Challenges of cancer therapy
Cancer is a leading cause of death worldwide, with projections estimating 13.1 million of deaths in 2030 [1–3]. There are many different cancer types, as well as complex metastatic processes, limiting the efficacy of broad stroke treatments [4–6]. Solid tumors account for up to 80 % of all cancers, and despite the great number of anticancer drugs available, treatment is still a challenge for the chemotherapist in pharmaceutical companies [7]. Cases of stomach and breast cancers have been illustrated in the Egyptian history (1500–3000 bc), and physicians used to remove surgically the mass of tumor as palliative. Then, surgery was the most used method to cancer treatment until chemotherapeutic drugs development [8–10]. Chemotherapy refers to the uses of conventional drugs to eradicate or eliminate the growth and development of tumors cells [11]. Earlier, cancer treatment began with Goodman and Gilman in 1941, using the nitrogen mustards, which had effect of leukocyte reduction in patients with lymphosarcoma. However, further studies with these substances identified their carcinogenic effects [10]. Many molecules with antiproliferative and cytocidal effects have been reported, especially antifolate and platinum-based drugs, as described by Rosenberg in 1970s. Cisplatin, carboplatin, and oxaliplatin are examples of compounds that are widely used for the treatment of different types of cancer until today. Other anticancer drugs have been released in 1990s, for treatment of solid tumors, including vinca alkaloids and the taxanes [12–14].
Significant advances have been done in all the currently available chemotherapeutic treatments for cancer, associated or not with other therapies, such as surgery and radiotherapy; however, there are still shortcomings, making them far from ideal options for the patient. Systemic chemotherapy is widely employed across the whole cancer spectrum [6, 15–17]. Traditional chemotherapeutic drugs prevent cell division (mitosis), trigger cell death (apoptosis), and/or reduce blood vessel growth (angiogenesis). Therefore, these are potent to kill cancer cells, but do not discriminate between tumor and normal cells. In addition, chemotherapy is frequently associated with systemic toxicity, and their low efficacy is related to the development of side effects, including emesis, neurotoxicity, and neutropenia [18–20]. Cisplatin is a well-known metal complex that exhibits high antitumor activity; however, it has elevated toxicity, as acute and chronic nephrotoxicity [13]. Other side effects of anticancer drugs include the number of blood cells decreasing (granulocytes, lymphocytes, erythrocytes, monocytes, and platelets), increasing risk of infection, bruising, and bleeding, as well as nausea and hair loss. Most of anticancer drugs remain in bloodstream for a short period of time due to high glomerular clearance [21]. Furthermore, the intravenous administration of chemotherapeutic drugs is painful because it requires a pharmaceutical formulation with organic solvents and/or classical surfactants. In order to overcome this drawback, oral administration has been applied; however, this route often leads to a low bioavailability of the active cytotoxic drug decreasing treatment efficiency [22].
The known limitations of anticancer agents include the following: (a) low selectivity, wherein the distribution volume of the anticancer agent provides an uncharacteristically high drug concentration in normal tissues; (b) low specificity, wherein the chemotherapeutic agent also affects the acts of metabolic pathways also presented in the normal cells; (c) low molecular weight, which would require a high-dose of administration because of a high rate of excretion; (d) small therapeutic index with dose-dependent side effects; (e) low water solubility that complicates the preparation of formulations that lead to a high rate of degradation that hinders oral administration; and/or (f) the rapid induction of chemoresistance, which is frequently associated with the overexpression of P-glycoprotein that rapidly removes positively charged xenobiotics [23, 24].
Moreover, there are numerous obstacles in vivo that can physically prevent the anticancer drug from reaching its intended site after injection or administration [25]. Intratumoral drug delivery is complex and depends on: (a) physicochemical properties, such as size, surface composition, hydrophobicity, and charge; (b) presence of a physiological barrier, including the blood–brain barrier; and/or (c) biophysical events, such as interstitial pressure, extravasation, and tissue access [24, 26–28]. These drawbacks need to be overcome to amend the chemotherapeutic treatment of solid tumors and to increase therapeutic efficacy, by improvement of drug loading and retention, circulation kinetics, tumor accumulation, target cell uptake, and intracellular drug release. Nanodelivery systems are novel promising approaches to overcome these known barriers and to improve the efficacy and safety of chemotherapy treatment [22–25].
Thus, this review will focus on different strategies to target tumors using nanodelivery systems based on biodegradable polymers, aspects of intellectual property and market. This study will be useful to make a strategic evaluation concerning the drug nanodelivery systems in cancer treatment.
Nanotechnology and molecular targets the treatment of solid tumors
Over the past 30 years, exploration of nanotechnology1 strategies has brought significant innovations to the fields of pharmacology. Systems based on liposomes were developed about 40 years ago, in order to improve the delivery of known compounds [29–35]. Furthermore, nanotechnology has contributed to the development of more effective diagnostic methods for the last 25 years, through the creation of contrasts and improve drug delivery by targeting to specific sites [36]. Targeted therapy is a recent development in cancer therapeutics and has now become an established paradigm in cancer treatment; however, further improvements could arise from progress in nanodelivery systems. Thus, targeted delivery of anticancer agents has been a key focus for drug delivery specialists, based on the already mentioned reasons. Current research has shown that, for nanosystems to penetrate the tumor and to be effective in cancer treatment, the ideal dimensions should be in the range of 10–100 nm, since anything smaller may be filtered out and then removed by the kidneys and particles larger can be taken up by macrophages [37–40].
Incorporation of anticancer drugs into nanodelivery systems is a promising way to overcome chemotherapy-related systemic toxicity. Drug nanodelivery systems have several advantages over systemic chemotherapy. First of all, their smaller size allows the accumulation of higher drug concentrations at the tumor sites [41]. Second, these nanometric systems improve the solubility of water-insoluble drugs, decreasing the toxic side effects associated with intravenous administration [42]. Third, nanodelivery systems can help to minimize drug degradation, which might improve drug bioavailability after oral administration, and finally, these systems could potentially reduce the frequency of administration improving patient compliance to the prescribed treatment regimen [43–46]. The generation of drug nanodelivery systems for specific molecular targets is essential to understand and take advantage of signaling pathways that are specifically activated in cancer. This knowledge has allowed to develop “smart drugs” that recognize and bind the target tissues using specific ligands [47, 48]. In this research area, one could find several successful examples of cancer-specific targets [49].
One of the most interesting targets in anticancer therapy is the angiogenic process, which is interconnected with the maintenance, growth, and spread of various types of tumors [50]. Angiogenesis is characterized by a variety of factors; however, its main targetable effectors include the vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor (VEGFR), the platelet-derived growth factor receptor (PDGFR), the αvβ3 integrins, the matrix metalloproteinase receptors, and the vascular cell adhesion molecule-1 (VCAM-1). Although there are target-based cancer drugs available in the market today, some therapies are still in clinical trials (Table 1), and further improvements could arise from progress in nanodelivery systems [49, 51–58]. One important example of nanodelivery system of anticancer drug available in the clinic is Abraxane®, which consist of nanoparticle albumin-bound paclitaxel that does not require the use of toxic solvents and seems to contribute to its favorable therapeutic index [59]. In addition, other examples of nanodelivery system are disclosed in the literature, such as ternary nanostructures polysaccharide–paclitaxel–folic acid as cancer target. In this work, it is shown that the ternary system is more effective than the Food and Drug Administration (FDA) approved paclitaxel formulation when tested against the highly FR-positive KB tumor model. The remarkable tumor shrinkage observed in groups treated with heparin–paclitaxel–folic acid was on pair with the best results reported in the literature found on polymer–drug conjugate investigation. The ternary nanostructures displayed remarkable antiangiogenic effect on tumor vasculature. Heparin–paclitaxel–folic acid was also very effective in drug resistant KB tumors, potentially overcoming multidrug resistance through targeting and tumor vasculature inhibition [60].
Table 1.
Examples inhibitors of angiogenesis
| Drugs | Target | Trial stage |
|---|---|---|
| Bevacizumab (Avastin®) | VEGF | FDA approved |
| Sorafenib (Nexavar®) | VEGFR-1, VEGFR-2, VEGFR-3, PDGFR | FDA approved |
| Sunitinib (Sutent®) | VEGFR-1, VEGFR-2, PDGFR | FDA approved |
| Vatalanib (PTK-87) | VEGFR-1, VEGFR-2, PDGFR | Phase III (colorectal), phase II (brain, breast, gynecological, non-small cell lung, lymphoma, melanoma, mesothelioma, myelodysplasia, neuroendocrine, pancreatic, prostate) |
| AMG-706 | VEGFR-1, VEGFR-2, VEGFR-3, PDGFR | Phase II (breast, gastrointestinal stromal tumors, gynecological, neuroendocrine, non-small cell lung, thyroid) |
| CP-547,632 | VEGFR-1, VEGFR-2, VEGFR-3 | Phase II (gynecological) |
| Pazopanib (Votrient® ) | VEGFR-1, VEGFR-2, VEGFR-3, PDGFR | FDA approved |
| ABT-869 | VEGFR-1, VEGFR-2, VEGFR-3, PDGFR | Phase II (breast, colorectal, liver, non-small cell lung, renal) |
| Cediranib (AXD)2171 | VEGFR-1, VEGFR-2, VEGFR-3 | Phase II (brain, breast, colorectal, leukemia, liver, non-small cell lung, small cell lung, melanoma, mesothelioma, myelodysplasia, ovarian, prostate, renal |
Another important process involved in the development of cancerous tissue is uncontrolled cell growth (mitosis). This process is characterized by the expression of several membrane receptors, such as human epidermal receptors (HERs), the transferrin receptor, and the folate receptor. Some small-molecules inhibitors that target HER were previously approved by FDA. Other drugs are currently under development and clinical trials (Table 2) [49, 52, 61–65].
Table 2.
Examples of small-molecule targeting HER
| Drugs | Target | Trial stage |
|---|---|---|
| Erlotinib (Tarceva®) | EGFR | FDA approved |
| Lapatinib (Tykerb®) | EGFR, HER2 | FDA approved |
| XL-647 | EGFR, HER2, Ephrin B4, VEGFR2, | Phase II (non-small cell lung) |
| BIBW-2992 | EGFR, HER2 | Phase II (breast, head and neck, non-small cell lung, prostate) |
| BMS-599626 | EGFR, HER2, HER4 | Phase I |
| BMS-690514 | EGFR, HER2, HER4, VEGFR2 | Phase I |
| PF-00299804 | EGFR, HER2, HER4 | Phase I |
| ARRY-334543 | EGFR, HER2 | Phase I |
Biodegradable polymers as nano-carriers for targeted delivery systems
Several drug nanocarriers have been investigated for cancer chemotherapy transporters, including metallic nanostructures, silica nanoparticles, quantum dots, carbon nanostructures, liposomes, dendrimers, and other polymeric systems, including nanoparticles and polymeric micelles [20, 66–69].
In polymeric nanoparticles, a drug is dispersed into the polymer matrix (nanospheres) or encapsulated in polymer (nanocapsules). On the other hand, polymeric micelles are formed of amphiphilic block copolymers consisting of a hydrophilic and a hydrophobic monomer, which assemble into a core–shell structure in aqueous solution [45, 70, 71]. Polymer systems offer significant advantages when compared to other applied drug delivery systems: (a) there are a variety of polymer matrices available; (b) some polymers respond to stimuli; (c) surface modification are possible; (d) drug is better protected against degradation; (e) factors can be included that modulate drug release; and (f) production of biocompatible and biodegradable polymers has been extensively investigated under current Good Manufacturing Practices guidelines [72].
These investigations include matrices for the preparation of drug nanodelivery systems, using FDA-approved aliphatic polyesters, including poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(ε-caprolactone) (PCL) [46, 73–76]. Additionally, other polymers and copolymers, including poly(lactic-co-glycolic) (PLGA), and those containing lactide, glycolide, and caprolactone, have been synthesized to modify chain properties (e.g., hydrophilicity, crystallinity, and molecular weight) to increase encapsulation rate and modulate drug release [30, 77–79]. In this sense, PLGA can be made with different proportions of lactide and glycolide and must be identified according to the ratio of the monomers present (e.g., PLGA 75:25 identifies the copolymer containing 75 % lactic acid and 25 % glycolic acid) [29, 78]. Generally, aliphatic polyesters degradation occurs through the cleavage of polymer esters bonds chain by hydrolysis [80].
Polymeric systems of aliphatic polyesters, when associated with other components, are efficient multifunctional devices for anticancer drug delivery systems. These systems demonstrated greater specificity and better drug delivery into cancer cells through the careful modulation of their size and surface properties [20, 23, 81–86].
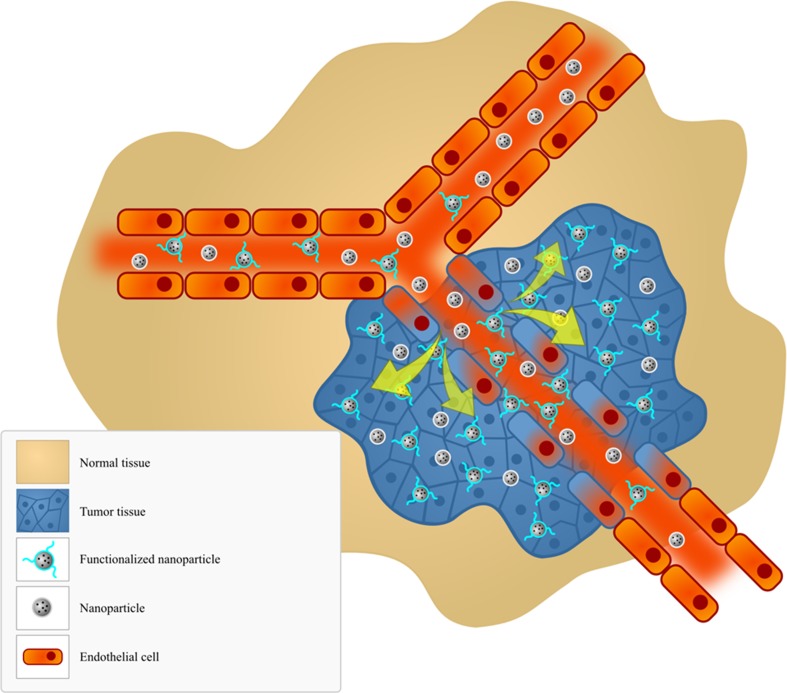
Passive targeting is based on the physiological differences between healthy and diseased tissues. Tumor growth depends on the formation of new blood vessels (i.e., neoangiogenesis), which brings fresh oxygen and nutritional support to the sites of rapid growth. During tumor angiogenesis, which differs from normal angiogenesis, vessels are rapidly formed that are leaky and have high permeability. Thus, nanoparticles depend on diffusion or convection to access the sites of tumor. Their movement is controlled by the phenomenon of enhanced permeability and retention (EPR). This process results in the accumulation of particles inside a solid tumor (Fig. 1) [45, 52, 87–92].
Fig. 1.
Passive and active targeting. This diagram shows the particular enhanced vascular permeability in regions of tumor, which allows the macromolecules to be retained in tumors for extended periods, through the enhanced permeability and retention (EPR) effect
In active targeting, particles are preloaded with drug and are targeted to the site of action by surface ligands that are attracted by tumor-specific receptors (Fig. 1). Success of this process is related to the careful selection of ligands, which would ideally provide high specificity, high affinity, and cause an increased cellular uptake of nanoparticles [6, 45, 93–98]. The most commonly used types of ligands include monoclonal antibodies, aptamers, cell-specific peptides, carbohydrates, and small molecules [99, 100]. Moreover, gene therapy presents new opportunities for cancer treatment once its development is associated with multiple changes on the genetic level of the cells. Cancer gene therapy aims to correct these genetic alterations called genetic dimorphisms through the suppression pathological genes and/or re-expression functional tumor suppressor genes. Thus, plasmid DNA, antisense oligodeoxynucleotides and small interfering RNA (siRNA) have also been studied to solid tumors targeting [101–104].
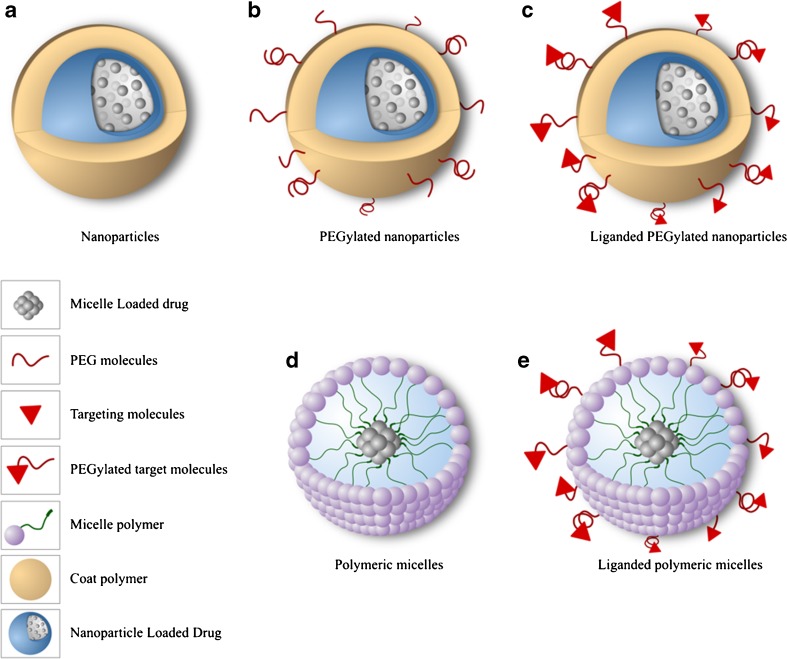
Recent studies have shown that functionalized polymeric systems are promising carriers to target anticancer drug delivery, since these systems have preferential internalization by diseased cells, thus leading to an overall greater efficiency of the therapeutic agent [105–110]. These polymeric systems are based on homopolymers and copolymers, such as micelles formed by amphiphilic block copolymers and contain poly(ethylene glycol) (PEG), because it is nontoxic, nonimmunogenic, and FDA approved for human intravenous, oral, and dermal applications. The hydrophilic polymers used in the surface modification (such as PEG) promote a greater particle biocirculation time and their functional groups enable ready conjugation with specific targeting molecules (Fig. 2) [72, 73, 80, 111].
Fig. 2.
Nanoparticles and micelles used in targeting cancer treatment: (a) Nanoparticles; (b) PEGylated nanoparticles; (c) Liganded PEGylated nanoparticles; (d) Polymeric micelles; (e) Liganded polymeric micelles
Strategies for modifying the surface of aliphatic polyesters
Materials science researchers are continuously challenged to develop new and better methods to modify and to functionalize the surface of aliphatic polyester nanoparticles and micelles with variety ligands. Different surface chemistries are associated with particular circulation times in bloodstream, rates of accumulation in the target cell, endocytosis and endosomal escape, and toxicity profiles in the body [112]. Thus, the control of surface properties, through surface change, is related to the ultimate success of the nanodevices for delivering and targeting cytotoxic drugs to the action site [82]. In order to modify the surface of aliphatic polyesters nanoparticles and micelles covalent and noncovalent attachments between the polymer and the desired ligands can be generated. Noncovalent attachment occurs by entrapment, migratory additives, plasma treatment, and adsorption process. On the other hand, covalent bonds are formed by chemical reactions using specific reagents [113, 114].
A widely used approach for performing noncovalent attachment is the adsorptive process using by coating the particle surface hydrophilic polymers, such as PEG, poly(l-lysine) (PLL), and polysaccharides. This strategy maximizes the accumulation of nanoparticles in solid tumors based on the significantly regulation time increasing in the system. This phenomenon is essentially related by making them “invisible” to the mononuclear phagocyte system. In this process, plasma proteins are then repelled from the particle surface due to mechanisms associated with steric stabilization and minimization of surface charge by attaching hydrophilic polymers. Then, those polymers reduce particle aggregation and electrostatic interactions with other components within the circulation resulting in less opsonization and clearance [39, 115, 116].
Strategy used by Chung et al. [117] to increase efficacy of the PLGA nanoparticles in tumor-targeting and to decrease their high localization in liver was to coat their surface using Pluronic® F127 (polyoxyethylene–polyoxypropylene triblock copolymer) functionalized with heparin or chitosan. The rationale behind this functionalization was driven by higher-affinity binding and internalization of heparin in dividing vascular endothelial cells and also because of the mucoadhesive properties of chitosan, which increases the retention time of nanoparticle in cancer cells. Cytotoxicity and cellular uptake of the nanoparticles were analyzed using normal cells (NIH/3T3 fibroblast) and tumor culture cells (SCC7). There was no significant cytotoxicity for nonfunctionalized and functionalized nanoparticles in contrast to the enhanced tumor cell uptake of the functionalized nanoparticles in comparison to the control nanoparticles coated with Pluronic®. In vivo accumulation and biodistribution of PLGA-based nanoparticles was evaluated in athymic mice after that SCC7 tumor model was developed. Higher accumulation in tumor of 2.2- and 2.4-fold for heparin and chitosan-conjugated Pluronic® nanoparticles was observed, respectively [117].
In a similar study conducted by Parveen and Sahoo [118], PLGA nanoparticles loaded with paclitaxel surface coated with chitosan, PEG, and chitosan/PEG blend were prepared in order to maximize the drug therapeutic effect and also to decrease the phagocytosis process of PLGA nanoparticles. Lower opsonization and macrophage uptake of PLGA-loaded paclitaxel nanoparticles associated with a combinatorial coating of chitosan and PEG was observed in comparison noncoated nanoparticles. This result could be due to lower blood protein adherence of the PLGA coated nanoparticles. Higher bioavailability was also observed as a consequence of lower liver nanoparticles catching process. In addition, higher efficacy of PLGA-coated nanoparticles loaded with paclitaxel in cancer cell including human retinoblastoma cell line (Y79 and WERI-Rb-1), breast cancer cell line (MCF 7), and pancreatic cancer cell line (MIA PaCa-2) was observed may be due to higher intracellular paclitaxel delivery in the cancer cells when compared to free paclitaxel [118].
Bilensoy et al. [119] developed PCL nanoparticles coated with chitosan (PCL-CS) and poly(l-lysine) (PCL-PLL) loaded with mitomycin C for bladder cancer treatment through intravesical drug delivery. Challenges in this treatment are to keep the drug therapeutic level because of periodical discharge of bladder. In order to overcome this problem, development of systems using bioadhesive polymers can lead effective devices. Higher cellular uptake of PCL-CS nanoparticles was observed, being selectively incorporated by a MB49 mouse urinary bladder carcinoma line in comparison to normal bladder epithelial cells [119].
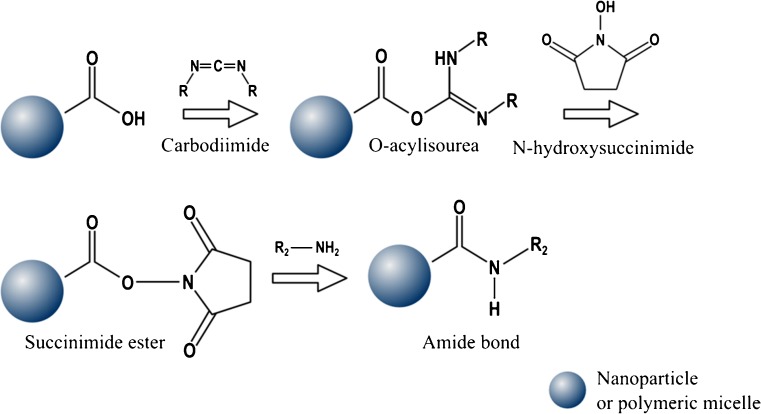
Second strategy used to modify the surface of aliphatic polyester polymers forms a covalent attachment through an amide bond formation between one carboxylic acid group and one amino group. Compounds containing carbodiimide functionality, such as coupling reagents, are used in this organic synthesis in order to activate the acid groups. In this sense, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N,N′-dicyclohexylcarbodiimide (DCC) are widely used. The reaction of the carbodiimide with one carboxylic acid group results in the formation of an unstable intermediate, so called O-acylisourea, which is stabilized using N-hydroxysuccinimide (NHS), thus leading to the succinimide ester. In this reaction, this last active ester intermediate reacts with the amine group to form an amide bond. A schematic of this reaction can be viewed in Fig. 3 [120–122].
Fig. 3.
Reaction between nanoparticles or micelles with terminal carboxylate groups and amine groups leading to amide bond formation using carbodiimide and N-hydroxysuccinimide
PEGylation techniques involve the incorporation of polymer conjugates (e.g., PLA-PEG) or the covalent attachment of amino- or carboxyl-terminated polymers. These techniques are described as a strategy for surface modification in order to increase the overall circulation time of the particles in the body [72, 108]. The reactions described above have been extensively used in the preparation of nanoscale anticancer drug release systems from aliphatic polyester matrices, as well as for the conjugation of ligands. Strategy developed by Cheng et al. [105] describes the preparation of core–shell structures, based on amphiphilic block copolymers of PLGA-PEG conjugated to A10 2′-fluoropyrimidine RNA aptamer (A10 RNA aptamer) for systemic drug delivery as an alternative approach for the intratumoral administration already developed for the same research group. A10 RNA aptamer has been used as a ligand, targeting prostate cancer cells that express prostate-specific membrane antigen (PSMA). Firstly, systems were synthesized from PLGA-PEG-COOH and COOH-PEG-NH2 through a reaction with EDC/NHS. Then, copolymer micelles were prepared, containing docetaxel or 14C-paclitaxel, and subsequently conjugated to an amine-terminated aptamer through an EDC/NHS reaction. Human xenograft prostate cancer tumors (LNCaP cells) were induced in balb/c nude mice and in vivo accumulation and biodistribution of micelles were carried out. Significantly enhanced 14C-paclitaxel delivery and greater uptake by cancer cells were observed for micelles conjugated with A10 RNA aptamer over control micelles without targeting aptamer (a 3.77-fold increase at 24 h) [105].
In another study developed by Dhar et al. [123], in order to obtain a device for prostate cancer treatment, core–shell structures based on PLGA-PEG conjugated to A10 RNA aptamer loaded with platinum (IV) compound c,t,c-[Pt(NH3)2(O2CCH2CH2CH2CH2CH3)2Cl2] (cisplatin prodrug) were prepared. Cisplatin is less effective to treat prostate cancer due to development of resistance and poor targeting. For this reason, cisplatin targeting was proposed as a way to increase the efficacy of treatment. In vitro tests used prostate cancer cells including LNCaP (express a high level of PSMA) and PC3 (negligible levels of PSMA) were developed to evaluate uptake and cytotoxicity of the platinum (IV) compound encapsulated within micelles. Higher cytotoxicity of bioconjugated PLGA-PEG-loaded cisplatin prodrug micelles on LNCaP cells was observed in comparison with nontargeted micelles and free cisplatin. Cellular uptake via endocytosis was observed only in the LNCaP cells confirming the differential binding and internalization of the micelles conjugated with A10 RNA aptamer in cells which express PSMA protein on their surfaces [123].
According to Zhao and Yung [124], core–shell structures of PLGA-PEG can also be prepared using folic acid (FOL) for tumor-specific targeting. Different types of cancer cells express folate receptors in greater quantities compared to normal tissues. In this sense, folate has been employed as a targeting molecule in drug delivery devices. Initially, PLGA-PEG-FOL was synthesized from NH2-PEG-NH2, PLGA, and FOL through a reaction with DCC/NHS and after doxorubicin was then incorporated into the system as a model drug. Higher cytotoxicity of doxorubicin-loaded PLGA-PEG-FOL micelles was observed than that of PLGA-PEG micelles and free doxorubicin. In addition, higher selectivity and cellular uptake of the PLGA-PEG-FOL micelles on KB an MATB III cancer cells, which overexpressed folate receptors, was observed than fibroblast cells [124].
In another study, Pan and Feng [125] prepared polymeric nanoparticles based on PLA and d-α-tocopheryl polyethylene glycol succinate (TPGS), a water-soluble derivative of natural vitamin E, surface modified with FOL, and loaded with paclitaxel. It was reported that TPGS has ability to increase the drug encapsulation loading and inhibited P-glycoprotein-mediated multidrug resistance. In this process, copolymer PLA-TPGS and TPGS-COOH were synthesized and blended together to prepare paclitaxel-loaded nanoparticles. Subsequently, FOL-NH2 was synthesized through an amination reaction of FOL in the presence of excess ethylenediamine and DCC/NHS. The conjugation of TPGS-COOH, present on the surface of the nanoparticles and FOL-NH2 was carried out using EDC/NHS. The therapeutic effect of paclitaxel formulated in nanoparticles was evaluated through in vitro tests using breast cancer cells (MCF-7) and brain cancer cells (C6). It was demonstrated higher cytotoxicity and cellular uptake of nanoparticle conjugated with FOL on MCF-7 and C6 than nanoparticles without FOL surface modification and Taxol®, gold standard formulation of paclitaxel [125].
Wheat germ agglutinin (WGA) was used to functionalize the surface of PLGA nanoparticles containing paclitaxel. WGA is considered as a good ligand to targeting since it specifically recognizes and binds to N-acetylglucosamine and sialic acid present in the cell membrane, thus promoting a greater internalization of nanoparticle-loaded drugs within colon cancer cells. WGA was conjugated to nanoparticles using EDC/NHS, which promotes the activation of free carboxylic groups of PLGA with subsequent binding to amine groups present in the molecules of WGA. Cellular uptake and cytotoxic activity of the PLGA-WGA nanoparticles on Caco-2 and HT-29 cancer cells was higher than nonmodified PLGA nanoparticles and Taxol® due to increased intracellular paclitaxel concentration. Moreover, higher selectivity of PLGA-WGA nanoparticles on colon cancer cells was also observed in comparison to the human fibroblast [126].
Kocbek et al. [106] described the preparation of PLGA nanoparticles conjugated with monoclonal antibody that specifically recognized cytokeratins expressed by cancer cells. These macromolecules have played an important role in site-selective delivery because some monoclonal antibodies are associated with specific signaling cascades which can potentiate the therapeutic effect for anticancer drugs. In this study, covalent and noncovalent modes of binding were used to attach the antibody to these nanoparticles. Covalent binding was made through a reaction with EDC, which conjugated the primary amino group present on the antibody with the carboxylic acid groups of PLGA. Noncovalent binding was achieved by adsorption. The ability of the nanoparticles to target antigen rich cells was investigated using human breast epithelial cells (MCF-7 and MCF-10A neoT). The particles with noncovalent bound antibody showed a greater ability to recognize cancer cells than the particles with covalently bound antibody. This fact can be explained by the possible self-polymerization of the antibody during the reaction with EDC, reducing their specific target affinity. Another possibility is associated with nonspecific covalent binding of PLGA to the antibody within the antigen recognition site as a consequence of proteins side reactions in presence of EDC [106].
Recently, Chakravarthi and Robinson [120] investigated the PLGA particles containing paclitaxel and their process of a adhering to mucus on cancer cell membranes. These particles were modified with chitosan, which were adsorbed onto the surface of the particle or covalently linked through a reaction with EDC/NHS. It was observed that conjugation of chitosan-PLGA nanoparticles decreased the IC50 2.5 fold, compared to control PLGA nanoparticles as well as paclitaxel solution and that the cellular incorporation of paclitaxel into 4T1 cells increased 4.5–10 times when chitosan was adsorbed or conjugated to PLGA nanoparticles [120].
Numerous studies have been conducted to investigate the uses of gene therapy for the treatment of cancer [16, 110, 127–129]. However, antisense oligonucleotides and plasmid DNA have inherently poor stability and problems to penetrate in the cell. These barriers can be overcome by encapsulation of biomolecules in polymeric systems [130]. In this sense, Xiong et al. [131] synthesized and evaluated polymeric micelles, based on amphiphilic copolymer poly-(ethylene oxide)-block-poly-(ε-caprolactone) grafted with polyamines, for the delivery of siRNAs to treat different types of cancer. The synthesis of this copolymer was performed by ring-opening polymerization of α-benzylcarboxylate-ε-caprolactone, using α-methoxy-PEO (PEO) as an initiator. Then, polyamines were conjugated through a reaction with DCC/NHS, and after the subsequent formation of amide bonds, siRNA was added to the system to form a copolymer/siRNA complex. These studies showed that the copolymers effectively bind to siRNA to form micelles and protected the RNA from its typical degradation by serum nuclease. It was also found that amphiphilic polycationic polymers with primary amine ends were efficient transducers of siRNA cellular uptake. In vitro tests indicated that these polymeric micelles exhibited efficient gene silencing of P-glycoprotein expression, which is the main cause of multidrug resistance and the major cause of chemotherapy failure in cancer patients [131].
Intellectual property and drug nanodelivery system patents
Intellectual property, primarily in the patents form, continues to be a key role for specialists and for the development of new generation of advanced drug nanodelivery systems [132, 133]. This is particularly true for the pharmaceutical companies that depend tightly on the exclusion of other competitors from the market, in order to ensure the recovery of their research and development (R&D) costs and investments. For this reason, it is economically essential to create an intellectual property strategy that will protect the technology and the commercial interests of the companies [94]. Furthermore, companies that seek to develop and commercialize new drug nanodelivery systems must ensure that they have broad freedom to operate and to avoid legal problems. On the other hand, the patent system is designed to disseminate knowledge and information to the public, through the publication of patent applications and granted patents. Publishing this information has previously stimulated innovation and the generation of new patents by designing around existing patents [134–136].
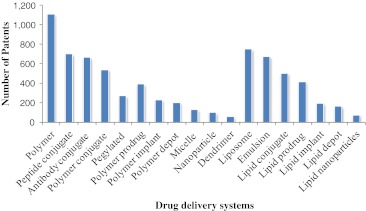
The patents are one of the key factors to protect the efforts and the investments in this new area of delivery systems for cancer. Thus, since 1975 until now, around 2,121 patents in advanced drug delivery in cancer have been issued, which most of these patents are related to polymer-based delivery systems (Fig. 4). Each one of 2,121 patents can have more than one variety of advanced drug delivery systems described [7]. According to Fraser-Moodie [137], a recent world patent analysis showed that more than 60 % of the healthcare nanotechnology patents originate in the USA, followed by Europe and Japan with 34 and 5 %, respectively. This scenario may account for the accumulated scientific knowledge and experience in the commercialization of healthcare products based on nanotechnology. However, share from USA has decreased significantly, due to increase in R&D efforts of healthcare players and the growth of market share from developed countries (Fig. 5) [137].
Fig. 4.
Issued USPTO patents of drug delivery systems for cancer, including biodegradable polymers since 1975 until now
Fig. 5.
Percentage of healthcare nanotechnology patent applications in 2008
There are at least three compulsory conditions to obtain a patent: (a) the invention must be novel, (b) the invention must contribute sufficient advancement in relation to the state-of-the-art (inventive step), and (c) the invention must have industrial application [138]. Therefore, mainly due to the high specificity of nanodelivery systems, there are clear opportunities for obtaining patent protection. Nanodelivery systems are a promising way to improve therapeutic activity and to reduce the toxicity of previously approved drugs, which in some cases can also provide a life-cycle extension of drugs after their patent expires [139]. According to Barton [7], the uses of advanced drug delivery technologies fulfills these challenges by providing new formulations of leading brands (e.g., cisplatin, paclitaxel, and doxorubicin), which are either off patent or face patent expiration in the near term. In January 2007, for example, Frasenius Kabi Oncology company launched the drug nanodelivery system used for the delivery of paclitaxel (Nanoxel®) [7]. Another example is the Avastin® (Bevacizumab; Roche/Genentech) that prevents tumor blood vessel growth (angiogenesis) through the targeting of VEGF and was approved in 2004. Avastin® is currently used as first-line therapy for metastatic colorectal cancer, as first-line therapy for nonsmall cell lung cancer in combination with chemotherapy and more recently as first-line treatment of metastatic breast cancer, advanced renal cell carcinoma, and glioblastoma. Its patent will expire in 2019. However, the literature discloses new nanotechnology advances to this currently used drug and probably will get new formulation patents [7, 140, 141]. One example of a nanotechnology advancement involving this drug is related to its encapsulation in PEGylated cationic liposomes, which enhanced tumor targeting [142].
Although it is still a challenge to distinguish between cancer and healthy cells effectively, several methods using nanodelivery systems are being developed, with promising results. One effort at addressing the issue of selective tumor destruction is disclosed in the issued US patent 7,521,066, entitled “Pharmaceutical and diagnostic compositions containing nanoparticles useful for treating targeted tissues and cells.” It is disclosed in the literature that nanoparticle encapsulation and polymer conjugation could also slow or inhibit internalization of drug into tumor cells. For example, systems of solid lipid nanoparticles for tumor targeting have critical problems related to undesired accumulation in tissues (liver and spleen) and low drug-loading capacities. To overcome these problems, the invention seems to provide a significant advance in the art, describing a process of producing lipid nanoparticles containing paclitaxel, camptothecin, carmustine, and etoposide to reach high drug uptake levels and elevated passive accumulation when applied to targeted tumor tissues and cells. In order to improve the loading capacity, surfactants were added such as eoxycholate and Tween80® [143].
Similarly, the issued US patent 7,976,825, entitled “Cancer cell diagnosis by targeting delivery of nanodevices,” described the preparation of nanoparticle self-assemblies formed from the reaction of polymeric nanoparticles and paramagnetic ions or metals through ion-ion interaction in an aqueous media used to detect tumors by magnetic resonance imaging (MRI). According to the inventors, paramagnetic contrast agents showed promising clinical results in MRI. Two properties must be considered in the design of MRI agents: first, the biocompatibility and second, the ability of relaxivity which is driven by the choice of metal and rotational correlation times. Usually, paramagnetic metal ions are most commonly selected for their potent relaxation enhancement. In this invention, nanoparticles were constructed by self-assembling chitosan, a polycation, and poly-gamma glutamic acid, a polyanion. The paramagnetic metal ion was encapsulated in the nanoparticles forming polycation–metal ion–polyanion complex that were capable of uptake forming a particle with suitable molecular relaxivity. In addition, the folic acid was linked to the nanoparticles in order to improve their ability to target tumor cells [144].
US patent application 2011077581, entitled “Targeted cellular delivery of nanoparticles,” discloses therapeutic strategies for the cellular delivery of functionalized gold nanoparticles against breast cancer. Usually, therapeutic applications of functionalized nanoparticles are related to the specificity cellular target, wherein different ligands have provided better binding affinity and consequently improvements in the therapeutic response. Gold nanoparticles are promising candidates for targeting strategies of cancer treatment due to their biocompatibly, inert, and relatively easy to tweak chemically. Changing the size and shape of the gold particle could respond to different wavelengths and could target and kill specific cancer cells by heating while leaving healthy cells alone [145]. In order to improve their specificity, there are some studies demonstrating their functionalization with ligands of cell surface receptors overexpressed by tumor cells [146, 147]. In this context, the present invention strategy demonstrates a synergistic antitumoral activity through a cojugation of gold nanoparticles and tamoxifen, which is a drug that compete with the estrogen for binding the receptors [148]. Tamoxifen ligand was conjugated to the thiol-poly(ethylene glycol) linker via an azide–alkyne coupling, and the gold nanoparticle core was covalently attached to the linker, which ensured the unit of the ligand to the core and provided specificity for cellular target. Results showed dramatic enhanced potency (IC50) of the functionalized gold nanoparticle in comparison of the free drug [149].
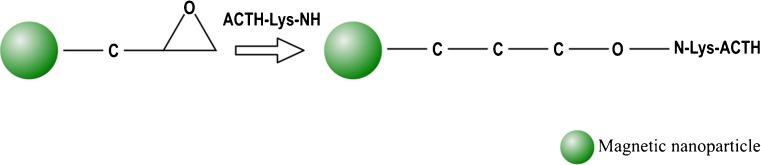
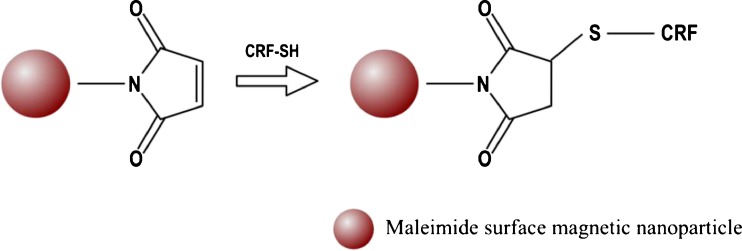
According to inventors of the US patent application 2011044911, entitled “Use of functionalized magnetic nanoparticles in cancer detection and treatment,” many diagnostic techniques are currently available to monitor and detect tumors; however, most of them present limitations, and sometimes, these are not able to differentiate between metastatic tumors and normal tissues. In order to overcome some of these challenges the inventors built functionalized magnetic nanoparticles comprising attached ligands capable to provide specificity and metabolic uptake into a cancer cell. Although many ligands have been claimed in this patent application, inventors provided examples. First of them used the ligand 2-deoxy-d-glucose (2DG), which is a component of anticancer drugs, and antibiotics with a lactonic ring. The 6-carbon of 2DG was attached to the dextran that coated the magnetic nanoparticles. Inventors showed sufficient uptake of the 2DG-dextran-coated magnetic nanoparticles by tumor cells. The second one was a conjugation of adrenocorticotropin hormone (ACTH) to dextran-coated and epoxy-surface-modified magnetic nanoparticles. ACTH was conjugated at its NH3+ group of lysine to the dextran-coated and the epoxy-surface-modified magnetic nanoparticles, as shown in Fig. 6. Another example in this invention disclosed a conjugation between corticotropin releasing factor (CRF) and magnetic nanoparticles for the detection of tumors, as tumors have increased receptors for CRF in comparison with normal tissues. CRF was conjugated via its sulfur atoms to magnetic nanoparticles by maleimide linkers or directly to the coating of the nanoparticle, as shown in Fig. 7 [150].
Fig. 6.
Epoxy-surface modified magnetic nanoparticles
Fig. 7.
CRF conjugation via its sulfur atoms to magnetic nanoparticles
The issued US patent 7,638,558, entitled “Polymeric micelles for drug delivery” disclosed several advantage points for cancer treatment by nanosystems of polymeric micelles, being the capacity to tune the core and shell components due to their amphiphilic portions, better stability when compared to liposomes, capacity of accumulation in solid tumors by the EPR effect, nonspecific uptake by the reticuloendothelial system, which are responsible to remove foreign particles from the bloodstream, and the possibility of surface functionalization with targeting ligands. However, the polymer micelles have also clinical limitations related to concentration dependence and reversibility. When the formulation was diluted to be administered and met the blood components, the dissociation of the drug-loaded micelle could occur before the target. To overcome these problems, the inventors have developed a method to self-assembly nanosized polymeric micelles in water. The nanosystem comprised a drug-loaded inner core polymeric micelle, a cross-linked poly(amino acid block), and a poly(amino acid block) outer core, and a hydrophilic shell designed to afford cross-linked polymer micelles a higher stability, for better dilution in the bloodstream. Moreover, the nanosystem also comprised better pH-sensitive drug targeting, wherein the drug is released in response to the specific pH range found in cancer cells [151].
US patent application 2010203149, entitled “Nanoparticles for cytoplasmic drug delivery to cancer cells,” disclosed three main challenges to be overcome in a nanodelivery system to cancer treatment, such as the premature release of anticancer drugs in bloodstream, slow drug release, and slow cellular uptake by cancer cells. First, the drug is immediately released upon intravenous administration, and only a small content of drug will reach the tumors, which causes low drug efficiency and toxicity to healthy tissues [152]. According to the inventors, a chemical bond between polymer and drug could reduce this problem; however, some nanodelivery systems showed low or no anticancer activity when these were covalently bonded. Fast release is caused by the low entrapment efficiency of the drugs that can be only adsorbed onto the particles surface and the large surface area of nanodelivery systems. Second, the diffusion of the anticancer drug from the core to the outside hampered by the polymer layer and the drug release is slow, which lead to low effectiveness because of low concentration of the drug inside the cancer cell. Third, to avoid the recognition and subsequent uptake by the reticuloendothelial system and to prolong the drug circulation in the bloodstream, PEGylation strategy has been used. However, PEGylated nanoparticles provided passive accumulation in cancer interstitium, but not interaction with target cancer cells. For this reason, several funcionalization strategies have been used to enhance the cellular uptake based on different targets, including transferrin receptor and folate receptor. In this context, the inventors disclosed nanodelivery systems containing an anticancer drug, specifically soluble at the pH of tumor cells, releases the anticancer drug that are uptaken quickly by cancer cells. The core of the nanoparticle comprised a layer of polymer chains that are insoluble at the pH of healthy cells, but soluble at the pH of cancer cells. The outside layer comprised water-soluble polymer chains to protect the nanoparticle from recognition by the reticuloendothelial system, thus providing a long circulation time in the bloodstream. A particle can be optionally conjugated to folic acid to target receptors on the surface of the cancer cell [153]. Layered nanoparticles further comprised a layer between the outer layer and the core inner layer that was insoluble at the pH of healthy tissue but soluble at the pH of a cancer interstitium and folate moieties in the outer layer.
The US patent application 20070519126, entitled “Breast cancer therapy based on hormone receptor status with nanoparticles comprising taxane,” claimed methods and kits for the treatment of breast cancer (related to the US patent number 6506405) which described the discovery of paclitaxel and albumin (Abraxane®). This invention showed that nanoparticles comprising paclitaxel and a carrier protein have significantly better safety profiles, such as lower toxicities than Taxol® and Taxotere® with significantly improved outcomes in efficacy. The patent also showed that these nanoparticles are more effective for the treatment of breast cancer, compared to monotherapies with non-nanoparticle formulations or combination chemotherapy. This efficacy was dependent on the hormone receptor status of the breast cancer [154]. Currently, efforts in nanodelivery systems have a potential role in the cancer treatment, and their complexity also provides huge grounds for patentability [139].
Market opportunities
The application of nanotechnology in healthcare represents an important advancement for the efficacy of old and new drugs, particularly anticancer drugs in order to get to market; all new drugs must receive approval from regulatory agencies, being FDA in the USA and the European Agency for the Evaluation of Medicinal products in Europe. Recently, FDA published its guidelines for an acceptable nanotechnology product application, which must demonstrate all features of safety, quality, characterization, and environmental impact [94].
In 2012, it is projected that about $4.8 billion will be earned with nanotechnology modifications of drug delivery systems, which would be a total market share of 5.2 % of nanotechnology field. If the current rate of development continues, this market share could increase to 7 % in 2015 and 10 % in 2020 [155]. Interestingly, no single product is dominant, as a large combination of therapies is used in cancer treatment [133]. As expected, North America remains the largest market for healthcare nanotechnology applications, reaching revenues of nearly $4 billion in 2008. Europe is considered to be the second largest market for healthcare nanotechnology applications, wherein France, Germany, and the UK are the countries with the largest markets. Brazil, Russia, India, and China known as BRICS, are increasing their focus and rate of investment in the exploitation of health care nanotechnology [133].
Application of nanotechnology in health care often requires the development of partnerships with experts outside the original company. It is recommended that this partnership begins at the earliest stage of the drug development process and that the management of intellectual property ownerships must be carefully considered throughout. According to Merchant [133], these collaborations and licensing agreements have been an important way to develop drug formulations and controlled drug delivery systems based on nanotechnology. In particular, agreements with universities have been important for nanoproduct development and patent filing. Universities and governmental research institutions also hold several patents, and it has become very common during the course of negotiation to transfer some of the technology to companies for commercialization [133].
Summary and future directions
This review discloses about different strategies to target tumors using nanodelivery systems based on biodegradable polymers, aspects of intellectual property, and market. The discussion considers technical aspects of functionalization, targeting, in vitro application, and the various phases of preclinical and clinical drug development. We also reviewed works in which not all challenges have yet been overcome, but the results show clearly the advantages and promising ability of nanodelivery systems in cancer treatment.
The application of nanotechnology for the development of drug nanodelivery systems has proven to be an important way to obtain novel methods, higher safety, and efficacy than conventional treatments for cancer. The use of nanodelivery systems based on biodegradable aliphatic polyesters for cellular targeting in cancer therapy is promising to minimize the toxic side effects of drugs and to improve the selectivity for diseased cells. The therapeutic advantages of employing polymeric systems are intrinsically linked to the tiny size of the devices that allow penetration into solids tumors and the possibility of surface modification for drug active targeting. The versatility of these nanodevices is related to different ligands that can be conjugated to their surface, through either chemical reactions or adsorptive processes, which then target the drug to various types of cancer cells.
Furthermore, it should be noted that drug nanodelivery systems will continue to play a vital role in drug discovery and development and that the management of the intellectual property rights will have a huge impact on the future of drug delivery science.
Acknowledgments
Authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Instituto Nacional de Ciência e Tecnologia Nanobiofar (INCT-MICT/CNPq-Fapemig), and Nanofar CNPq network for financial support. The authors thank Eônio Paulo Júnior (Software Engineering, for figures design), Arshad Islam (Ph.D candidate at UFMG), Dr. Frederico B. De Sousa and Carlos Peñaranda for helpful comments during this manuscript preparation.
Abbreviations
- 2DG
2-Deoxy-d-glucose
- A10 RNA aptamer
A10 2′-fluoropyrimidine RNA aptamer
- ACTH
Adrenocorticotropin hormone
- CRF
Corticotropin releasing factor
- CS
Chitosan
- DCC
N,N′-dicyclohexylcarbodiimide
- EDC
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- EPR
Enhanced permeability and retention
- FOL
Folic acid
- HER
Human epidermal receptors
- MRI
Magnetic resonance imaging
- NHS
N-Hydroxysuccinimide
- PCL
Poly(ε-caprolactone)
- PDGFR
Platelet-derived growth factor receptor
- PEG
Poly(ethylene glycol)
- PET
Positron emission tomography
- PGA
Poly(glycolic acid)
- PLA
Poly(lactic acid)
- PLGA
Poly(lactic-co-glycolic)
- PLL
Poly(l-lysine)
- PSMA
Prostate-specific membrane antigen
- siRNA
Small interfering RNA
- SPECT
Single photon emission computed tomography
- TPGS
d-α-tocopheryl polyethylene glycol succinate
- VCAM-1
Vascular cell adhesion molecule-1
- VEGFR
Endothelial growth factor receptors
- WGA
Wheat germ agglutinin
Footnotes
Nanotechnology is a multidisciplinary science that studies materials, devices, and systems at the atomic, molecular, and supramolecular levels. The sizes of these technologies can vary from a few nanometers to several hundred nanometers, according to their intended use. The use of nanotechnology in medicinal applications is called “nanomedicine,” and is an area that has been revolutionary for medical care. This particular field has led to the development of sophisticated drug nanodelivery systems, particularly with regard to diagnosis and treatment of cancer.
All authors contributed equally to the paper.
References
- 1.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63(10–11):943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Shen H, You J, Zhang G, Ziemys A, Li Q, Bai L, Deng X, Erm DR, Liu X, Li C, Ferrari M. Cooperative, nanoparticle-enabled thermal therapy of breast cancer. Adv Healthc Mater. 2012;1(1):84–89. doi: 10.1002/adhm.201100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (2008) http://www.who.int. Accessed 21 March 2012
- 4.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson TA, Choi J, Green DR. Armed response: how dying cells influence T-cell functions. Immunol Rev. 2011;241:77–88. doi: 10.1111/j.1600-065X.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veiseh O, Kievit FM, Ellenbogen RG, Zhang M. Cancer cell invasion: treatment and monitoring opportunities in nanomedicine. Adv Drug Deliv Rev. 2011;63(8):582–596. doi: 10.1016/j.addr.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton CL (2011) Innovations in the delivery of cancer therapies. Business Insights
- 8.Chabner BA, Roberts TG. Timeline—chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 9.Lewis LD. Cancer pharmacotherapy: 21st century ‘magic bullets’ and changing paradigms. Br J Clin Pharmacol. 2006;62(1):1–4. doi: 10.1111/j.1365-2125.2006.02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira LA, Fonseca CO (2007) De doença desconhecida a problema de saúde pública: o INCA e o controle do câncer no Brasil. http://www.inca.gov.br Acessed 28 Aug 2012
- 11.ACS (2012) http://www.acs.org. Accessed 10 Jan 2012
- 12.Rosenberg B, Camp L, Grimley E, Thomson A. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum (IV) complexes. J Biol Chem. 1967;242(25):1347–1352. [PubMed] [Google Scholar]
- 13.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 14.Zhang CX, Lippard SJ. New metal complexes as potential therapeutics. Curr Opin Chem Biol. 2003;7(4):481–489. doi: 10.1016/s1367-5931(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29(4):402–417. doi: 10.1016/j.biotechadv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Haile S. Cancer metastasis and in vivo dissemination of tissue-dwelling pathogens: extrapolation of mechanisms and exchange of treatment strategies thereof. Med Hypotheses. 2008;70(2):375–377. doi: 10.1016/j.mehy.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Rowinsky E, Donehower R. Paclitaxel (Taxol) N Engl J Med. 1995;332(15):1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 19.Soppimath KS, Liu LH, Seow WY, Liu SQ, Powell R, Chan P, Yang YY. Multifunctional core/shell nanoparticles self-assembled from pH-induced thermosensitive polymers for targeted intracellular anticancer drug delivery. Adv Funct Mater. 2007;17(3):355–362. [Google Scholar]
- 20.Wang H, Zhao Y, Wu Y, Hu YL, Nan KH, Nie GJ, Chen H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32(32):8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Takahara PM, Rosenzweig AC, Frederick CA, Lippard SJ. Crystal-structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature. 1995;377(6550):649–652. doi: 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]
- 22.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarvand R, Sepehri N, Manoochehri S, Rouhani H, Atyabi F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int J Nanomed. 2011;6:877–895. doi: 10.2147/IJN.S18905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergaro V, Scarlino F, Bellomo C, Rinaldi R, Vergara D, Maffia M, Baldassarre F, Giannelli G, Zhang X, Lvov YM, Leporatti S. Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv Drug Deliv Rev. 2011;63(9):847–864. doi: 10.1016/j.addr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Peer D, Karp JM, Hong S, FaroKhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 26.Alexandrakis G, Brown EB, Tong RT, McKee TD, Campbell RB, Boucher Y, Jain RK. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat Med. 2004;10(2):203–207. doi: 10.1038/nm981. [DOI] [PubMed] [Google Scholar]
- 27.Geng Y, Dalhaimer P, Cai SS, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophys J. 2002;83(3):1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Alexis F, Rhee J-W, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol Oncol-Semin Ori Inv. 2008;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 32.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 33.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol-Semin Ori Inv. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Thei DP, Eric Drexler JK, et al. Nanotechnology. Nat Nano. 2006;1(1):8–10. [Google Scholar]
- 35.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62(2):90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomed-Nanotechnol Biol Med. 2007;3(1):20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 39.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuo C. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol Med. 2010;16(12):594–602. doi: 10.1016/j.molmed.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 42.Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004;90(2):304–305. doi: 10.1038/sj.bjc.6601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. Acs Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh K, Kanapathipillai M, Korin N, McCarthy JR, Ingber DE. Polymeric nanomaterials for islet targeting and immunotherapeutic delivery. Nano Lett. 2012;12:203–208. doi: 10.1021/nl203334c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8:147–166 [DOI] [PubMed]
- 46.Prakash S, Malhotra M, Shao W, Tomaro-Duchesneau C, Abbasi S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv Drug Deliv Rev. 2011;63(14–15):1340–1351. doi: 10.1016/j.addr.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin. 2008;29(1):1–20. doi: 10.1111/j.1745-7254.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 48.Mahmud A, Xiong XB, Aliabadi HM, Lavasanifar A. Polymeric micelles for drug targeting. J Drug Target. 2007;15(9):553–584. doi: 10.1080/10611860701538586. [DOI] [PubMed] [Google Scholar]
- 49.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59(2):111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 50.Dhanikula AB, Panchagnula R. Localized paclitaxel delivery. Int J Pharm. 1999;183(2):85–100. doi: 10.1016/s0378-5173(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 51.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 54.Francavilla C, Maddaluno L, Cavallaro U. The functional role of cell adhesion molecules in tumor angiogenesis. Semin Cancer Biol. 2009;19(5):298–309. doi: 10.1016/j.semcancer.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8(9):918–921. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14(4):377–386. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- 57.Li LY, Wartchow CA, Danthi SN, Shen ZM, Dechene N, Pease J, Choi HS, Doede T, Chu P, Ning SC, Lee DY, Bednarski MD, Knox SJ. A novel antiangiogenesis therapy using an integrin antagonist or anti-FLK-1 antibody coated Y-90-labeled nanoparticles. Int J Radiat Oncol Biol Phys. 2004;58(4):1215–1227. doi: 10.1016/j.ijrobp.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 58.Zhao YS, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, Clement-Lacroix P, Clezardin P. Tumor alpha(nu)beta(3) integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67(12):5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 59.Guarneri V, Dieci MV, Conte P. Enhancing intracellular taxane delivery: current role and perspectives of nanoparticle albumin-bound paclitaxel in the treatment of advanced breast cancer. Expert Opin Pharmacother. 2012;13(3):395–406. doi: 10.1517/14656566.2012.651127. [DOI] [PubMed] [Google Scholar]
- 60.Kim JG. Cancer nanotechnology: engineering multifunctional nanostructures for targeting tumor cells and vasculatures. Atlanta: Georgia Institute of Technology; 2007. [Google Scholar]
- 61.Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor. Part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121(2):159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases—preface. Adv Drug Deliv Rev. 2004;56(8):1055–1058. doi: 10.1016/j.addr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Lu YJ, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother. 2002;51(3):153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richardson DR, Kalinowski DS, Lau S, Jansson PJ, Lovejoy DB. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta, Gen Subj. 2009;1790(7):702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: a review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol. 2003;4(7):397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 66.Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34(7):592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kateb B, Chiu K, Black KL, Yamamoto V, Khalsa B, Ljubimova JY, Ding H, Patil R, Portilla-Arias JA, Modo M, Moore DF, Farahani K, Okun MS, Prakash N, Neman J, Ahdoot D, Grundfest W, Nikzad S, Heiss JD. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? NeuroImage. 2011;54(1):S106–S124. doi: 10.1016/j.neuroimage.2010.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat. 2011;14(3):150–163. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong J, Chen M, Zheng Y, Wang S, Wang Y (2012) Polymeric micelles drug delivery system in oncology. J Control Release 159:312–323 [DOI] [PubMed]
- 71.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 72.van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007;24(8):1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 73.Chan JM, Zhang LF, Yuet KP, Liao G, Rhee JW, Langer R, Farokhzad OC. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30(8):1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 74.Mansour HM, Sohn M, Al-Ghananeem A, DeLuca PP. Materials for pharmaceutical dosage forms: molecular pharmaceutics and controlled release drug delivery aspects. Int J Mol Sci. 2010;11(9):3298–3322. doi: 10.3390/ijms11093298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohamed F, van der Walle CF. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J Pharm Sci. 2008;97(1):71–87. doi: 10.1002/jps.21082. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, Zhang LJ, Webster TJ. Nanobiomaterials: state of the art and future trends. Adv Eng Mater. 2011;13(6):B197–B217. [Google Scholar]
- 77.Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci. 2008;33(11):1088–1118. [Google Scholar]
- 78.Seyednejad H, Ghassemi AH, van Nostrum CF, Vermonden T, Hennink WE. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J Control Release. 2011;152(1):168–176. doi: 10.1016/j.jconrel.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 79.Vroman I, Tighzert L. Biodegradable polymers. Materials. 2009;2(2):307–344. [Google Scholar]
- 80.Park J, Mattessich T, Jay SM, Agawu A, Saltzman WM, Fahmy TM. Enhancement of surface ligand display on PLGA nanoparticles with amphiphilic ligand conjugates. J Control Release. 2011;156(1):109–115. doi: 10.1016/j.jconrel.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaucher GV, Marchessault RH, Leroux J-C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J Control Release. 2010;143(1):2–12. doi: 10.1016/j.jconrel.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B-Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 83.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. Acs Nano. 2008;2(5):889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park JH, Lee S, Kim JH, Park K, Kim K, Kwon IC. Polymeric nanomedicine for cancer therapy. Prog Polym Sci. 2008;33(1):113–137. [Google Scholar]
- 85.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12(1):39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Q, Guo X, Chen T, Zhang Z, Shao SJ, Luo C, Li JR, Zhou SB. Target-specific cellular uptake of folate-decorated biodegradable polymer micelles. J Phys Chem B. 2011;115(43):12662–12670. doi: 10.1021/jp207951e. [DOI] [PubMed] [Google Scholar]
- 87.Danquah MK, Zhang XA, Mahato RI. Extravasation of polymeric nanomedicines across tumor vasculature. Adv Drug Deliv Rev. 2011;63(8):623–639. doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Jang SH, Wientjes MG, Lu D, Au JLS. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 89.Liang CY, Yang YB, Ling Y, Huang YS, Li T, Li XM. Improved therapeutic effect of folate-decorated PLGA-PEG nanoparticles for endometrial carcinoma. Bioorg Med Chem. 2011;19(13):4057–4066. doi: 10.1016/j.bmc.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 90.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 91.Maeda H, Greish K, Fang J (2006) The EPR effect and polymeric drugs: a paradigm shift for cancer chemotherapy in the 21st century. In: SatchiFainaro R, Duncan R (eds) Polymer therapeutics II: Polymers as drugs, conjugates and gene delivery systems. Adv Polym Sci 193:103–121. doi::10.1007/12_026
- 92.Xu J, Ganesh S, Amiji M (2011) Non-condensing polymeric nanoparticles for targeted gene and siRNA delivery. Int J Pharm 427:21–34 [DOI] [PMC free article] [PubMed]
- 93.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23(7):1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 95.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 96.Lammers T, Kiessling F, Hennink WE, Storm G (2012) Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J Control Release 161:175–187 [DOI] [PubMed]
- 97.Lee ES, Gao ZG, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132(3):164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vladimir T. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71(3):431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc Chem Res. 2011;44(10):1061–1070. doi: 10.1021/ar2001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahato R, Tai WY, Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliv Rev. 2011;63(8):659–670. doi: 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94(1):1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Guo P, Coban O, Snead NM, Trebley J, Hoeprich S, Guo S, Shu Y. Engineering RNA for Targeted siRNA delivery and medical application. Adv Drug Deliv Rev. 2010;62(6):650–666. doi: 10.1016/j.addr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu C-MJ, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 104.Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367(1–2):195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28(5):869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release. 2007;120(1–2):18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Li K, Pan J, Liu B, Feng S-S. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials. 2010;31(2):330–338. doi: 10.1016/j.biomaterials.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 108.Park J, Fong PM, Lu J, Russell KS, Booth CJ, Saltzman WM, Fahmy TM. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomed-Nanotechnol Biol Med. 2009;5(4):410–418. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sutton D, Nasongkla N, Blanco E, Gao JM. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24(6):1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 110.Zhou J, Patel TR, Fu M, Bertram JP, Saltzman WM. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33(2):583–591. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Efthimiadou EK, Tapeinos C, Bilalis P, Kordas G. New approach in synthesis, characterization and release study of pH-sensitive polymeric micelles, based on PLA-Lys-b-PEGm, conjugated with doxorubicin. J Nanoparticle Res. 2011;13(12):6725–6736. [Google Scholar]
- 112.Yoo J-W, Doshi N, Mitragotri S. Adaptive micro and nanoparticles: temporal control over carrier properties to facilitate drug delivery. Adv Drug Deliv Rev. 2011;63(14–15):1247–1256. doi: 10.1016/j.addr.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 113.Rasal RM, Janorkar AV, Hirt DE. Poly(lactic acid) modifications. Prog Polym Sci. 2010;35(3):338–356. [Google Scholar]
- 114.Thamake SI, Raut SL, Ranjan AP, Gryczynski Z, Vishwanatha JK. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology. 2011;22(3):035101. doi: 10.1088/0957-4484/22/3/035101. [DOI] [PubMed] [Google Scholar]
- 115.Sussman EM, Clarke MB, Shastri VP. Single-step process to produce surface-functionalized polymeric nanoparticles. Langmuir. 2007;23(24):12275–12279. doi: 10.1021/la701997x. [DOI] [PubMed] [Google Scholar]
- 116.Wiradharma N, Zhang Y, Venkataraman S, Hedrick JL, Yang YY. Self-assembled polymer nanostructures for delivery of anticancer therapeutics. Nano Today. 2009;4(4):302–317. [Google Scholar]
- 117.Chung YI, Kim JC, Kim YH, Tae G, Lee SY, Kim K, Kwon IC. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J Control Release. 2010;143(3):374–382. doi: 10.1016/j.jconrel.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 118.Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol. 2011;670(2–3):372–383. doi: 10.1016/j.ejphar.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 119.Bilensoy E, Sarisozen C, Esendagli G, Dogan AL, Aktas Y, Sen M, Mungan NA. Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. Int J Pharm. 2009;371(1–2):170–176. doi: 10.1016/j.ijpharm.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 120.Chakravarthi SS, Robinson DH. Enhanced cellular association of paclitaxel delivered in chitosan-PLGA particles. Int J Pharm. 2011;409(1–2):111–120. doi: 10.1016/j.ijpharm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 121.Khandare J, Minko T. Polymer–drug conjugates: progress in polymeric prodrugs. Prog Polym Sci. 2006;31(4):359–397. [Google Scholar]
- 122.Thanh NTK, Green LAW. Functionalisation of nanoparticles for biomedical applications. Nano Today. 2010;5(3):213–230. [Google Scholar]
- 123.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A. 2008;105(45):17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao H, Yung LYL. Selectivity of folate conjugated polymer micelles against different tumor cells. Int J Pharm. 2008;349(1–2):256–268. doi: 10.1016/j.ijpharm.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 125.Pan J, Feng SS. Targeted delivery of paclitaxel using folate-decorated poly(lactide)—vitamin E TPGS nanoparticles. Biomaterials. 2008;29(17):2663–2672. doi: 10.1016/j.biomaterials.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 126.Wang C, Ho PC, Lim LY. Wheat germ agglutinin-conjugated PLGA nanoparticles for enhanced intracellular delivery of paclitaxel to colon cancer cells. Int J Pharm. 2010;400(1–2):201–210. doi: 10.1016/j.ijpharm.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 127.Heidel JD, Davis ME. Clinical developments in nanotechnology for cancer therapy. Pharm Res. 2011;28(2):187–199. doi: 10.1007/s11095-010-0178-7. [DOI] [PubMed] [Google Scholar]
- 128.Lee SH, Mok H, Lee Y, Park TG. Self-assembled siRNA-PLGA conjugate micelles for gene silencing. J Control Release. 2011;152(1):152–158. doi: 10.1016/j.jconrel.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 129.Susa M, Milane L, Amiji MM, Hornicek FJ, Duan ZF. Nanoparticles: a promising modality in the treatment of sarcomas. Pharm Res. 2011;28(2):260–272. doi: 10.1007/s11095-010-0173-z. [DOI] [PubMed] [Google Scholar]
- 130.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6(4):319–327. [Google Scholar]
- 131.Xiong XB, Uludag H, Lavasanifar A. Biodegradable amphiphilic poly(ethylene oxide)-block-polyesters with grafted polyamines as supramolecular nanocarriers for efficient siRNA delivery. Biomaterials. 2009;30(2):242–253. doi: 10.1016/j.biomaterials.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 132.Bastani B, Fernandez D. Intellectual property rights in nanotechnology. Thin Solid Films. 2002;420:472–477. [Google Scholar]
- 133.Merchant M (2010) Nanotechnology in Healthcare. Business Insights, 16–218
- 134.Koppikar V, Maebius S, Rutt S. Current trends in nanotech patents: a view from inside the patent office. Nanotechnol Law Bus. 2004;1(1):24–30. [Google Scholar]
- 135.Laurie A, Axford GRL. Patent drafting considerations for nanotechnology inventions. Nanotechnol Law Bus. 2006;3(3):305. [Google Scholar]
- 136.O’Neill S, Hermann K, Klein M, Landes J, Bawa R. Broad claiming in nanotechnology patents: is litigation inevitable? Nanotechnol Law Bus. 2007;4(1):595–606. [Google Scholar]
- 137.Fraser-Moodie I (2008) Delivery mechanisms for large molecule drugs. Business Insights, 10–138
- 138.EPO (2010) Guidelines for examination in the European Patent Office. Part C—guidelines for substantive examination—Chapter IV patentability.
- 139.Burgess P, Hutt PB, Farokhzad OC, Langer R, Minick S, Zale S. On firm ground: IP protection of therapeutic nanoparticles. Nat Biotechnol. 2010;28(12):1267–1271. doi: 10.1038/nbt.1725. [DOI] [PubMed] [Google Scholar]
- 140.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 141.Shepherd FA, Pereira JR, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu DS, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L, Natl Canc Inst Canada Clin T Erlotinib in previously treated non-small-cell lung cancer. New Eng J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 142.Kuesters GM, Campbell RB. Conjugation of bevacizumab to cationic liposomes enhances their tumor-targeting potential. Nanomedicine. 2010;5(2):181–192. doi: 10.2217/nnm.09.105. [DOI] [PubMed] [Google Scholar]
- 143.Shorr R, Rodriguez R (2007) Pharmaceutical and diagnostic compositions containing nanoparticles useful for treating targeted tissues and cells. US patent no. 7,521,066
- 144.Borbely J, Bodnar M, Hartmann JF, Hajdu I, Kollar J, Vamosi G (2008) Cancer cell diagnosis by targeting delivery of nanodevices. US patent no. 7,976,825
- 145.Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine. 2007;2(1):125–132. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem. 2006;17(3):603–609. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 147.El-Sayed IH, Huang XH, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 148.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8(3):235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 149.Oyelere Adegboyega K, EL-Sayed Mostafa A, Dreaden EC (2009) Targeted cellular delivery of nanoparticles. US Patent application no. 2011077581
- 150.Akhtari M, Engel J (2009) Use of functionalized magnetic nanoparticles in cancer detection and treatment. US Patent application no. 2011044911
- 151.Layton B (2008) Recent patents in bionanotechnologies: nanolithography, bionanocomposites, cell-based computing and entropy production. US patent no. 7,638,558 [DOI] [PubMed]
- 152.Liu L, Li C, Li X, Yuan Z, An Y, He B. Biodegradable polylactide/poly(ethylene glycol)/polylactide triblock copolymer micelles as anticancer drug carriers. J Appl Polym Sci. 2001;80(11):1976–1982. [Google Scholar]
- 153.Radosz M, Xu P, Shen Y (2005) Nanoparticles for cytoplasmic drug delivery to cancer cells. US Patent application no. 2010203149
- 154.Desai NP, Soon-Shiong P (2007) Breast cancer therapy based on hormone receptor status with nanoparticles comprising taxane. US Patent application no. 20070519126
- 155.Moradi M. Global developments in nano-enabled drug delivery market. Nanothecnology Law & Business. 2005;2(2):139–148. [Google Scholar]