Abstract
Pluripotent stem cells derived from testis is a new, natural, and unlimited source for cell therapy in regenerative medicine and represent a possible alternative to replacing of all cells in the body. Here, we designed a simple co-culture system of spermatogonia cells with Sertoli cells for the generation of embryonic stem-like cells from mouse testis. The importance of our simple method will be clear when we compared it with other complex and time-consuming methods. Embryonic stem-like colonies with sharp border confirmed by real-time PCR, immunocytochemistry and flow cytometry assessments. Embryonic stem-like colonies were immunopositive for pluripotency markers. Transition of spermatogonia cells to embryonic stem-like cells was accompanied by extensive changes in gene expression. These changes included significant increase in pluripotency genes expression and significant decrease in germ cell-specific genes expression. Also, we proved the differentiation capacity of embryonic stem-like cells to neuroepithelial-like cells which were immunoreactive to Nestin and Neurofilament 68. Evaluation of genes expression during in vitro differentiation into neuroepithelial-like cells showed high-level expression of Nestin whether this gene approximately has no expression in undifferentiated embryonic stem-like cells. Also, expression of pluripotency genes has significantly decreased in neuroepithelial-like cells compared with embryonic stem-like cells. This study shows that embryonic stem-like cells derived from testis are capable to differentiate into neuroepithelial-like cells that may provide a cellular reservoir usable for neurodegenerative disorders.
Keywords: Spermatogonia cells, Embryonic stem-like cell, Reprogramming, Pluripotency, Neuroepithelial cells
Introduction
Nervous system has the limited capacity to repair the affected neuronal or glial loss (Bjartmar et al. 1999). Several types of stem cells are used to conquer this problem as a source for neurons and glia (Gage 2000; Barberi et al. 2003).
These cells can improve neurogenesis and repair the injury of the central nervous system (Gage 2000).
Among different sources for cell therapy, such as bone marrow, hematopoietic or embryonic stem cells, some evidences have suggested extensive proliferation activity and pluripotency of spermatogonial stem cells (Kanatsu-Shinohara and Shinohara 2007; Takahashi and Yamanaka 2006).
Spermatogonial stem cells can form embryonic stem-like cells (ES-like cells) and embryoid body structure in vitro that can differentiate to three germ layers (Nayernia 2004; Damjanov 1982).
This novel pluripotent stem cell has no ethical limitation associated with embryonic stem cell (ESC) and decrease immune rejection problems of transplantation therapies, so it can be useful in regenerative medicine (Damjanov 1982). Spermatogonia cells may represent a possible alternative to embryonic stem cells for cell therapy in replacing of neurons and glia (Mizrak et al. 2010).
The process of neural differentiation accompanied with neuroepithelial-like cells (NELC) generation which are multipotent stem cells that give rise to all the cells of central nervous system, including various types of neurons, astrocytes, and oligodendrocytes (Turnpenny et al. 2005). Today, different sources and methods are currently used for derivation neuroepithelial-like cells in vitro (Silani et al. 2004).
In relation to previous results showing the pluripotency of ES like cells derived from testis, the aim of this study was to evaluate the differentiation capacity and alteration in genes expression patterns during in vitro differentiation of mouse spermatogonial cells into neuroepithelial-like cells using Retinoic acid (RA) induction.
Materials and methods
Generation and culture of ES-like cells
ES-like cells were generated from neonatal mouse testis. This study performed after obtaining animal ethical clearance from Tarbiat Modares University. Neonatal mouse was killed and both testes were removed via small insecure in lower part of the abdomen. After decapsulation of the testis, tissue was mechanically dissected and dissociated via a two-step mechanical and enzymatic digestion with Dulbecco’s modified Eagle’s medium (DMEM) which contained; 0.5 mg/ml collagenase-dispase, 0.5 mg/ml trypsin, and 0.05 mg/ml DNase (with shaking and pipetting) at 37 °C for 30–45 min.
The obtained dissociated cells that included spermatogonia and Sertoli cells were incubated together at 37 °C and 5 % CO2, and in a humidified atmosphere, they were cultured in DMEM (Invitrogen) supplemented with 15 % Fetal Bovine Serum (FBS; Invitrogen), 1 mM l-glutamine (Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma Aldrich), and 1,000 units/ml Leukemia Inhibitory Factor (LIF; Sigma Aldrich).
Differentiation of ES-like cells to neuroepithelial-like cells
ES-like cells differentiated to neuroepithelial-like cells according to neural differentiation methods of ESC (Okabe et al. 1996; Brüstle et al. 1999). Cells were passaged and propagated on gelatin-coated dishes (0.1 %; Sigma) to eliminate Sertoli cell then were transferred to bacteriological six-well plate at a cell density of 50,000 cells/cm2. In this condition, ES-like cells form embryoid bodies (EB). After 4 days, retinoic acid was added to induce EB colonies and the morphological changes of EB cells were monitored every 12 h.
At day 8th, EBs were disaggregated using trypsin/EDTA and plated on 0.01 % gelatine-coated dishes in neural stem cells medium that consists of neurobasal medium added N2 supplement and B27 supplement (Gibco) for 4 days. Half of the medium was changed every 2 days.
Quantitative real-time polymerase chain reaction (RT-qPCR)
To confirm generation of ES-like cells, real-time PCR was performed to analyze the expression of a subset of pluripotency markers as well as germ cell-specific genes. Nanog and C-myc were evaluated as the pluripotency genes in ES-like cells and the spermatogonial markers of Stra8, Mvh, and Piwill2 were evaluated. For evaluation of alteration in genes expression patterns during in vitro differentiation of ES-like cells into neuroepithelial-like cells after induction with RA, all above-mentioned genes and Nestin were evaluated.
Total RNA was extracted from Spermatogonia cells, ES-like cells, and neuroepithelial-like cells groups using RNXPlusTM (Cinnagen, Iran) according to the manufacturer’s recommendations. In order to remove genomic contamination, RNA was treated with DNase I using a kit from Fermentas (Lithuania) based on the protocol described by the manufacturers. Concentrations of RNA were determined using UV spectrophotometer (DPI-1, Qiagen).
The cDNAs were synthesized from 500 ng DNase-treated RNA samples with a Revert AidTM first-strand cDNA synthesis kit (Fermentas, Lithuania) using oligo (dT) primers. For PCRs, primers were adapted from others (21, 25) and synthesized by Cinnagen and Genefanavaran Companies (Iran, Table 1).
Table 1.
Quantitative real-time PCR primer sequences
| Gene | Primer (forward/reverse) | Significance |
|---|---|---|
| Mvh | 5′-CGGAGAGGAACCTGAAGC-3′ | Spermatogonial marker |
| 5′-CGCCAATATCTGATGAAGC-3′ | ||
| Stra8 | 5′-ACGACGCGTCGCTATTCCCTCTCACATCTTC-3′ | Spermatogonial marker |
| 5′-AGCGAGCTCGATGCACCTTCGACACTTG-3′ | ||
| Piwill2 | 5′-GCACAGTCCACGTGGTGGAAA-3′ | Spermatogonial marker |
| 5′-TCCATAGTCAGGACCGGAGGG-3′ | ||
| C-myc | 5′-CCCTCAGTGGTCTTTCCCTAC-3 | Pluripotency marker |
| 5′-CCACAGACACCACATCAATTTC-3′ | ||
| Nanog | 5′-CTGGGAACGCCTCATCAA-3′ | Pluripotency marker |
| 5′-CGCATCTTCTGCTTCCTGG-3′ | ||
| β Actin | 5′-CTTCTTGGGTATGGAATCCTG-3′ | Internal control |
| 5′-GTGTTGGCATAGAGGTCTTTAC-3′ |
PCRs were carried out using Master Mix (Cinnagen, Iran) and SYBR Green I (Fluka, Switzerland) in a Rotor-Gene3000 thermocycler (Corbett, Australia). The PCR program started with an initial melting cycle, 4 min at 94 °C, to activate the polymerase and followed by 40 cycles as follows: a melting step (20 s at 94 °C), an annealing step (30 s at 57 °C), and an extension step (30 s at 72 °C). After completing the PCR run, the quality of the reactions was confirmed by melting curve analyses.
Efficiency was determined for each gene using a standard curve (the logarithmic dilution series of testis cDNA). For each sample, the reference gene (b2M) and the target gene were amplified in the same run. Ratio of gene expression was determined using the comparative CT (cycle threshold) method.
Statistical analysis
The statistical analysis was performed with SPSS 13.0 software using T test with P values less than 0.05 which was considered significant. Each point represents the average of three separate experiments.
Immunocytochemistry analysis
For immunocytochemistry, cells in each group were washed with phosphate-buffered saline (PBS) at pH 7.4 and fixed in 4 % paraformaldehyde (PFA) for 30 min at room temperature (RT): Fixed cells were permeabilized with 0.2 % Triton X-100 for 10 min at RT followed by three washes with PBS. To block unspecific binding of the antibody, cells were incubated with 10 % goat serum for 30 min at RT.
Then, cells were incubated with primary antibodies overnight at 4 °C. Primary antibodies including: stage-specific embryonic antigen 1 (SSEA1) (1:200; Abcam), Sox2 (1:500; Abcam) and Oct4 (1:200; Abcam), Nestin (1:100; Abcam), NF68 (1:200; Abcam). The following day, cells were washed twice with PBS and incubated with the appropriate secondary antibody. Antigens were visualized using appropriate fluorochrome-conjugated secondary antibodies including: Phycoerythrin (PE)-conjugated Donkey anti-Rabbit polyclonal IgG (1:1,000; Abcam), goat anti-rabbit IgG-fluorescein isothiocyanate FITC (1:1,000; Abcam), goat anti-mouse IgG-fluorescein isothiocyanate FITC (1:1,000; Abcam).
After two washes with PBS for 5 min, cells were mounted with 4,6-diamidino-2-phenylindole (DAPI)/PBS and Images were captured with an Olympus phase contrast microscope (BX51, Olympus, Tokyo, Japan).
Flow cytometry
Cells were washed with PBS and treated with trypsin/EDTA for 5 min to form single cells.
The suspension collected by centrifugation at 2,000 rpm for 5 min were fixed in 4 % PFA for 10–15 min at 4 °C for stabilizing proteins, followed by permeablizing of cells in detergent (0.2 % Triton X-100). Fixation/permeabilization procedures had to be on ice. Then, it was washed by adding 2 ml of PBS, and centrifuged at 2,000 rpm for 5 min. The supernatant was discarded and the cells were resuspended in goat serum for 45 min to block nonspecific antibody binding. Cells were labeled with primary antibodies including: Oct4 (1:200; Abcam), Sox 2 (1:500; Abcam) Nestin (1;100), NF68 (1:200, Abcam system) overnight at 4 °C in dark, then washed 3-times by centrifugation at 2,000 rpm for 5 min and resuspended in ice-cold PBS. The following day, cells were incubated with the appropriate secondary antibody and antigens were visualized using appropriate fluorochrome-conjugated secondary antibodies included Phycoerythrin (PE)-conjugated Donkey anti-Rabbit polyclonal IgG, goat anti-rabbit IgG-fluorescein isothiocyanate (FITC), goat anti-mouse IgG-fluorescein isothiocyanate FITC (1:1,000; Abcam). This incubation had to be done in dark, followed by 3 washes by centrifugation at 2,000 rpm for 5 min and resuspension in ice-cold PBS. Analysis was performed as soon as possible using a BD FACS Caliber (Becton–Dickinson, San Jose, CA, USA) and FlowJo software (WinMDI 2.9, J. Trotter).
Results
Generation of ES-like cells
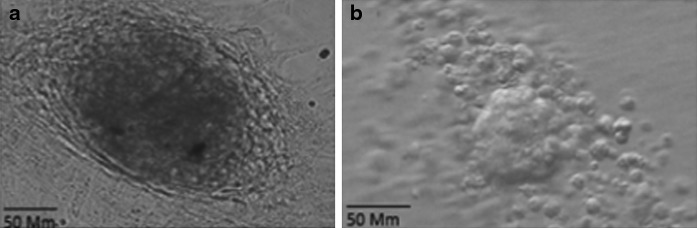
Our result showed after several days, spermatogonia formed colonies and these colonies were passaged every 4 days (Fig. 1). Finally, ES-like colonies with sharp edge that resembled ES cell appeared within 3 weeks (at passages 5). ES-like cells were confirmed by immunoflorescent staining and quantitative real-time PCR analysis.
Fig. 1.
a The morphology of a spermatogonia cell colony. b Embryoid bodies formation of ES-like cells after transferring to bacteriological plate
Immunoflorescent staining
ES-like cells were confirmed with Immunoflorescent staining. Embryonic stem cells were considered as a control group. ES-like cells expressed the cell-surface marker SSEA-1 (stage-specific embryonic antigen-1), Sox2 and Oct 4 that are expressed in undifferentiated mouse ES cells (Fig. 2).
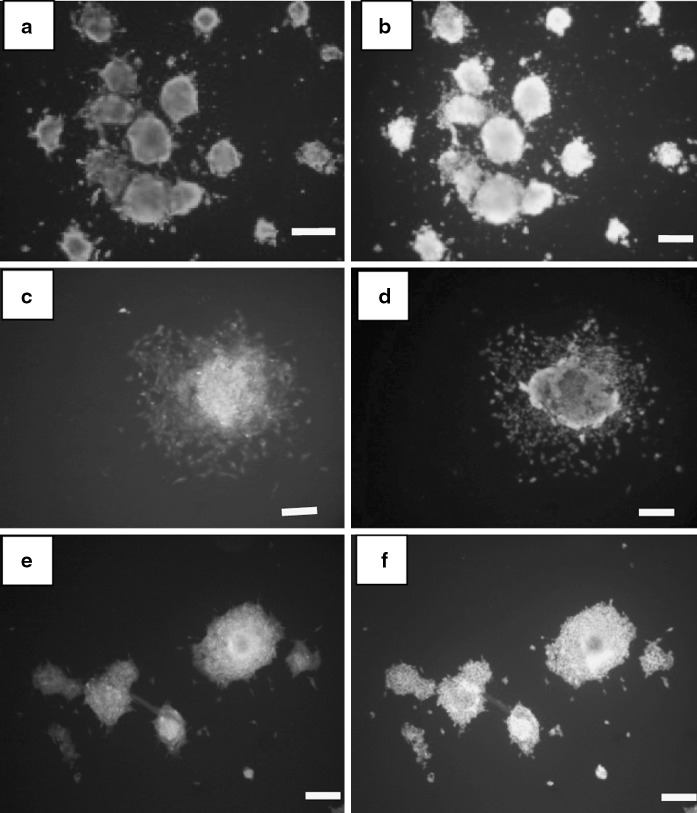
Fig. 2.
Immunostaining of ES-like cells derived from mouse spermatogonial cell for SSEA-1, Oct4, and Sox2. a ES-like cells show positive reaction to SSEA-1. b DAPI for ES-like cells. c ES-like cells show positive reaction to Oct 4. d DAPI for ES-like cells. e ES-like cells show positive reaction to Sox2. f DAPI for ES-like cells. Scale bars 50 μm
Quantitative real-time PCR
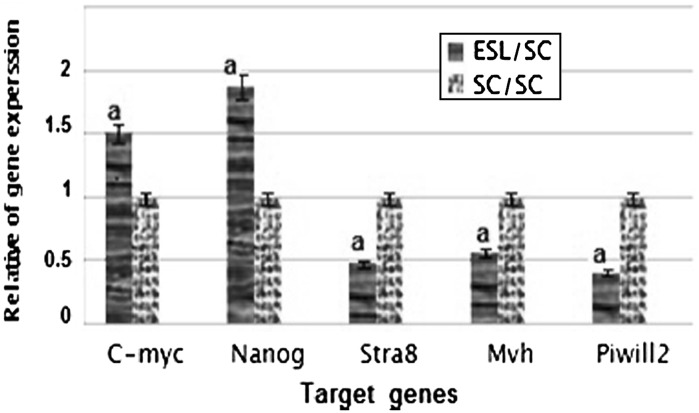
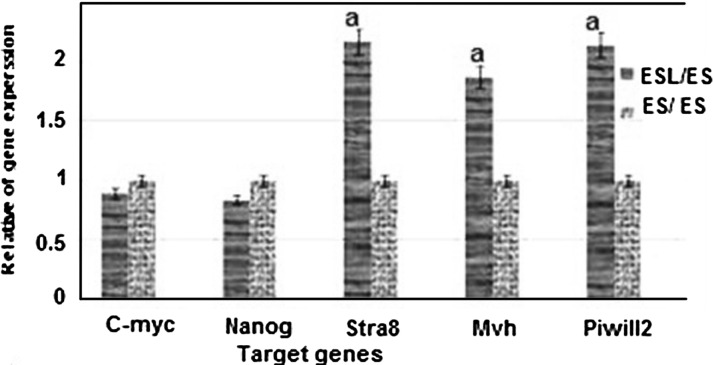
Real-time PCR was performed in the isolated ES-like cells to analyze the expression of a subset of pluripotency markers, as well as germ cell-specific genes. Results demonstrated that the expression of pluripotency genes Nanog and C-myc has significantly increased in ES-like cells in comparison with spermatogonia cells. Also, the expression of Nanog and C-myc was similar to ES cells. The Spermatogonial genes Stra8, Mvh and Piwill2 were down regulated during transition of spermatogonia cells to ES like cells (Figs. 3, 4).
Fig. 3.
The ratio of pluripotency genes expression: Nanog, C-myc, and spermatogonial genes: Stra 8, Mvh, and Piwill2 in ES-Like cells in comparison with spermatogonia cells a significant increase or decrease in gene expression level of ES-Like cells in comparison to spermatogonia cells
Fig. 4.
The ratio of pluripotency genes expression: Nanog, C-myc, and spermatogonial genes: Stra8, Mvh, and piwill2 in ES-Like cells in comparison with ES cells a significant increase in gene expression level of ES-Like cells in compare with ES cells
The ability of EB formation in vitro is one of the characteristics of pluripotent ES-like cells. EB formation was detected after 2–4 days of hanging drop method (Fig. 1c, d).
Neural differentiation of ES-like cells
After exposure to differentiation conditions, ES-like cells showed the potential to differentiate into neuroepithelial-like cells.
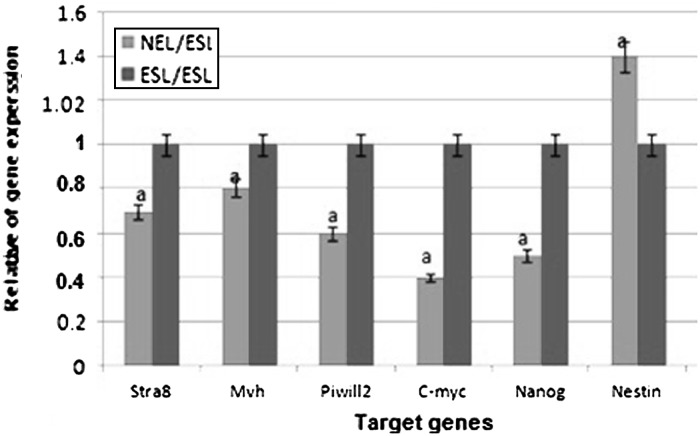
The expression of pluripotency genes Nanog and C-myc were downregulated after differentiation of ES-like cells to neuroepithelial-like cells, but expression of Nestin gene was significantly increased (Fig. 5).
Fig. 5.
The ratio of pluripotency genes expression: Nanog, C-myc, Stra8, Mvh, piwill2, and Nestin in neuroepithelial-like cells in comparison with ES-like cells a significant increase or decrease in gene expression level of neuroepithelial-like cells in comparison to ES like cells
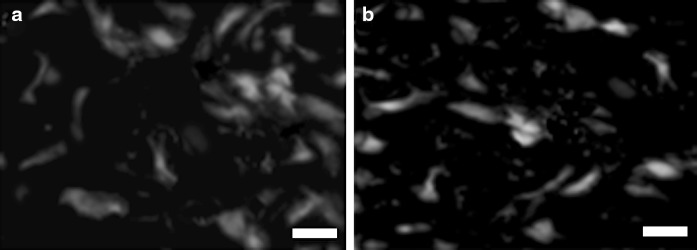
We confirmed differentiation into neuroepithelial-like cells by immunostaining with Nestin and NF68 (Fig. 6).
Fig. 6.
Immunostaining of neuroepithelial-like cells for Nestin and NF68. a Neuroepithelial-like cells show positive reaction to Nestin. b Neuroepithelial-like cells show positive reaction to NF68. Scale bars 20 μm
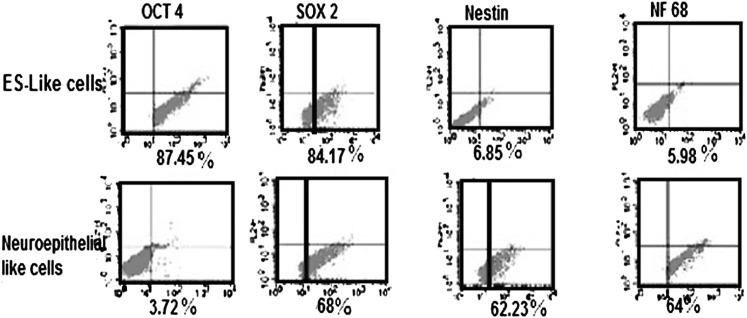
Flow cytometry result showed that 87.45% of undifferentiated ES-like cells were positive for Oct4 and 84.17 % were immunopositive for Sox2 but very few of the cells in this stage expressed detectable markers of neuroepithelial-like cells such as Nestin and NF68. After induction with RA, we dissociated the cells and stained them for the expression of Nestin and NF68 protein to identify neuroepithelial-like cells. Oct4 and Sox2 antigens were used to identify residual, undifferentiated ES cells. As shown in Fig. 7, this protocol resulted in the significant increase in Nestin and NF68 expression and a decrease in Oct4 expression but not in Sox2 expression. The proportion of Nestin-positive cells and NF68-positive cells in this way was 62.23 and 64 %, and there were 3.72 % residual Oct4-positive cells present in the culture. The high expression of Sox2 (68 %) which is either the pluripotency or neural progenitor marker, confirm the neuroepithelial characterization of this cells (Fig. 7). Expression of Nestin in neuroepithelial cells could also be confirmed by real-time PCR analysis, our results show that the expression of Nestin gene significantly increased in neuroepithelial-like cells in comparison to undifferentiated ES like cells. In neuroepithelial-like cells, expression of pluripotency genes such as Nanog and C-myc has significantly decreased in comparison with ES-like cells (Fig. 6).
Fig. 7.
Flow cytometry of Oct4, Sox2, Nestin and NF68 in ES-like cells and neuroepithelial-like cell
Discussion
Generating ES-like cells from testis is useful in cell line production from a specific patient and can produce sufficient cells for regenerative therapy (Nayernia 2004).
New research has shown the derivation of pluripotent stem cells from neonatal and adult mouse testis that can differentiate to all cell types (Guan et al. 2007; Nayernia 2007). These studies prove that ES-like cells were generated without genetic manipulation but under specific culture conditions (De Rooij and Mizrak 2008).
The results obtained from mice studies may have preclinical and clinical applications in other species particularly in humans (De Rooij and Mizrak 2008).
Following pervious investigations, in this study, we isolated ES-like cells from mouse testis but under simple co-culture condition with Sertoli cells which is a novelty of our research. In fact, the importance of our simple method will be clear when we compared it with other complex and time-consuming methods.
Our results showed that spermatogonia cells can proliferate and form colonies on the feeder layer of Sertoli cells.
Co-culture of spermatogonia cells with Sertoli cells as a feeder layer provide a cell model for in vitro culture of spermatogonia cells (Kanatsu-Shinohara et al. 2003). ES-like cell colonies with sharp border had a morphology similar to that of embryonic stem cells and formed embryoid bodies structures, in suspension.
Transition of spermatogonia stem cells to ES-like cells is accompanied by extensive changes in gene expression. Our research proved that ES-like cells originated from spermatogonia cells, express high levels of the pluripotency genes Nanog and C-myc, that is, similar to ES. Furthermore, germ cell-specific genes such as Stra8, Mvh, and Piwill2 are downregulated which confirms pervious results (Kanatsu-Shinohara et al. 2003; Kubota and Brinster 2006).
ES-like cells have the same molecular profiling as embryonic stem cells (Mardanpour et al. 2008; Kossack et al. 2009).
Our results showed that ES-like cells derived from spermatogonial stem cells share many molecular and cellular characteristics with embryonic stem cells; on cellular level, these cells have the same morphology with the sharp edge and can form embryoid body structure after transfer to bacteriological plate (De Rooij and Mizrak 2008).
The applicability of spermatogonial stem cells to be used for individual cell therapy without ethical problem, make them more applicable for cell transplantation than ES cells (Nayernia 2004). These characteristics propose the therapeutic use of spermatogonial stem cells in regenerative medicine especially as a new and unlimited source for neurodegenerative disease treatment and may represent a possible alternative to replace neurons and glia.
Differentiation of spermatogonial stem cells into neuroepithelial-like cells (neuro-glial progenitor cells are capable of giving rise to both differentiated neuronal and glial cells) is an important step in cell therapy for patients suffering from neurodegenerative diseases (Nayernia 2004).
In this study, we used spermatogonial cells for generation of neuroepithelial-like cells and examined RA treatment for the production of neuroepithelial phenotype.
There are different methods to induce neuroepithelial-like cells; the results of our study showed that in the presence of RA, ES-like cells differentiated to neuroepithelial-like cells, which were immunolabeled for Nestin and NF68. RA, which was used in our study, is a derivative of vitamin A and is an essential factor in the normal cellular growth and development (Wagner et al. 1992). In fact, RA receptors are found in different tissues, in particular in the nervous system and their numbers increase at the time of neural differentiation (Maden et al. 1998). Our result confirmed previous studies showed that RA induced neural differentiation in both ESC- and ES-like cells (Maden et al. 1998; Nayernia 2004).
Our results proved very high expression levels of Oct4 and Sox2 and low detectable levels of Nestin and NF68 in undifferentiated ES-like cells but an increase in the expression of Nestin and NF68 in neuroepithelial-like cells.
In fact, alteration in the expression pattern of these proteins after induction confirms the hypothesis that these cells are pluripotent and thus can retain the ability for neuronal differentiation.
At a molecular level, our data show that the expression of Nestin gene significantly increased in neuroepithelial-like cells in comparison to undifferentiated ES like cells. Expression of pluripotency genes such as Nanog and C-myc has significantly decreased in neuroepithelial-like cells compared with ES-like cells.
This study shows that our co-culture system is useful to generate ES-like cells from testis and that these cells are capable to generate neuroepithelial-like cells that can provide a cellular reservoir usable for the treatment of neurodegenerative disorders of the central nervous system.
Acknowledgments
This research is derived from PhD thesis of Anatomical science with funding support from Medical Faculty of Tarbiat Modares University and Iranian council of stem cell technology.
Contributor Information
Maryam Nazm Bojnordi, Email: bojnordi@modares.ac.ir.
Mansoureh Movahedin, Phone: +98-21-82884503, FAX: +982182884555, Email: Mansoure@modares.ac.ir.
References
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 21:1200–1207 [DOI] [PubMed]
- Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. J Neurocytol. 1999;28:383–395. doi: 10.1023/A:1007010205037. [DOI] [PubMed] [Google Scholar]
- Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD (1999) Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 285:754–756 [DOI] [PubMed]
- Damjanov I. Immunohistochemical localization of murine stage-specific embryonic antigens in human testicular germ cell tumors. Am J Pathol. 1982;108:225–230. [PMC free article] [PubMed] [Google Scholar]
- De Rooij DJ, Mizrak C. Deriving multipotent stem cells from mouse spermatogonial stem cells: a new tool for developmental and clinical research. Development. 2008;135:2207–2213. doi: 10.1242/dev.015453. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Guan K, Wagner S, Unsold B, Maier LS, Kaiser D, Hemmerlein B, Nayernia K, Engel W, Hasenfuss G. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–1625. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Shinohara T. The germ of pluripotency. Nat Biotechnol. 2007;24:663–664. doi: 10.1038/nbt0606-663. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen H, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA (2009) Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem cells 27:138–149 [DOI] [PMC free article] [PubMed]
- Kubota H, Brinster R. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Prac Endocrinol Metab. 2006;2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M, Sonneveld E, van der Saag PT, Gale E. The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development. 1998;125:4133–4144. doi: 10.1242/dev.125.21.4133. [DOI] [PubMed] [Google Scholar]
- Mardanpour P, Guan K, Nolte J, Lee J, Hasenfuss G, Engel W, Nayernia K. Potency of germ cells and its relevance for regenerative medicine. J Anat. 2008;213:26–29. doi: 10.1111/j.1469-7580.2008.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak SC, Chikhovskaya JV, Sadri Ardekani H, van Daalen S, Korver CM. Embryonic stem cell-like cells derived from adult human testis. Reprod Biol. 2010;25:158–167. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- Nayernia K. Stem cell based therapeutical approach of male infertility by teratocarcinoma derived germ cells. Hum Mol Genet. 2004;13:1451–1460. doi: 10.1093/hmg/ddh166. [DOI] [PubMed] [Google Scholar]
- Nayernia K. Stem cells derived from testis show promise for treating a wide variety of medical conditions. Cell Res. 2007;17:895–897. doi: 10.1038/cr.2007.96. [DOI] [PubMed] [Google Scholar]
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD (1996) Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev 59:89–102 [DOI] [PubMed]
- Silani V, Cova L, Corbo M, Ciammola A, Polli E. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet. 2004;364:200–202. doi: 10.1016/S0140-6736(04)16634-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Turnpenny L, Cameron I, Spalluto C, Hanley K, Wilson D, Hanley N. Human embryonic germ cells for future neuronal replacement therapy. Brain Res Bull. 2005;68:76–82. doi: 10.1016/j.brainresbull.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wagner M, Han B, Jessell TM. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992;116:55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]