Abstract
Citral, 3,7-dimethyl-2,6-octadienal, is a key component of the essential oils extracted from several lemon-scented herbal plants. Besides its antifungal activity, the anticancer effect of citral was studied in recent years. In this study, we investigated the effect of citral on the acute promyelocytic leukemia cell line NB4. Citral treatment had an antiproliferative effect in NB4 cells via the induction of apoptosis assessed by morphology, proliferation assay, DNA electrophoresis, Annexin V-FITC/PI staining and caspase-3 activation. And citral induced apoptosis of NB4 cells in a dose- and time-dependent manner. In addition, citral treatment induced decreased mitochondrial membrane potential, indicating that citral induced apoptosis via the mitochondrial pathway. Bax up-regulation and Bcl-2 down-regulation on mRNA level and NF-κB down-regulation on protein level was found in this study, suggesting that Bcl-2, Bax and NF-κB may be involved in the mechanism of the apoptotic effect of citral on NB4 cells. These data suggest that citral has a potential therapeutic effect on leukemia.
Keywords: Citral, Apoptosis, Acute promyelocytic leukemia, Bax, NB4
Introduction
Apoptosis is the process of programmed cell death which occurs during physiological conditions. It is characterized by morphological features including membrane blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation and formation of apoptotic bodies (Cohen 1999). Unlike the process of necrosis, the formation of apoptotic bodies, which phagocytes are able to remove quickly, can prevent the contents of the apoptotic cell spilling out and causing damage to surrounding tissues.
Tumorigenesis is strongly implicated in apoptosis. During tumorigenesis, genetic alterations in components of the apoptosis pathway have been found, which confer resistance to a number of physiological (oncogene-induced cell death, loss of adhesion, growth under hypoxia) and therapeutic (chemotherapy and radiation) death triggers. Meanwhile, inducing tumor cell apoptosis is thought to be the main mechanism of antitumor therapies (McGill and Fisher 1997; Karin and Greten 2005).
Apoptosis is primarily classified into two pathways, which include extrinsic pathway (or receptor-mediated pathway) and intrinsic pathway (or mitochondria-mediated pathway). The extrinsic pathway is initiated by interaction between death receptors on the surface of cell membranes and their respective ligands. This interaction activates the initiator caspase, caspase-8, which subsequently leads to the activation of the downstream effector caspase, caspase-3. The intrinsic pathway is initiated by a number of receptor-independent stimuli (e.g. radiation, free radicals, viral infections, chemotherapeutics etc.), and involved with an imbalance between proapoptotic (e.g. Bax etc.) and antiapoptotic (e.g. Bcl-2 etc.) proteins from Bcl-2 family in mitochondria and cytosol. This imbalance causes changes in the inner mitochondrial membrane permeability, the loss of mitochondrial transmembrane potential and the subsequent release of two groups of proapoptotic mitochondrial proteins. One group of released proteins, such as cytochrome c (cyt c) and Smac/DIABLO (second mitochondria-derived activator of caspases), may activate caspase dependent pathway. For example, Cyt c can activate caspase-9 and subsequently leads to the activation of the executioner caspases-3 and -7. The other group of proteins acts in a caspase-independent manner to execute cell death, including apoptosis inducing factor (AIF) and endonuclease G (Endo G). Because caspase-3 might be activated by both extrinsic and intrinsic pathway, it is thought to be a good indicator of apoptosis (Adams 2003; Indran et al. 2011).
Nuclear factor-κB (NF-κB) is a widely expressed family of transcription factors, which plays a key role as regulator of inflammation, cell proliferation and early pathogen response (Karin and Greten 2005; Vallabhapurapu and Karin 2009). Additionally, NF-κB activation is very strongly implicated in many aspects of tumorigenesis, including control of apoptosis, cell cycle, differentiation, and cell migration. The activation of NF-κB may prevent cells from apoptosis by inducing expression of genes encoding antiapoptotic proteins (Wang et al. 1998; Micheau et al. 2001), and several members of NF-κB family have been found amplified in some cancer cell lines (Gilmore et al. 1996; Rayet and Gelinas 1999; McKeithan et al. 1997). Moreover, the inhibition of NF-κB may prove to be therapeutic in certain leukemias or lymphomas (e.g. Hodgkin’s lymphoma), where NF-κB appears to play a unique survival role (Bargou et al. 1997).
Citral, 3,7-dimethyl-2,6-octadienal, is a key component of essential oils extracted from several lemon-scented herbal plants (e.g. Lemon grass etc.). Because of its particular aroma, substantial antibacterial, antifungal and insecticidal effects, low toxicity and low carcinogenecity, it has been widely used as food additive and fragrance material in cosmetics. In recent years, several studies reported that citral was capable of inducing apoptosis in certain tumor cell lines, such as MCF-7, U937 and HL60. (Chaouki et al. 2009; Dudai et al. 2005). The aim of our study was to investigate the apoptotic effect of citral on the acute promyelocytic leukemia cell line NB4 and the mechanism involved in this effect.
Materials and methods
Reagents
Citral (purity ≥ 95 %) was purchased from SIGMA company (St. Louis, MO, USA) and stored at 4 °C. As2O3 was purchased from Harbin Yida Pharmaceutical Company, Haerbin, Heilongjiang, China.
Cell line, and culture
The acute promyelocytic leukemia cell line NB4, donated by Miss Chen Saijuan from Shanghai Rui Jin Hospital, was seeded at a density of 1–5 × 105/mL in 75 cm2 culture flasks and grown in RPMI1640 (Gibco BRL, Cergy-Pontoise, France) supplemented with 10 % calf bovine serum (Sijiqing Company, Hangzhou, China). Cultures were maintained in a humidified atmosphere with 5 % CO2 at 37 °C.
Morphological study
NB4 cells were cultured in the presence of citral on various concentrations, and the negative control was cultured without citral for 16 h. Afterwards, smears of treated and control cells were stained with Wright-Giemsa solution, and cell morphology was subsequently investigated by light microscopy.
Proliferation assay
Cells were seeded into 96-well plates at 2 × 104 cells per well and treated with citral (0–20 μg/mL), and the positive control was treated with As2O3 (0.2 μg/mL) for 12, 24, and 48 h respectively. The anti-proliferative effect of citral was assessed with cell Count Kit-8 (Beyotime Biotechnology Company, Jiangsu, China).
DNA fragmentation analysis
NB4 cells were treated with serum-free medium for 24 h, followed by a treatment with medium containing various concentrations (5, 10, 20, 40 μg/mL) of test agents for 16 h. Cells were washed twice with PBS, and DNA was extracted with Apoptotic DNA Ladder Detection Kit (Bodataike Company, Beijing, China) according to the manufacturer’s instruction. We kept extracted DNA at 4 °C overnight, and mixed 8.5 μL of DNA sample with 1.5 μL of 6 × buffer solution. Then, the DNA sample was electrophoresed on 2 % agarose gel containing ethidium bromide at 80 V, and observed through the DBT-08 gel image analysis system.
Annexin V-binding assay
An Annexin V-binding assay was used according to the manufacturer’s protocol. Briefly, approximately 5 × 105/mL NB4 cells in 24-well plates were treated with various concentrations of citral (0, 5, 10, 20 μg/mL) for 24 h or with 5 μg/mL citral for various duration (0, 12, 24, 48 h). In the other assay, various concentrations of the cells were then harvested and stained with Annexin V-FITC/PI. A flow cytometer was used to analyze the expression of AnnexinV+/PI− (early apoptosis) and AnnexinV+/PI+ (late apoptosis) cells.
Mitochondrial membrane potential assay
To evaluate mitochondrial membrane potential(MMP), NB4 cells were incubated with 5 μg/mL citral for 0, 12, 24 and 48 h. The treated cells were then stained with Rh123 and PI and analyzed with a flow cytometer (EPICS XL-MCL 4C, Beckman, Brea, CA, USA).
Reverse transcription and polymerase chain reaction (RT-PCR)
The expression of Bcl-2 and Bax mRNA was detected by RT-PCR. Briefly, total RNA samples were prepared from NB4 cells in exponential phase which were treated by different concentrations of citral for 48 h, using the TRIzol (Invitrogen, Carlsbad, CA, USA). 1 μg of total RNA sample was then reverse transcribed into cDNA with a First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, MD, USA). The following gene-specific primers were used:
GAPDH-F: 5′-CAAGGTCATCCATGACAACTTTG-3′,
GAPDH-R: 5′- GTCCACCACCCTGTTGCTGTAG-3′;
BCL-2-F: 5′-TGGGAAGTTTCAAATCAGC-3′,
BAX-F: 5′-GCAAACTGGTGCTCAAGG-3′,
BCL-2-R: 5′-GCATTCTTGGACGAGGG-3′;
BAX-R: 5′-ACTCCCGCCACAAAGA-3′.
The PCR conditions were as follows: for GAPDH, at 94 °C for 1 min, at 60 °C for 40 s, and 72 °C for 50 s; for Bcl-2, 94 °C for 30 s, 56 °C for 40 s, 72 °C for 50 s; and for Bax, 94 °C for 30 s, 55.5 °C for 40 s, 72 °C for 50 s. Thirty-five cycles of amplification were used. PCR products (10 μl) were analyzed by electrophoresis on 2 % (w/v) agarose gel.
Flow cytometry analysis
NB4 cells were incubated with various concentrations of citral (0, 5, 10, 20 μg/mL) for 48 h and washed by PBS twice. Then, cells were treated with Fixation and Permeabilization Kit (Invitrogen), according to the manufacturer’s instruction, and were stained respectively with FITC labeled anti-activated caspase-3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and FITC labeled anti-activated NF-κb antibody (Santa Cruz Biotechnology) to detect the activation of capsase-3 and NF-κb with flow cytometer.
Statistical analysis
The median and standard deviation (SD) were calculated by using SPSS. Statistical analysis of differences was carried out by t test. A p value of less than 0.05 was considered to indicate significance.
Results
Inhibition of NB4 cell proliferation by citral
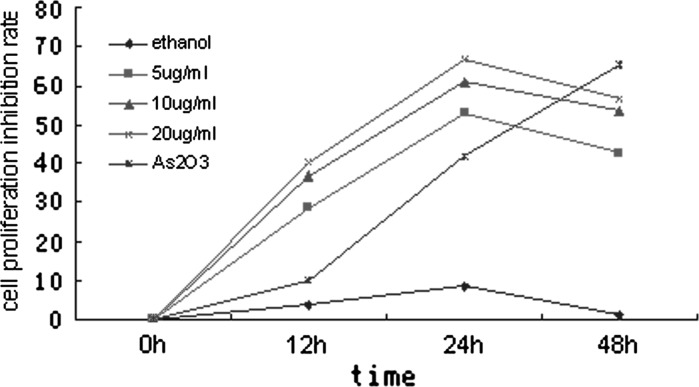
Cell Counting Kit-8 (CCK-8, Beyotime Biotechnology) was used to detect the proliferation of NB4 cells which were treated with citral (0–20 μg/mL) for 0, 12, 24 and 48 h respectively. Results showed that citral had a significant cytotoxic effect on NB4 cells in a dose and time dependent manner between 0–24 h, and after 24 h, the inhibition rate decreased (Fig. 1). The IC50 value was 3.995 μg/mL at 24 h.
Fig. 1.
NB4 cell proliferation inhibition rate due to being induced by citral of various concentrations for 0, 12, 24, 48 h. Results showed that citral had a significant cytotoxic effect in NB4 cells in a dose and time dependent manner between 0 and 24 h. After 24 h, the inhibition rate decreased
Citral-induced apoptosis and caspase-3 activation in NB4 cell line
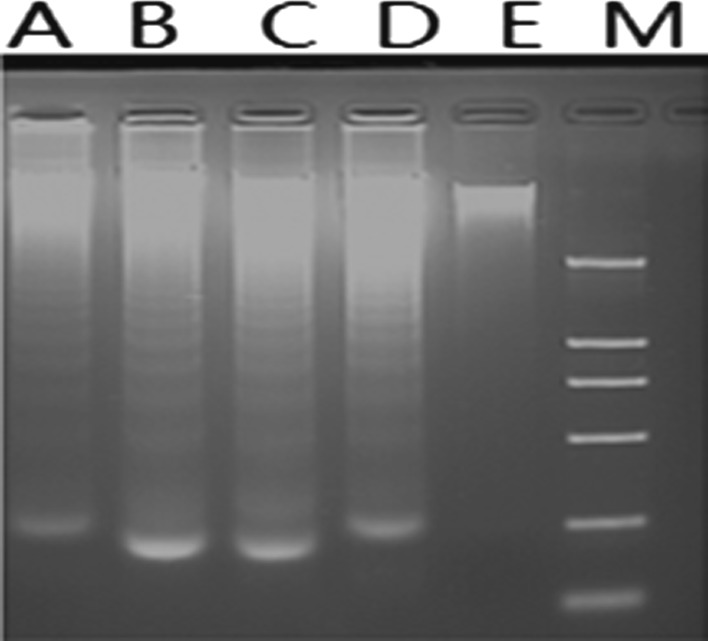
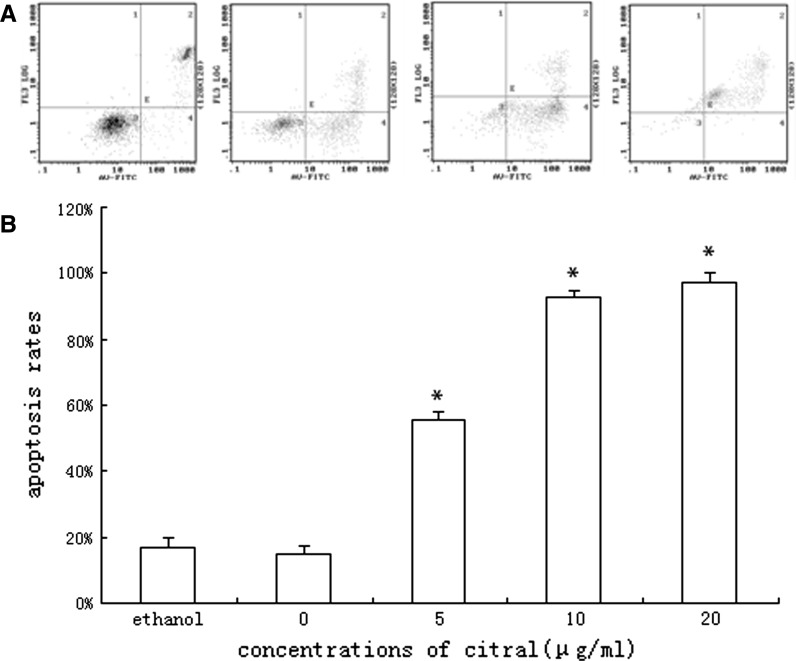
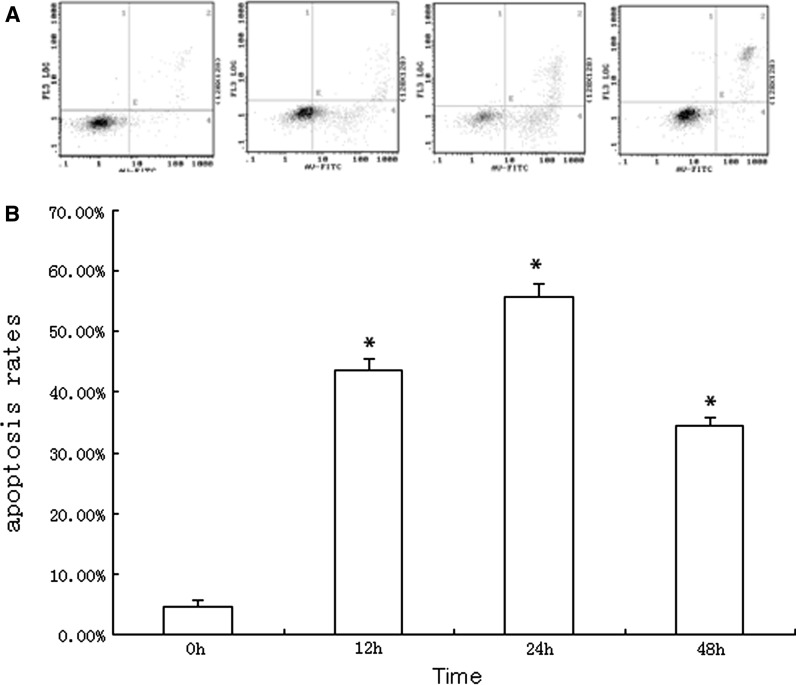
To determine if growth inhibition induced by citral was a result of apoptosis, the pro-apoptotic effect was examined by using Wright-Giemsa, DNA fragmentation analysis and Annexin-V/PI staining. Morphological changes under Wright-Giemsa staining indicating apoptosis (e.g. cell shrinkage, nuclear condensation etc.) were observed by light microscope of NB4 cells treated by various concentrations of citral. No observable changes were obtained in the morphology of NB4 cells in control group (Fig. 2). The typical DNA ladders were observed with DNA electrophoresis for NB4 cells treated by citral (Fig. 3). And apoptosis was also confirmed by Annexin V-FITC/PI staining using flow cytometry (Figs. 4, 5). Treatment with citral at 5, 10, 20 μg/mL for 24 h resulted in apoptosis rates of 55.67 ± 2.13 %, 92.90 ± 1.78 %, and 97.33 ± 2.89 %, respectively (Fig. 4). Furthermore, flow cytometry also showed that citral induced caspase-3 activation, one hallmark of apoptosis (Fig. 6). And Annexin V-FITC/PI staining and flow cytometry showed that citral induced apoptosis of NB4 cells in a dose- and time-dependent (between 0 and 24 h) manner.
Fig. 2.
Photomicrographs of NB4 cells treated with citral for 16 h in Wright-Giemsa staining. a Untreated NB4 cells b NB4 cells treated with 10 μg/mL of citral c NB4 cells treated with 20 μg/mL of citral. Original magnification, ×1,000 (Olymps IX70)
Fig. 3.
Effect of citral on NB4 cell’s DNA integrity. LaneA, B, C, D-DNA from cells treated respectively by 5, 10, 20, 40 μg/mL of citral. Lane E-DNA from untreated control cells, Lane M-DNA marker
Fig. 4.
NB4 cells treated by 0, 5, 10, 20 μg/mL of citral for 24 h were assessed by Annexin V-FITC/PI staining using flow cytometry; the 4 diagrammes from left to right stand for the 0, 5, 10, 20 μg/ml group, respectively (a). The results showed that apoptosis of NB4 cell was induced by citral in a dose-dependent manner (b). (* means compared with group 0 μg/mL, p < 0.05)
Fig. 5.
NB4 cells treated by 5 μg/mL of citral for 0, 12, 24, 48 h were assessed by Annexin V-FITC/PI staining using flow cytometry; the 4 diagrammes from left to right stand for the 0, 12, 24, 48 h group, respectively (a). The results showed that citral induced apoptosis of NB4 cell in a time-dependent manner between 0 and 24 h (b). (* means compared with group 0 h, p < 0.05)
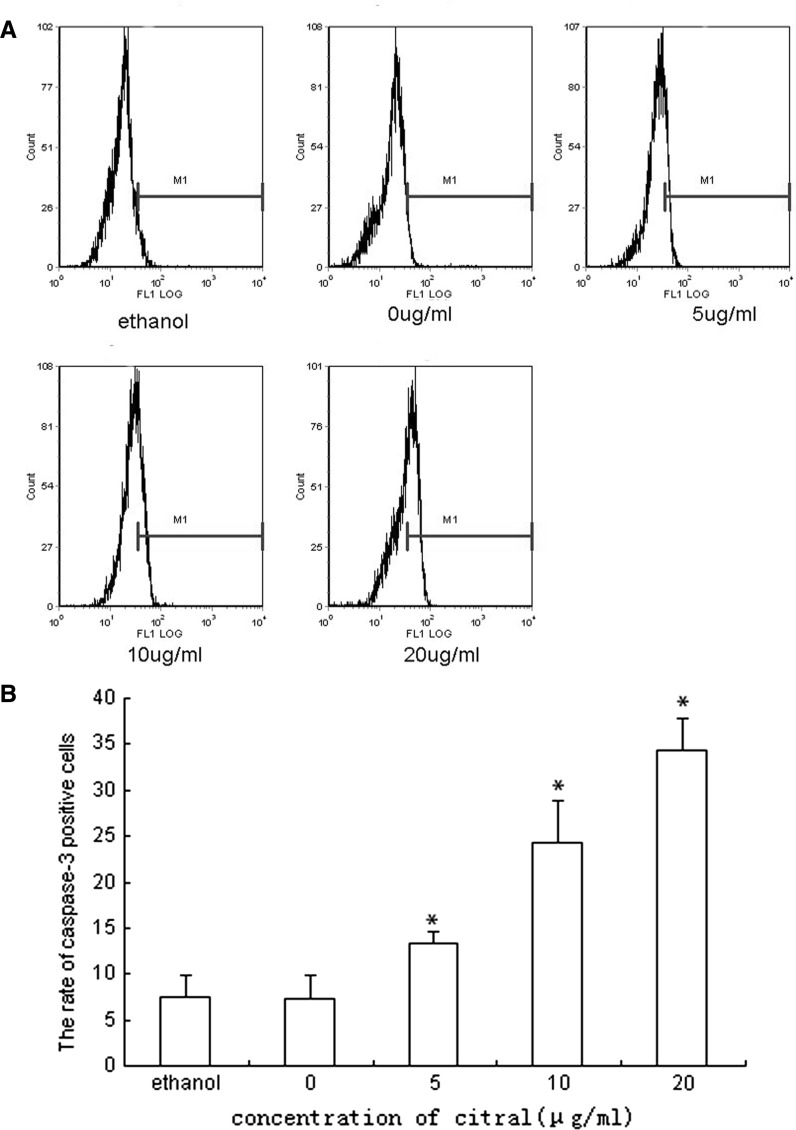
Fig. 6.
The caspase-3 activation of NB4 cells treated by 0, 5, 10, 20 μg/mL of citral for 24 h. The results showed that citral induced caspase-3 activation, one hallmark of apoptosis, in a dose-dependent manner. Due to the insolubility of citral in RPMI-1640, ethanol was used as cosolvent in this experiment, we also incubated NB4 cells in the presence of ethanol (the final concentration in medium was 5 %V/V, equivalent to concentrations in other citral groups) to investigate its apoptotic effect to cells. No significant effect of ethanol was observed (a indicates the results from flow cytometry and b indicates the statistical results) (* compared with group 0 μg/mL, p < 0.05)
Influence of citral on the expression of Bcl-2, Bax and NF-κB and mitochondrial membrane potential (MMP) of NB4 cells
The mechanisms underlying citral-induced apoptosis were investigated. The Bcl-2 family plays a significant role in the regulation of cell apoptosis, effects of citral on mRNA levels of Bcl-2 and Bax were therefore assessed by RT-PCR. Bcl-2 mRNA level was significantly down-regulated in NB4 cell lines, while Bax mRNA level was significantly up-regulated. In addition, results demonstrated that citral affected expressions of Bcl-2 and Bax in a dose-dependent manner (Fig. 7). These findings suggested that down-regulation of Bcl-2 and up-regulation of Bax contributed to citral-induced apoptosis. Because NF-κB was strongly implicated with tumors, especially with several hematological malignancies like leukemia and lymphoma as described in “Introduction”, effect of citral on the expression of NF-κB was assessed by flow cytometry as well. We found that citral down-regulated NF-κB protein level, indicating that NF-κB was involved in citral-induced apoptosis as well (Fig. 8). Furthermore, we investigated changes of mitochondrial membrane potential (MMP) of NB4 cells treated by citral. Exposure of the NB4 cells to 5 μg/mL of citral for various durations led to a significant reduction in the MMP in a time-dependent manner (Fig. 9), suggesting that mitochondria-dependent pathway might be one of the mechanisms of citral-induced apoptosis.
Fig. 7.
Citral down-regulated Bcl-2 mRNA level and up-regulated Bax mRNA level in a dose-dependent manner. The 4 lanes of the electrophoresis from 1 to 4 stand for the 0, 5, 10 , 20 μg/ml group respectively, and the lane 5 stand for the ethanol group. Three independent experiments were performed with similar results, and representative data are shown (* compared with group 0 μg/mL, p < 0.05)
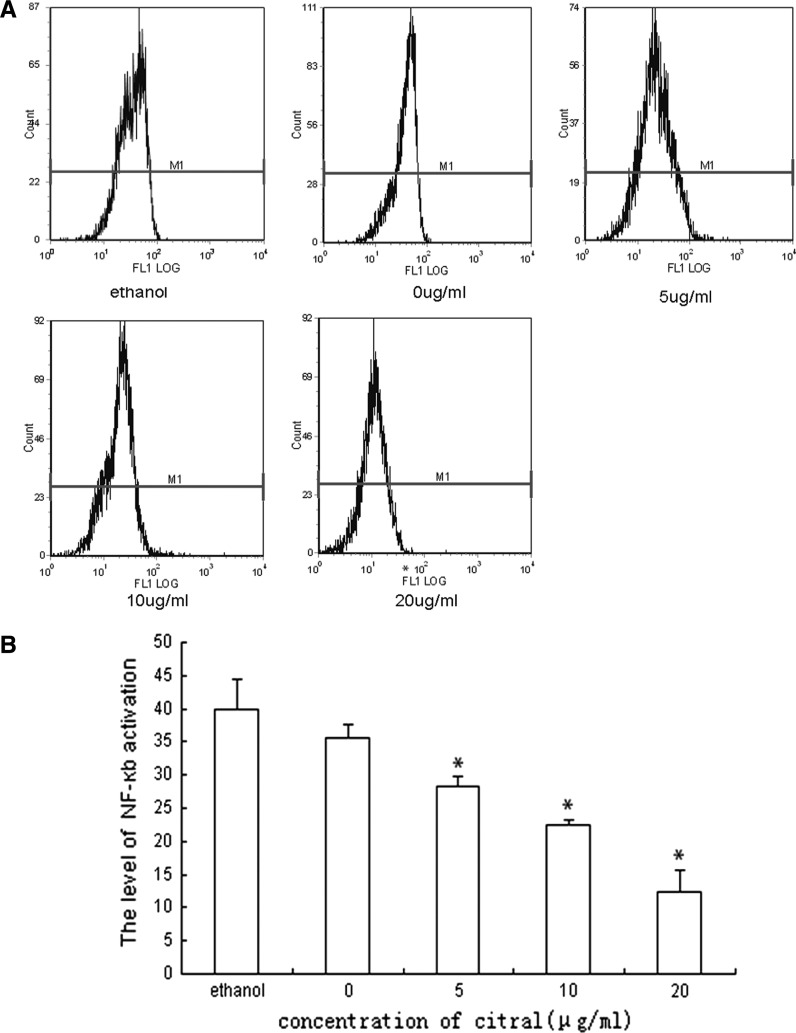
Fig. 8.
Influence of citral on the NF-κB protein level of NB4 cells. NB4 cells were treated by 0, 5, 10, 20 μg/mL of citral for 24 h and NF-κB protein regulation level was estimated by flow cytometry (a). The result showed that NF-κB protein regulation level decreased after treatment with citral in a dose dependent manner, and no significant effect of ethanol was observed (b). The results shown are representative of three independent experiments (* compared with group 0 μg/mL, p < 0.05)
Fig. 9.
Influence of citral on the MMP of NB4 cells. NB4 cells were treated by 5 μg citral for 0, 12, 24, 48 h. MMP was estimated by flow cytometry; the 4 diagrammes from left to right stand for the 0, 12, 24, 48 h group, respectively (a). The result showed that the percentage of Rh123-/PI- cells (MMP-decreased cells) increased after the treatment of citral in a time-dependent manner, indicating that citral induce an MMP decrease of NB4 cells in a time-dependent manner (b). The results shown are representative of three independent experiments. (* means compared with group 0 h, p < 0.05)
Discussion
Citral is a key component of essential oils extracted from several lemon-scented herbal plants. It has been demonstrated to be an effective antifungal agent (Mendonca Ade et al. 2009; Luo et al. 2004). In recent years, it was reported that citral had an anti-proliferative effect in certain tumor cell lines and could be a potential anticancer drug (Chaouki et al. 2009; Dudai et al. 2005). The main goal of the present study was to evaluate the anti-proliferative effect of citral on the NB4 cell line and to understand mechanisms involved in this effect well.
Initially, in the proliferation assay, we found that citral treatment dramatically induced decrease in proliferation in the cells, especially at 24 h after drug treatment. We could estimate the IC50 at 24 h by a regression analysis at 3.955 mg/l.
To identify the mechanism involved in the citral-induced inhibition in NB4 cell proliferation, further studies were performed. We demonstrated that decrease of proliferation in NB4 cells treated by citral is due to the induction of apoptosis. Typical apoptotic morphological changes (e.g. cell shrinkage, nuclear condensation etc.) and DNA ladder were observed by Wright-Giemsa staining and DNA electrophoresis, respectively. Flow cytometry confirmed citral-induced apoptosis as well. The apoptosis rates of NB4 cells treated by citral at 5, 10, 20 μg/mL for 24 h were 55.67 ± 2.13 %, 92.90 ± 1.78 %, and 97.33 ± 2.89 %, respectively. This result had an agreement with a previous study showing that citral had an antiproliferative effect on the human breast cancer cell line (MCF-7) via the induction of apoptosis and cell cycle arrest in G2/M phase. We also detected the activation of caspase-3, which further confirmed results of Wright-Giemsa staining and DNA electrophoresis. Nativ Dudai et al. (2005) reported that citral had an anticancer effect on several hematopoietic cell lines, such as U937, HL60, RL12 and BS-24-1, via induction of apoptosis and activation of caspase-3, which agrees with our results.
We subsequently investigated the expression of Bcl-2, Bax and mitochondrial membrane potential (MMP) of NB4 cells. The Bcl-2 family and the change of MMP were very strongly implicated in the intrinsic apoptosis pathway as described in the “Introduction”. In our study, RT-PCR showed that Bcl-2 mRNA level was significantly down-regulated in NB4 cell lines, while Bax mRNA level was significantly up-regulated. In addition a significant reduction in MMP was detected by flow cytometry. All these findings suggested citral might induce apoptosis in NB4 cells via the intrinsic apoptosis pathway.
In addition, the expression of NF-κB was detected by flow cytometry as well. Nuclear factor-κB (NF-κB) was considered as a key regulator of inflammation, cell proliferation and early pathogen response (Karin and Greten 2005). NF-κB activation is very strongly implicated in tumors, especially in several hematological malignancies (Zhu et al. 2011). The present study showed that NF-κB protein level was down-regulated in NB4 cells treated by citral, suggesting that NF-κB was involved in the proapoptotic effect of citral.
In summary, this study demonstrated that citral induced apoptosis on NB4 cells in association with activation of caspase-3, down-regulation of Bcl-2 and NF-κB expression and up-regulation of Bax expression. And mitochondria-mediated pathway might be one of the mechanism involved in this apoptotic effect. Citral, thus, could be a potential agent for leukemia treatment.
Acknowledgments
We thank Dr Lixin Zhu for her help in English writing and technical supporting and Dr Saijuan Chen for her kindly supply of human tumor cell lines NB4. This experiment was supported by the national science foundation of PR China.
Footnotes
Hailong Xia, Wei Liang, and Qin Song contribute equally to this paper.
References
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A, Scheidereit C, Dorken B. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Investig. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouki W, Leger DY, Liagre B, Beneytout JL, Hmamouchi M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam Clin Pharmacol. 2009;23:549–556. doi: 10.1111/j.1472-8206.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- Cohen JJ. Apoptosis: mechanisms of life and death in the immune system. J Allergy Clin Immunol. 1999;103:548–554. doi: 10.1016/S0091-6749(99)70222-8. [DOI] [PubMed] [Google Scholar]
- Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005;71:484–488. doi: 10.1055/s-2005-864146. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Koedood M, Piffat KA, White DW. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- Indran IR, Tufo G, Pervaiz S. Brenner C Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Luo M, Jiang LK, Huang YX, Xiao M, Li B, Zou GL. Effects of citral on Aspergillus flavus spores by quasi-elastic light scattering and multiplex microanalysis techniques. Acta Biochim Biophys Sin. 2004;36:277–283. doi: 10.1093/abbs/36.4.277. [DOI] [PubMed] [Google Scholar]
- McGill G, Fisher DE. Apoptosis in tumorigenesis and cancer therapy. Front Biosci. 1997;2:d353–d379. doi: 10.2741/a197. [DOI] [PubMed] [Google Scholar]
- McKeithan TW, Takimoto GS, Ohno H, Bjorling VS, Morgan R, Hecht BK, Dube I, Sandberg AA, Rowley JD. BCL3 rearrangements and t (14;19) in chronic lymphocytic leukemia and other B-cell malignancies: a molecular and cytogenetic study. Genes Chromosomes Cancer. 1997;20:64–72. doi: 10.1002/(SICI)1098-2264(199709)20:1<64::AID-GCC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Mendonca Ade L, da Silva CE, de Mesquita FL, Campos Rda S, Do Nascimento RR, Ximenes EC, Sant’Ana AE. Antimicrobial activities of components of the glandular secretions of leaf cutting ants of the genus Atta. Antonie Van Leeuwenhoek. 2009;95:295–303. doi: 10.1007/s10482-009-9312-0. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS, Jr. (1998) NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683 [DOI] [PubMed]
- Zhu BS, Xing CG, Lin F, Fan XQ, Zhao K, Qin ZH. Blocking NF-kappaB nuclear translocation leads to p53-related autophagy activation and cell apoptosis. World J Gastroenterol. 2011;17:478–487. doi: 10.3748/wjg.v17.i4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]