Abstract
Mature adipocyte-derived dedifferentiated fat (DFAT) cells rapidly differentiate into osteoblasts under three-dimensional culture conditions. However, it has not been demonstrated that DFAT cells can differentiate into osteoblasts in a rigid scaffold consisting of titanium fiber mesh (TFM). We examined the proliferation and osteogenic differentiation ability of DFAT cells using TFM as a scaffold. DFAT cells derived from rabbit subcutaneous fat were seeded into TFM and cultured in osteogenic medium containing dexamethasone, l-ascorbic acid 2-phosphate and β-glycerophosphate for 14 days. In scanning electron microscopy (SEM) analysis, well-spread cells covered the titanium fibers on day 3, and appeared to increase in number from day 3 to 7. Numerous globular accretions were found and almost completely covered the fibers on day 14. Cell proliferation, as measured by DNA content in the TFM, was significantly higher on day 7 compared with that of day 1. Osteocalcin and calcium content in the TFM were significantly higher on day 14 compared to those of days 1, 3, and 7, indicating DFAT cells differentiated into osteoblasts. We theorize that globular accretions observed in SEM analysis may be calcified matrix resulting from osteocalcin secreted by osteoblasts binding calcium contained in fetal bovine serum. In this study, we demonstrated that DFAT cells differentiate into osteoblasts and deposit mineralized matrices in TFM. Therefore, the combination of DFAT cells and TFM may be an attractive option for bone tissue engineering.
Keywords: Dedifferentiated fat cells, Ceiling culture, Titanium fiber mesh, Bone tissue engineering
Introduction
Bone reconstruction is essential to repair bone defects resulting from serious injury, inflammation, tumor resection, skeletal deformity and other causes. Multiple approaches using autografts, allografts, xenografts and biomaterials are currently used to repair bone defects, but are also associated with numerous problems. Autograft transplantation suffers from insufficient supply and significant surgical morbidity of the donor site (Shimizu et al. 2007). Allografts and xenografts have inherent risks such as transmission of disease from donor to recipient and immunogenic responses. Synthetic bone substitute biomaterials, such as calcium phosphate ceramics, are usually not osteogenic (Hamada et al. 2008). To overcome these problems, various bone tissue engineering approaches have been attempted. The general approach of bone tissue engineering is to combine biomaterial scaffolds and cells to promote bone tissue regeneration. Therefore, selection of appropriate cell sources and scaffolds is considered very important.
Adipose-derived stem/stromal cells (ASCs) can differentiate along multiple lineages including adipocytes, osteoblasts, chondrocytes, myocytes, neuronal cells, endothelial cells and hepatocytes (Schäffler and Büchler 2007). ASCs are extensively used for bone tissue engineering, and their utility has been widely reported (Li et al. 2007; Lin et al. 2008; Yoon et al. 2007). However, ASCs are a heterogeneous cell population, particularly at early passages. ASCs at passage 0 contain contaminating endothelial and smooth muscle cells as well as pericytes (Zuk et al. 2001). Therefore, other stem cell sources with high purity are needed for clinical application.
In contrast to ASCs, mature adipocyte-derived dedifferentiated fat (DFAT) cells are a highly homogeneous cell population. Because of a high proliferative potential and their ability to differentiate into multiple cell types, including osteoblasts, chondrocytes and adipocytes, DFAT cells are thought to be useful as donor cells for tissue engineering (Matsumoto et al. 2008). In previous studies, we used a combination of DFAT cells and self-assembling peptide hydrogel RADA16 for bone tissue engineering (Kishimoto et al. 2008, 2011). However, hydrogels such as RADA16 are fragile after gel formation, and therefore a stronger scaffold is necessary to resist loading forces during the initial bone-healing period, for example, bone reconstruction after jaw resection in cranio-facial surgery.
Sintered titanium fiber mesh (TFM) has excellent mechanical properties, can be molded to the required shape and has been shown to be biocompatible (Jansen et al. 1992; Vehof et al. 2000, 2003). TFM can be used as a carrier for growth factors and bone marrow stromal cells (BMSCs) to induce bone formation (Vehof et al. 2000, 2003). We previously reported that DFAT cells differentiate into osteoblasts as early as day 7 of culture under three-dimensional (3D) culture conditions (Kishimoto et al. 2011). Using 3D culture with the combination of TFM, a rigid scaffold, and DFAT cells, which rapidly differentiate in a 3D environment, could be attractive in the field of bone tissue engineering. However, it has not been demonstrated that DFAT cells can differentiate into osteoblasts and deposit mineralized matrices in a 3D TFM scaffold. The objective of this study is to evaluate the proliferation and osteogenic differentiation ability of DFAT cells combined with TFM as a scaffold.
Materials and methods
Rabbit DFAT cell isolation and culture
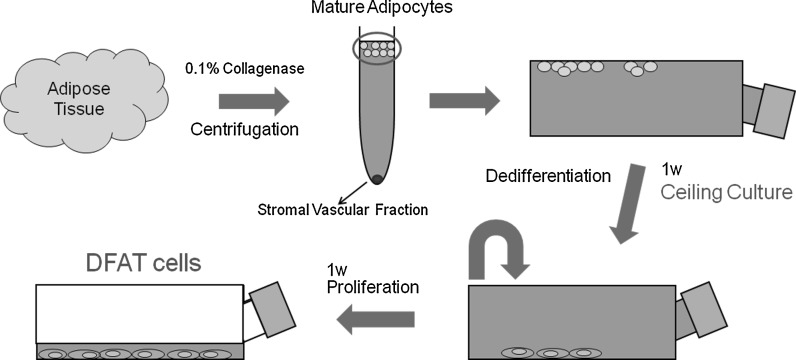
This study was approved by the animal research committee of Osaka Dental University (approval No. 11-03041). Fat tissue was collected from the inguinal region of 7-week-old male Japanese white rabbits to isolate mature adipocytes. Approximately 1 g fat tissue was minced into small pieces and dissolved in 0.1 % (w/v) collagenase solution (Collagenase type I; Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 37 °C for 1 h with gentle agitation. The cell suspension was filtered through 150 and 250 μm nylon meshes to allow cells to pass through and exclude unwanted stromal cells and tissue. Floating mature adipocytes in the top layer were collected and centrifuged at 135 g for 3 min. Isolated mature adipocytes were seeded into a 25 cm2 culture flask (SUMILON; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) completely filled with a standard medium consisting of DMEM (NACALAI TESQUE, INC., Kyoto, Japan) supplemented with 20 % (v/v) fetal bovine serum (FBS) (lot number 1412447, Invitrogen, Life Technologies Corp., Carlsbad, CA, USA), and antibiotics and antimycotics (an antibiotic/antimycotic mixed stock solution consisting of 10,000 units/mL penicillin, 10,000 μg/mL streptomycin and 25 μg/mL amphotericin B; NACALAI TESQUE, INC.), and were incubated at 37 °C with 5 % CO2. The flask was placed with the adhesive surface facing upward, so that floating adipocytes containing lipid droplets attached to the inner ceiling surface of the flask. This method is referred to as “ceiling culture” (Fig. 1). After 7 days, medium was removed and the flask was inverted. Fresh medium was added to barely cover the bottom of the flask. Medium was then exchanged biweekly. After reaching confluency, cells were passaged and used for experiments.
Fig. 1.
Isolation of rabbit dedifferentiated fat (DFAT) cells by “ceiling culture”. A small piece of adipose tissue was digested with 0.1 % collagenase. After centrifugation, suspended adipocytes in the top layer were collected and cultured in flasks completely filled with DMEM supplemented with 20 % fetal bovine serum for 7 days. During culture, cells attached and flattened on the upper surface of flasks, followed by dedifferentiation into DFAT cells. Flasks were then inverted and DFAT cells were cultured using conventional methods
Titanium fiber mesh (TFM)
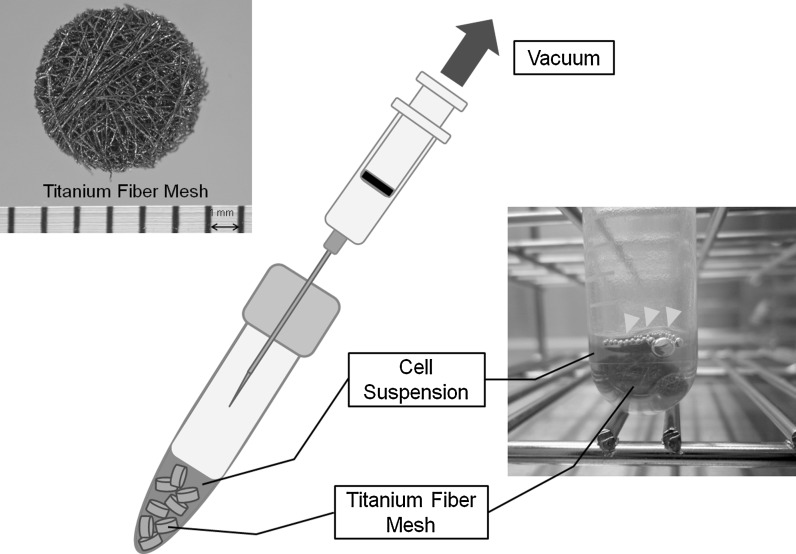
TFM (Titanium Web Cell-House; HI-LEX CORPORATION, Hyogo, Japan) with an 87 % volumetric porosity and 50 μm fiber diameter was used as scaffold material (Fig. 2). Prepared titanium fiber discs were shaped with a 5 mm diameter and 1.5 mm thickness. Meshes were sonicated for 10 min in isopropanol and then sterilized at 180 °C for 1 h.
Fig. 2.
Technique for cell seeding into titanium fiber mesh (TFM). Prepared titanium fiber discs were shaped with a 5 mm diameter and 1.5 mm thickness. DFAT cells were collected and suspended at 1 × 107 cells/mL in osteogenic medium. One milliliter of cell suspension and eight TFMs were transferred into a tube and a partial vacuum was applied by retracting a syringe plunger with the needle inserted into the tube cap. Yellow arrowheads indicate the air removed from the TFM by partial vacuum
Cell seeding technique
For cell seeding into TFM, we used a method described elsewhere (Tadokoro et al. 2008). Briefly, DFAT cells were detached with 0.25 % trypsin in 1 mM EDTA solution (NACALAI TESQUE, INC.) and centrifuged at 135 g for 5 min. Collected cells were resuspended at 1 × 107 cells/mL in osteogenic medium consisting of DMEM supplemented with 10 % FBS, 100 nM dexamethasone (Sigma-Aldrich, St, Louis, MO, USA), 50 μM L-ascorbic acid 2-phosphate (Sigma-Aldrich) and 10 mM β-glycerophosphate (Sigma-Aldrich). One milliliter of cell suspension and eight TFMs were transferred into a 13 mL tube (sterilized two position tube; SARSTEDT AG & CO. Nuremberg, Germany) and a partial vacuum was applied by retracting a syringe plunger with the needle inserted into the tube cap (Fig. 2). Air in the TFM was removed by the partial vacuum, and the cell suspension could flow into the TFM. Then, TFMs were incubated in the 13 mL tube with the cap loose at 37 °C with 5 % CO2 for 30 min, followed by transferring into a 24 well plate (Becton–Dickinson Labware, Franklin Lakes, NJ, USA), DFAT cells seeded into the TFM were cultured in osteogenic medium for 14 days. Medium was exchanged biweekly.
Scanning electron microscopy
After 3, 7 and 14 days of culture, TFMs were washed twice with PBS, and samples were fixed with 2 % glutaraldehyde in 0.1 M phosphate buffer for 1 h followed by 1 % OsO4 in 0.1 M phosphate buffer for 1 h. After dehydration through an ethanol series, samples were placed in t-butyl alcohol and dried using a critical point dryer (CRITICAL POINT DRYER HCP-1, Hitachi, Tokyo, Japan), followed by platinum ion coating (E-1030, Hitachi) and examination by SEM (S-4000, Hitachi) at a 3 kV accelerating voltage.
DNA analysis
DNA content was measured on days 1, 3, 7 and 14. Medium was removed from each mesh and the cell layer was washed twice with PBS. Each mesh was transferred into a 1.5 mL tube containing 300 μL 0.2 % Triton X-100 and then mixed by pipetting. A 5 mm hardened steel ball was placed into each tube, and then tubes were agitated on a shaker (Mixer Mill Type MM 301, Retsch Gmbh & Co. KG, Haan, Germany) at 29 Hz for 40 s to homogenize the sample. DNA content was measured with a Quant-iT™ PicoGreen® dsDNA Reagent and Kit (Invitrogen). Fifty microliters of sample was mixed with a DNA-binding fluorescent dye solution (0.5 μL Picogreen reagent in 100 μL 1× TE buffer), and the fluorescent intensity was measured with a microplate reader (Ex 450 nm/Em 510 nm; SpectraMax® M5, Molecular Device, Sunnyvale, CA, USA).
Osteocalcin measurement
A Gla-Type Osteocalcin EIA Kit (Takara Bio Inc., Shiga, Japan) was used to determine the expression of osteocalcin, an osteoblast-specific protein. On days 1, 3, 7 and 14 of culture, after washing with PBS, each mesh was transferred into a 1.5 mL tube containing 300 μL 10 % formic acid and mixed by pipetting. Tubes were then agitated on a shaker (Mixer Mill Type MM 301, Retsch Gmbh & Co. KG) under the same conditions used for DNA measurement to homogenize the sample. Samples (100 μL) were added to each well of an anti-osteocalcin antibody-coated microtiter plate and incubated at room temperature for 2 h. After washing three times with PBS, 100 μL peroxidase-conjugated anti-osteocalcin antibody solution was added to each well and incubated at room temperature for 1 h. After washing four times with PBS, 100 μL substrate solution (tetramethylbenzidine) was added to each well and incubated at room temperature for 15 min. Then 100 μL stop solution was added to each well, absorbance was measured at 450 nm with a microplate reader (SpectraMax® M5, Molecular Device).
Calcium measurement
Calcium content was measured with a Calcium E-test Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Two milliliters of monoethanolamine buffer was added to 50 μL of the same homogenized sample used for osteocalcin measurement, and then mixed thoroughly. After 1 mL methylxylenol blue coloring agent was added to the mixture, absorbance was measured at 610 nm with a microplate reader (SpectraMax® M5, Molecular Device).
Statistical analysis
Data were expressed as the mean ± standard deviation and analyzed using non-repeated measures analysis of variance, followed by the Student-Newman–Keuls test. Differences were considered significant at P < 0.05.
Results
Microscopy analysis
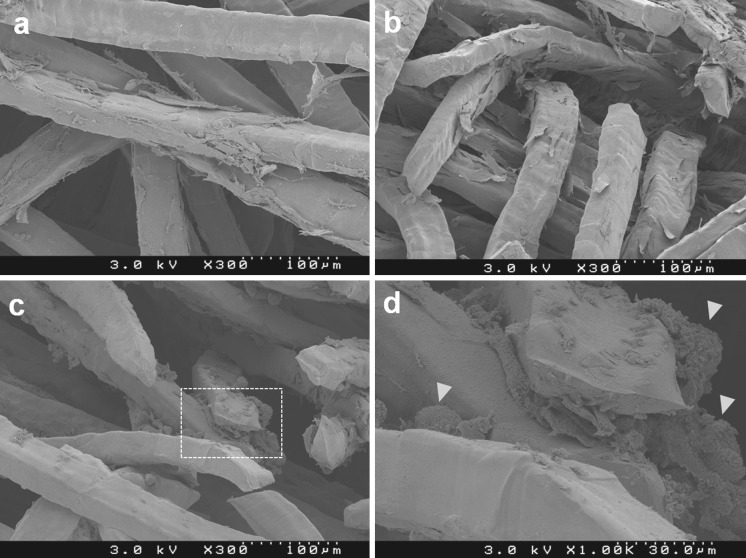
In SEM analysis, well-spread cells covered the titanium fibers on day 3 (Fig. 3a), and appeared to increase in number from day 3 to 7 (Fig. 3b). Numerous large and small globular accretions (indicated by yellow arrowheads in Fig. 3d) were found and almost completely covered the fibers on day 14 (Fig. 3c).
Fig. 3.
Scanning electron microscopy analysis. a Well-spread cells covered titanium fibers on day 3. b Cells appeared to increase in number on day 7. c Numerous large and small globular accretions (indicated by yellow arrowheads in d) were found and almost completely covered the fibers on day 14. d is an enlargement of the white dotted frame in c
DNA
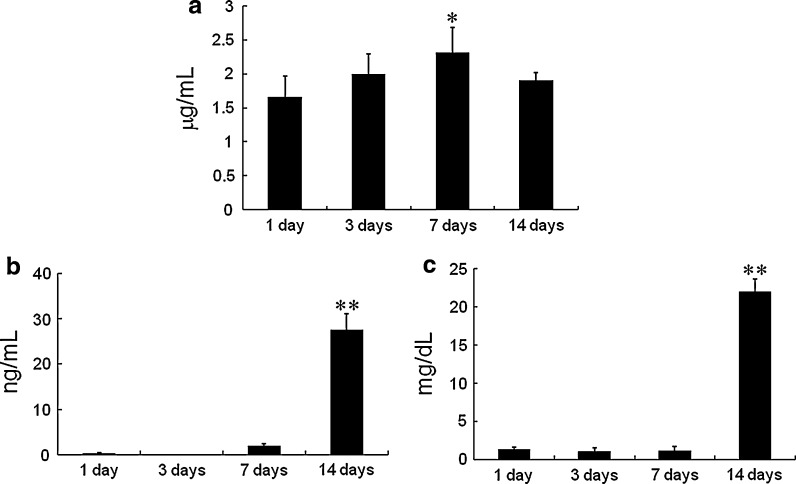
DNA content in the TFM was significantly higher on day 7 compared with that of day 1. However, no significant difference was found on day 14 compared with that of all other time points (Fig. 4a).
Fig. 4.
a DNA content in the DFAT cell/TFM construct after 1, 3, 7 and 14 days of culture in osteogenic medium. b Osteocalcin content in the DFAT cell/TFM construct after 1, 3, 7 and 14 days of culture in osteogenic medium. c Calcium content in the DFAT cell/TFM construct after 1, 3, 7 and 14 days of culture in osteogenic medium. Data are the mean ± standard deviation. *P < 0.05 versus day 1, **P < 0.01 versus days 1, 3 and 7
Osteocalcin and calcium
Osteocalcin and calcium content in the TFM were significantly higher on day 14 compared with those of days 1, 3 and 7 (Fig. 4b and c).
Discussion
In the present study, we evaluated the proliferation and osteogenic differentiation ability of DFAT cells seeded into TFM. DFAT cells proliferated in the TFM because numerous well-spread cells were found around titanium fibers and appeared to increase in number from day 3 to 7 in SEM analysis. DNA content, as an indicator of cell proliferation, also increased. DFAT cells differentiated into osteoblasts in TFM because osteocalcin (Zhao et al. 2009) and calcium (Lian and Stein 1992) as late and terminal stage markers of osteoblast differentiation, respectively, increased remarkably on day 14. Osteocalcin is a vitamin K-dependent protein produced by osteoblasts and strongly binds to calcium and hydroxyapatite (Park et al. 2009). Many globular accretions observed in SEM analysis resembled the morphology of calcified matrix deposited around titanium fibers in a previous report (Van Dolder et al. 2002). We theorize that these globular accretions are calcified matrix resulting from osteocalcin secreted by osteoblasts binding calcium contained in FBS. There was no significant difference in DNA content between day 14 and all other time points, which increased from day 1 to 7, and may be related to osteoblastic differentiation of DFAT cells. In general, there is an inverse relationship between proliferation and differentiation in bone cell cultures because of a decline of the nutritional state during mineralized matrix deposition (Owen et al. 1990). In this study, inhibition of cell proliferation may have increased due to increasing osteocalcin and calcium content, followed by calcified matrix deposition. The sudden increase of osteocalcin and calcium content on day 14 appeared to coincide with a change from proliferation to differentiation during day 7–14.
In this study, we used TFM as a scaffold for seeding DFAT cells. TFM possesses excellent strength, has been demonstrated to be osteoconductive and supports the attachment and growth of BMSCs (Vehof et al. 2001, 2002). Van den Dolder and colleagues reported on the utility of the combination of TFM and BMSCs for bone tissue engineering (Van Dolder et al. 2002, 2003a, b, c). In their previous studies, various techniques were used to show that cell seeding into TFM could be achieved by rotating a 10 mL tube containing a cell suspension on a rotation plate at 2 rpm (Van Dolder et al. 2002, 2003b), a droplet technique in which the cell suspension was placed as a droplet on top of the TFM (Van Den Dolder et al. 2003c), a cell suspension technique with the TFM in a 24 well plate (containing cell suspension) that was manually shaken every half hour (Van Den Dolder et al. 2003c) and a flow perfusion culture system (Van Den Dolder et al. 2003a). However, the rotation plate, droplet and cell suspension techniques showed limited cell penetration into TFM (Van Den Dolder et al. 2003a, c). Special equipment such as a bioreactor is necessary for a flow perfusion culture system, thus, it is not a simple and easy technique. Therefore, we used a vacuum method in this study for seeding DFAT cells into TFM. The vacuum method is economical, can be easily performed and is widely used for bone tissue engineering (Bruder et al. 1998; Kadiyala et al. 1997; Tadokoro et al. 2008). Applying a mild vacuum for a short period to remove air from a porous scaffold enhances contact between the scaffold material and cell suspension (Vehof et al. 2000). In this study, DFAT cell seeding in TFM using the vacuum method was able to efficiently attach DFAT cells to the scaffold, which may subsequently promote proliferation and differentiation of DFAT cells.
Van den Dolder and colleagues emphasized the variance in results between experimental runs using BMSCs and TFM. It was proposed that this variance was due to BMSCs being a heterogeneous cell population, and a homogeneous cell population is necessary to produce reliable and standardized tissue-engineered bone constructs (Van Dolder et al. 2002, 2003c). DFAT cells comprise a highly homogeneous cell population because DFAT cells originate from a fraction of highly homogeneous mature adipocytes. ASCs are derived from the stromal-vascular fraction that can be isolated by centrifugation of collagenase-digested fat tissue (Fig. 1). ASCs are a minor population of cells in fat tissue, and cultured ASCs are a heterogeneous cell population. It was reported that ASCs contain 18.6 % smooth muscle cells, 12.8 % lymphocytes, 13.3 % monocytes and 2.7 % endothelial cells. In contrast, these cells are highly infrequent in DFAT cell populations (0.00–0.07 %), and it has been reported that DFAT cells are a much more homogeneous population than ASCs (Matsumoto et al. 2008). Therefore, we believe that DFAT cells are more appropriate compared with that of BMSCs and ASCs as a cell source for bone tissue engineering. In this study, we demonstrated the combination of DFAT cells, which possess the beneficial characteristics mentioned above, and TFM is useful for bone tissue engineering in vitro. This study is a crucial process to validate further in vivo study. In future studies, we intend to transplant the construct of DFAT cells and TFM into rabbit calvarial bone defects, and evaluate the capacity of DFAT cells to regenerate bone tissue in vivo. It might be effective for transplantation of DFAT cells using TFM as a scaffold because TFM possesses excellent strength and can resist loading forces during the initial bone-healing period.
In summary, we demonstrated that DFAT cells differentiate into osteoblasts and deposit mineralized matrices in TFM. Using DFAT cells as a homogeneous cell source may provide standardization and improve the reliability of bone tissue engineering.
Acknowledgments
We thank Yoichiro Taguchi (Department of Periodontology, Osaka Dental University) and Hideaki Hori (Institute of Dental Research, Osaka Dental University) for the valuable suggestion of SEM analysis. This study was supported by a Grant-in-Aid for Scientific Research (B) No. 11023866 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Hamada K, Hirose M, Yamashita T, Ohgushi H. Spatial distribution of mineralized bone matrix produced by marrow mesenchymal stem cells in self-assembling peptide hydrogel scaffold. J Biomed Mater Res A. 2008;84:128–136. doi: 10.1002/jbm.a.31439. [DOI] [PubMed] [Google Scholar]
- Jansen JA, Von Recum AF, Van der Waerden JPCM, De Groot K. Soft tissue response to different types of sintered metal fibre-web materials. Biomaterials. 1992;13:959–968. doi: 10.1016/0142-9612(92)90121-4. [DOI] [PubMed] [Google Scholar]
- Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1016/S0963-6897(96)00279-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto N, Momota Y, Mori R, Hashimoto Y, Imai K, Omasa T, Kotani J. Bone regeneration using dedifferentiated fat cells with PuraMatrix™. J Oral Tissue Engin. 2008;6:127–134. [Google Scholar]
- Kishimoto N, Momota Y, Hashimoto Y, Omasa T, Kotani J. Self-assembling peptide RADA16 as a scaffold in bone tissue engineering using dedifferentiated fat cells. J Oral Tissue Engin. 2011;8:151–161. [Google Scholar]
- Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun. 2007;356:836–842. doi: 10.1016/j.bbrc.2007.02.165. [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3:269–305. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- Lin Y, Tang W, Wu L, Jing W, Li X, Wu Y, Liu L, Long J, Tian W. Bone regeneration by BMP-2 enhanced adipose stem cells loading on alginate gel. Histochem Cell Biol. 2008;129:203–210. doi: 10.1007/s00418-007-0351-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Park KH, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108:530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Ito A, Honda H. Mag-seeding of rat bone marrow stromal cells into porous hydroxyapatite scaffolds for bone tissue engineering. J Biosci Bioeng. 2007;104:171–177. doi: 10.1263/jbb.104.171. [DOI] [PubMed] [Google Scholar]
- Tadokoro M, Hirose M, Ohgushi H. Preliminary study for evaluating bone forming ability of porous bioceramics using rat mesenchymal stem cells to be used for international standard. Key Eng Mater. 2008;361–363:1161–1164. doi: 10.4028/www.scientific.net/KEM.361-363.1161. [DOI] [Google Scholar]
- Van Den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PHM, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J Biomed Mater Res A. 2003;64:235–241. doi: 10.1002/jbm.a.10365. [DOI] [PubMed] [Google Scholar]
- Van Den Dolder J, Farber E, Spauwen PHM, Jansen JA. Bone tissue reconstruction using titanium fiber mesh combined with rat bone marrow stromal cells. Biomaterials. 2003;24:1745–1750. doi: 10.1016/S0142-9612(02)00537-9. [DOI] [PubMed] [Google Scholar]
- Van Den Dolder J, Spauwen PHM, Jansen JA. Evaluation of various seeding techniques for culturing osteogenic cells on titanium fiber mesh. Tissue Eng. 2003;9:315–325. doi: 10.1089/107632703764664783. [DOI] [PubMed] [Google Scholar]
- Van Dolder JD, Vehof JWM, Spauwen PHM, Jansen JA. Bone formation by rat bone marrow cells cultured on titanium fiber mesh: effect of in vitro culture time. J Biomed Mater Res. 2002;62:350–358. doi: 10.1002/jbm.10189. [DOI] [PubMed] [Google Scholar]
- Vehof JWM, Spauwen PHM, Jansen JA. Bone formation in calcium-phosphate-coated titanium mesh. Biomaterials. 2000;21:2003–2009. doi: 10.1016/S0142-9612(00)00094-6. [DOI] [PubMed] [Google Scholar]
- Vehof JWM, De Ruijter AE, Spauwen PHM, Jansen JA. Influence of rhBMP-2 on rat bone marrow stromal cells cultured on titanium fiber mesh. Tissue Eng. 2001;7:373–383. doi: 10.1089/10763270152436436. [DOI] [PubMed] [Google Scholar]
- Vehof JWM, Haus MTU, De Ruijter AE, Spauwen PHM, Jansen JA. Bone formation in Transforming Growth Factor beta-1-loaded titanium fiber mesh implants. Clin Oral Implants Res. 2002;13:94–102. doi: 10.1034/j.1600-0501.2002.130112.x. [DOI] [PubMed] [Google Scholar]
- Vehof JWM, Van Den Dolder J, De Ruijter JE, Spauwen PHM, Jansen JA. Bone formation in CaP-coated and noncoated titanium fiber mesh. J Biomed Mater Res A. 2003;64:417–426. doi: 10.1002/jbm.a.10288. [DOI] [PubMed] [Google Scholar]
- Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GRD. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- Zhao J, Shinkai M, Takezawa T, Ohba S, Ui C, Nagamune T. Bone regeneration using collagen type I vitrigel with bone morphogenetic protein-2. J Biosci Bioeng. 2009;107:318–323. doi: 10.1016/j.jbiosc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]