameter. However, no symptoms were found. She was not an alcoholic and had no history of pancreatitis. The patient underwent a pancreatoduodenectomy, and three well-demarcated solid nodules measuring 1.7 cm, 0.4 cm, and 0.3 cm in diameter were found in the pancreatic head. Microscopically, the lesions were composed of non-neoplastic, disarranged acinar cells and ducts embedded in a sclerotic stroma with elongated spindle cells that lacked discrete islets. The stromal spindle cells were immunoreactive for CD34 and CD117. The histological diagnosis was multiple solid hamartomas of the pancreas. There has been no recurrence 30 mo after surgery. So far, 18 cases of pancreatic hamartoma have been reported in the English literature, including our case. Six out of these 18 cases seemed to fit the criteria of solid pancreatic hamartoma. Although the number of cases was limited, solid pancreatic hamartomas seem to be benign tumor-like lesions, which are found incidentally in healthy middle-aged adults, but occasionally involve the whole pancreas, resulting in a poor prognosis. Solid pancreatic hamartoma was sometimes associated with minor pancreatic abnormality, and multiple small lesions other than the main tumors were detected in a small number of cases. From these findings, one may speculate that solid pancreatic hamartoma could be the result of a malformation during the development of the pancreas.
Keywords: Pancreatic tumor, Hamartoma, Multiple, CD117, CD34
INTRODUCTION
Non-neoplastic tumor-like lesions in the pancreas are uncommon and include hamartoma[1-12]. Mass-forming pancreatitis which is associated with autoimmune pancreatitis, congenital arteriovenous malformation, intrapancreatic accessory spleen, and others[13,14]. Solid pancreatic hamartoma was first reported by Pauser et al[9] to be pancreatic tumors that had features in common with both hamartomas and gastrointestinal stromal tumors. Pancreatic hamartomas are divided into two subgroups: solid and cystic lesion and solid lesion[9]. Including the cases reported by Pauser et al[9] and Nagata et al[10], only three cases of solitary solid pancreatic hamartoma have been reported in the English literature, and the etiology of hamartoma remains unknown.
Here, we report on a patient with multiple solid pancreatic hamartomas. We also review the cases that were reported as pancreatic hamartomas to reclassify them into the two subgroups described above and summarize the clinicopathological features of solid pancreatic hamartoma.
CASE REPORT
A 78-year-old Japanese woman with no symptoms was found to have a pancreatic mass on computed tomography (CT) at her follow-up for a cyst of the pancreatic head, which had been detected by transabdominal sonography at her yearly health screening 2 years before admission. The mass was located at the tail side of the known cyst, and there was no connection between the two. She was referred to our hospital for further examination. She had a past history of uterine leiomyoma, which had been treated by hysterectomy 30 years earlier. She had been undergoing medical treatment for atrial fibrillation for 5 years. She did not smoke or consume alcohol. She had no history of pancreatitis. Physical examination found no abnormal signs, and laboratory data showed no elevation of tumor markers. Dynamic CT demonstrated a well-circumscribed nodule, measuring 1.8 cm × 1.2 cm, in the head of the pancreas, which showed weak enhancement in the portal venous phase (Figure 1). On magnetic resonance imaging, the nodule had a low signal on T1-weighted images (T1WI) and a high signal on T2WI. However, the pancreatic mass did not show any intense fluorine 18 fluorodeoxyglucose (FDG) by positron emission tomography (PET). The main pancreatic duct showed neither dilatation nor stenosis on endoscopic retrograde pancreatography. She underwent a pancreatoduodenectomy after the preoperative diagnosis of pancreatic cancer. The postoperative course was uneventful. She had no recurrence for 30 mo after surgery.
Figure 1.
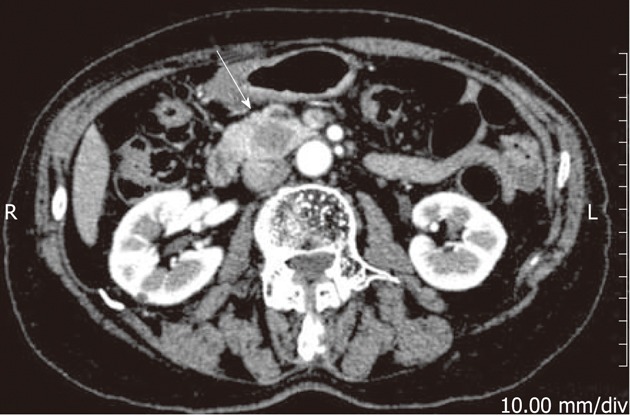
Dynamic computed tomography scan of the abdomen. An arterial-phase image shows a relatively well-circumscribed nodule, measuring 1.8 cm in the pancreatic head (white arrow). R: Right; L: Left.
Macroscopically, a firm, solid mass was noted in the pancreatic head, which was not exposed to the serosal surface of the pancreas. On a cut section, it was a well-demarcated, homogeneously white- to yellow-colored solid nodule, measuring 1.7 cm at the largest diameter (Figure 2A). In addition, two smaller nodules, measuring 0.4 cm and 0.3 cm, which were similar to the main lesion, were observed (Figure 2B). These small nodules were located in the pancreatic tail side of the main lesion. They were not in communication with the pancreatic duct.
Figure 2.
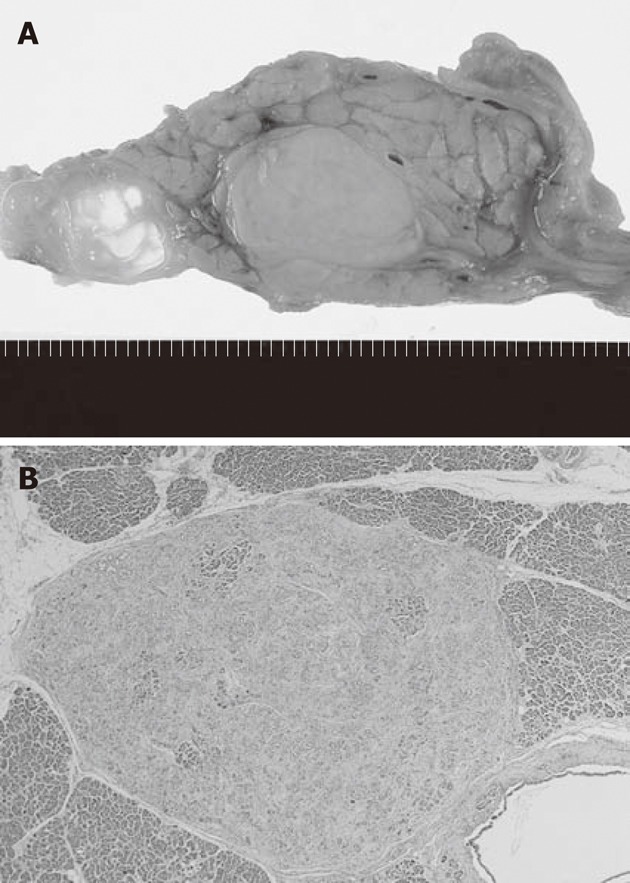
Macroscopic and loupe slide images of the tumors. A: On a cut section, the tumor is a well-demarcated, white- to yellow-colored solid nodule, measuring 1.7 cm; B: One of two small nodules measuring 0.3 cm is observed on the loupe slide (HE stain, 20x).
Microscopically, the lesions were composed of non-neoplastic acinar and ductal cells embedded in a hypocellular fibrous stroma with some amount of adipose tissue (Figure 3A). The acinar cells were well-differentiated, but the normal lobular structures were lost at the center of the mass. The ducts were mainly small and lined by cuboidal to columnar epithelium without atypia. These two components were embedded in a sclerotic, hypocellular stroma containing elongated spindle cells without nuclear atypia. Small numbers of lymphocytes and mast cells had infiltrated the stroma. Islets of Langerhans were not evident within the lesion. Immunohistochemically, both acinar cells and ductal cells were positive for epithelial markers (CAM5.2, AE1/AE3 and EMA), and the acinar cells were positive for exocrine markers (amylase and trypsin). The stromal spindle cells were positive for CD34 (Figure 3B) and CD117 but negative for S-100 protein, α-SMA, desmin, and bcl-2. Based on these findings, the histological diagnosis of multiple solid hamartomas of the pancreas was made.
Figure 3.
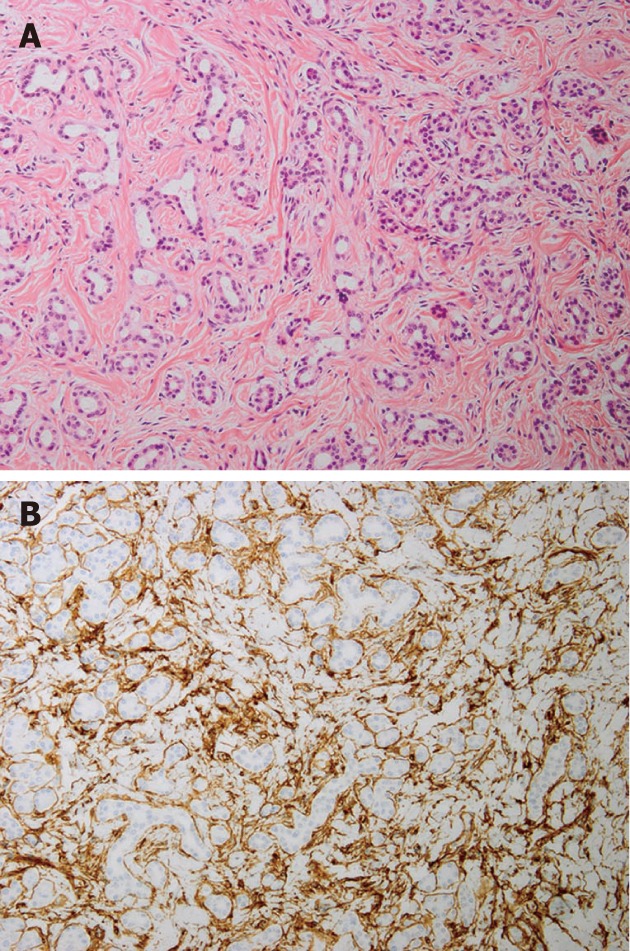
Microscopic images of the tumors. A: The lesion is composed of non-neoplastic acinar and ductal cells embedded in hypocellular fibrous stroma (HE stain, 100 x); B: Immunohistochemically, stromal spindle cells are positive for CD34 (100 x).
Pancreatic intraepithelial neoplasia 1B (PanIN-1B) was observed in the adjacent normal pancreatic tissue. The known cyst at the uncinate process of the pancreas was a retention cyst measuring 1.4 cm in the largest diameter histologically.
DISCUSSION
The term hamartoma refers to an excessive, focal overgrowth of cells and tissues native to the organ in which it occurs[15]. Each component consists of mature well-differentiated cells without atypia. Thus, hamartoma seems to be a malformation rather than a true neoplasm. To our knowledge, 17 cases of pancreatic hamartoma have been reported so far in the English literature, and the present case is the 18th[1-12]. In 2005, Pauser et al[9] divided pancreatic hamartomas into two subgroups: solid and cystic lesions and solid lesions, and the authors reported two cases of solid hamartoma of the pancreas as a cellular hamartoma resembling a gastrointestinal stromal tumor. They described the main features of the tumors as focally exocrine and endocrine tissue elements with stromal cells with coexpression of CD34, CD117, and bcl-2[9].
When we reviewed the cases reported as pancreatic hamartomas to reclassify them into two subgroups and identify the solid pancreatic hamartomas among them, we excluded the cases with evidence of chronic pancreatitis, according to the criteria of pancreatic hamartoma, because chronic pancreatitis with depletion of acinar cells in the fibrous stroma mimicked hamartomas lacking acinar cells in Pauser et al[9] and Nagata et al10]. Especially for multiple mass-forming lesions of the pancreas, we should consider the possible diagnosis of chronic alcoholic pancreatitis (CAP) and pancreatitis of unknown etiology more carefully than for solitary mass lesions because the fibrosis of CAP and pancreatitis of unknown etiology have been shown to be dense in the interlobular or perilobular areas, not uniformly distributed, and having multinodular cirrhosis-like appearances[16]. In the present case, minimal infiltration of lymphocytes and mast cells was noted in the sclerotic stroma. In addition, the islets of Langerhans, usually preserved in the case of chronic pancreatitis, were completely lost within the lesion. Neither squamous metaplasia of the ductal epithelium nor pancreatic lithiasis was observed. Only PanIN-1B was observed in the adjacent normal pancreatic tissue. The only other differential diagnosis of multiple mass-forming lesions of the pancreas is a patchy type of fibrosis of the pancreas in aged non-alcoholics[17] because the atrophic acini with fibrosis may mimic hamartoma. However, islets of Langerhans are also preserved in such cases.
Of the 17 cases of pancreatic hamartoma, 5 cases seemed to fit the criteria of solid pancreatic hamartoma[1,3,9,10]. In 1977, Anthony et al. reported three cases of pseudotumors of the pancreas. One of them, taken from a 46-year-old man, showed a well-defined ovoid mass, 1.6 cm in the largest diameter, consisting of lobulated connective tissue enclosing irregular, branching pancreatic ducts, acinar tissue, and islet cells in a disorderly arrangement without evidence of pancreatitis. In addition, a few tiny nodules of similar appearance in the other areas of the pancreas were also noted[1]. In 1983, Burt et al. reported a solid pancreatic hamartoma in a premature infant with hypoglycemia and hypocalcemia. Her entire pancreas consisted of ductal elements with a minority of islets and acinar components, which were surrounded by delicate bands of connective tissue fibers[3]. Three other cases of solid pancreatic hamartomas, reported by Pauser et al. in 2005 and by Nagata et al. in 2007, showed histologically well-circumscribed lesions composed of non-neoplastic aciar and ductal cells embedded in a fibrous stroma and lacking islets, which was the same histology as that in our case.
Table 1 shows the clinical features of the reported cases of solid hamartoma of the pancreas, which includes our case. The median age of the patients was 52.5 years (range 0-78 years). Four patients were asymptomatic, whereas the remaining two had slight abdominal discomfort[9] and hypoglycemia and hypocalcemia[3], respectively. The median size of the main tumor was 2.0 cm (range 1.6-11.5 cm). Two cases, including our case, revealed one index tumor with multiple tiny lesions[1]. Regarding the outcome, all of the patients except one[3] were alive and well during the entire duration of their follow-up periods. The one remaining patient experienced a prolonged postoperative complication related to metabolic problems, hepatic dysfunction and pulmonary disease and died 3 mo after the operation[3]. Clinical imaging detailing findings of pancreatic hamartoma was described in 2 cases including our case. Solid pancreatic hamartoma showed some degree of enhancement in the delayed phase in both cases[10]. In addition, the FDG-PET image did not show intense uptake in the tumor of our case. Based on the imaging findings, endocrine tumor of the pancreas might be the differential diagnosis, as well as pancreatic cancer.
Table 1.
Clinicopathological features of reported solid pancreatic hamartomas
| Case | Sex | Age (yr) | Symptom | Location | Operation | Size (cm) | Pathology | Outcome | Ref. |
| 1 | Male | 46 | - | Head | PD | 1.6, a few tiny nodules | Solid hamartoma, multiple | Unknown | [1] |
| 2 | Female | 0 | + | Entire pancreas | TP | 11.5 | Solid hamartoma, diffuse | Died 3 mo later | [3] |
| 3 | Male | 51 | - | Tail | LR | 3 | Solid hamartoma, solitary | Alive and well at 2 yr | [9] |
| 4 | Female | 54 | + | Body | DP | 2 | Solid hamartoma, solitary | Alive and well at 4 yr | [9] |
| 5 | Female | 58 | - | Body | DP | 1.9 | Solid hamartoma, solitary | Alive and well at 4 mo | [10] |
| 6 | Female | 78 | - | Head | PD | 1.7, 0.4, 0.3 | Solid hamartoma, multiple | Alive and well at 20 mo | Present case |
PD: Pancreatoduodenectomy; TP: Total pancreatectomy; LR: Local resection; DP: Distal pancreatectomy.
Table 2 summarizes the histological features of solid pancreatic hamartoma. Histologically, all six cases showed well-circumscribed solid lesions composed of non-neoplastic acinar and ductal cells embedded in a fibrous stroma. Four cases lacked islets[9,10]. In contrast with pancreatic solid and cystic hamartoma, the tumors did not show macroscopic cystic change even in a focal area[1,3,9,10] and showed a characteristic spindle cell stroma with an immunohistochemical coexpression of CD34 and CD117[9,10].
Table 2.
Pathological findings of reported solid pancreatic hamartomas
| Case | Acini | Ducts | Islets | CD34 | CD117 | bcl-2 | Ref. |
| 1 | Yes | Yes | Yes | ND | ND | ND | [1] |
| 2 | Yes | Yes | Yes | ND | ND | ND | [3] |
| 3 | Yes | Yes | No | + | + | + | [9] |
| 4 | Yes | Yes | No | + | + | + | [9] |
| 5 | Yes | Yes | No | + | + | - | [10] |
| 6 | Yes | Yes | No | + | + | - | Present case |
ND: Not done.
Although the number of cases is limited, solid pancreatic hamartomas seem to be benign tumor-like lesions, which are found incidentally in healthy middle-aged adults but occasionally involve the whole pancreas, leading to a poor prognosis. One case was associated with other minor pancreatic abnormalities, namely pancreas divism[10]. Multiple small lesions other than the main tumors were detected in two cases[1]. From these findings, one may speculate that solid pancreatic hamartoma could be the result of a malformation during the development of the pancreas.
CD34, a myeloid stem cell marker, is known to be expressed by fibrocytes in neoplastic and inflammatory pancreatic lesions[18]. It seems to play an important role in maintaining stromal integrity and inhibiting tumor cell migration[19]. CD117, a transmembrane tyrosine kinase receptor of stem cell factor, is encoded by the protooncogene c-kit. The interaction between the kinase receptor and its ligand is essential for the development of several non-epithelial cells[20-24]. Although the precise etiology remains unknown, the presence of CD34- and CD117-positive stromal cells may involve the pathogenesis of solid pancreatic hamartoma.
Footnotes
Peer reviewer: Cosimo Sperti, MD, Department of Medical and Surgical Sciences, Clinica Chirurgica IV, via Giustiniani 2, Padova 35128, Italy
S- Editor Wang JL L- Editor A E- Editor Xiong L
References
- 1.Anthony PP, Faber RG, Russell RC. Pseudotumours of the pancreas. Br Med J. 1977;1:814. doi: 10.1136/bmj.1.6064.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noltenius H, Colmant HJ. [Excessive hyperplasia of the exocrine pancreatic tissue and Wernicke’s encephalopathy (author’s transl)] Med Klin. 1977;72:2155–2158. [PubMed] [Google Scholar]
- 3.Burt TB, Condon VR, Matlak ME. Fetal pancreatic hamartoma. Pediatr Radiol. 1983;13:287–289. doi: 10.1007/BF00973350. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty MJ, Benjamin DR. Multicystic pancreatic hamartoma: a distinctive lesion with immunohistochemical and ultrastructural study. Hum Pathol. 1992;23:1309–1312. doi: 10.1016/0046-8177(92)90301-i. [DOI] [PubMed] [Google Scholar]
- 5.Izbicki JR, Knoefel WT, Müller-Höcker J, Mandelkow HK. Pancreatic hamartoma: a benign tumor of the pancreas. Am J Gastroenterol. 1994;89:1261–1262. [PubMed] [Google Scholar]
- 6.Wu SS, Vargas HI, French SW. Pancreatic hamartoma with Langerhans cell histiocytosis in a draining lymph node. Histopathology. 1998;33:485–487. doi: 10.1046/j.1365-2559.1998.0491c.x. [DOI] [PubMed] [Google Scholar]
- 7.McFaul CD, Vitone LJ, Campbell F, Azadeh B, Hughes ML, Garvey CJ, Ghaneh P, Neoptolemos JP. Pancreatic hamartoma. Pancreatology. 2004;4:533–537; discussion 533-537. doi: 10.1159/000080528. [DOI] [PubMed] [Google Scholar]
- 8.Pauser U, Kosmahl M, Kruslin B, Klimstra DS, Klöppel G. Pancreatic solid and cystic hamartoma in adults: characterization of a new tumorous lesion. Am J Surg Pathol. 2005;29:797–800. doi: 10.1097/01.pas.0000157748.18591.d7. [DOI] [PubMed] [Google Scholar]
- 9.Pauser U, da Silva MT, Placke J, Klimstra DS, Klöppel G. Cellular hamartoma resembling gastrointestinal stromal tumor: a solid tumor of the pancreas expressing c-kit (CD117) Mod Pathol. 2005;18:1211–1216. doi: 10.1038/modpathol.3800406. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Yamaguchi K, Inoue T, Yamaguchi H, Ito T, Gibo J, Tanaka M, Tsuneyoshi M. Solid pancreatic hamartoma. Pathol Int. 2007;57:276–280. doi: 10.1111/j.1440-1827.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- 11.Thrall M, Jessurun J, Stelow EB, Adsay NV, Vickers SM, Whitson AK, Saltzman DA, Pambuccian SE. Multicystic adenomatoid hamartoma of the pancreas: a hitherto undescribed pancreatic tumor occurring in a 3-year-old boy. Pediatr Dev Pathol. 2007;11:314–320. doi: 10.2350/07-04-0260.1. [DOI] [PubMed] [Google Scholar]
- 12.Sampelean D, Adam M, Muntean V, Hanescu B, Domsa I. Pancreatic hamartoma and SAPHO syndrome: a case report. J Gastrointestin Liver Dis. 2009;18:483–486. [PubMed] [Google Scholar]
- 13.Klöppel G, Sipos B, Zamboni G, Kojima M, Morohoshi T. Autoimmune pancreatitis: histo- and immunopathological features. J Gastroenterol. 2007;42 Suppl 18:28–31. doi: 10.1007/s00535-007-2048-6. [DOI] [PubMed] [Google Scholar]
- 14.Kurosaki M, Hattori K, Minato Y, Shiigai T, Ohashi I, Umehara I, Marumo F, Sato C. Asymptomatic arteriovenous malformation of the pancreas. Demonstration by Doppler ultrasonography and magnetic resonance imaging. Dig Dis Sci. 1993;38:1342–1346. doi: 10.1007/BF01296088. [DOI] [PubMed] [Google Scholar]
- 15.Maitra A. Diseases of Infancy and Childhood. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease, 8th ed. Philadelphia: Saunders Elsevier; 2010. pp. 447–483. [Google Scholar]
- 16.Suda K, Takase M, Fukumura Y, Suzuki F, Jim A, Kakinuma C, Tanaka T, Matsugu Y, Miyasaka K, Funakoshi A. Histopathologic difference between chronic pancreatitis animal models and human chronic pancreatitis. Pancreas. 2004;28:e86–e89. doi: 10.1097/00006676-200404000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800–805. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 18.Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A. CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch. 2002;440:128–133. doi: 10.1007/s00428-001-0551-3. [DOI] [PubMed] [Google Scholar]
- 19.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 20.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 21.Isozaki K, Hirota S, Nakama A, Miyagawa J, Shinomura Y, Xu Z, Nomura S, Kitamura Y. Disturbed intestinal movement, bile reflux to the stomach, and deficiency of c-kit-expressing cells in Ws/Ws mutant rats. Gastroenterology. 1995;109:456–464. doi: 10.1016/0016-5085(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 23.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 24.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]


