Abstract
Stem cells present an important tool in livestock assisted reproduction and veterinary therapeutic field such as tissue engineering. We report for the first time isolation of pluripotent stem cell-like cells expressing pluripotency markers (alkaline phospahatase, OCT-4, NANOG and SOX-2) from the amnion of water buffalo (Bubalus bubalis). The cells showed no apparent abnormalities in their chromosomal profiles before and after cryopreservation. The cytochemical staining revealed that pluripotent cells were capable of undergoing directed differentiation in vitro into osteocytes. It could be inferred that amnion-derived pluripotent stem cell-like cells can be isolated, cultured for many passages and differentiated into mesoderm lineage, and may be an alternative source to mesenchymal stem cells. These cells can have applications in assisted reproduction, developmental biological and regenerative medicine.
Keywords: Amnion cells, Buffalo, Differentiation, Pluripotency, Stem cells
Introduction
It is generally accepted that mammalian amnion is derived from the extra-embryonic endoderm or extra-embryonic trophectoderm. However, Miki and Strom (2006) have shown that amnion is derived from the epiblasts, the pluripotent cells which eventually give rise to all of the cell types of the embryo. The amniotic membrane (AM) is composed of three major layers: a single epithelial layer, an avascular mesenchyme and a thick basement membrane. The compact layer of stromal matrix adjacent to the basement membrane forms the fibrous skeleton of the AM (Parry and Strauss 1998). It is adjacent to the trophoblast cells and lines the amniotic cavity. Amnion can be separated from the underlying chorion, as chorion and amnion are not truly fused at cellular level, because AM has anti-adhesive properties and is felt to promote epithelialization and decrease inflammation, neovascularization, and fibrosis.
The AM has been regarded as a promising source of non-invasive isolation of mesenchymal stem (MS) cells, and poses no ethical concerns as fetal membranes are discarded at birth. Further, the amnion-derived MS cells are reported to express certain key pluripotency markers including Oct-4 (Prusa et al. 2003), and possess features of differentiation into cells like cardiomyocytes (Zhao et al. 2005). This makes them more suitable candidates for cell therapy with less chances of immuno-rejection (Lovati et al. 2011). Among animals, amniotic fluid (AF) derived cells have been reported in rodents (Marcus et al. 2008), porcine (Zheng et al. 2009) and buffalo (Yadav et al. 2011). Review of the literature reveals the increasing demand of research on stem cells in livestock assisted reproduction and veterinary applications (Dev et al. 2011; Nowak-Imialek et al. 2011; Singh et al. 2011; Yadav et al. 2012). Further, the availability of an easily accessible and reproducible cell source may greatly facilitate the development of stem cell-based tissue engineering and therapies for regenerative veterinary medicine. Present study was undertaken for isolation, characterization and differentiation of these cells from the AM of water buffalo, a mainstay of dairy, meat and agriculture in several countries across the globe (Nanda and Nakao 2003; Singh et al. 2009; Yadav et al. 2011).
Materials and methods
Culture media and supplements
All chemicals, reagents, culture media and antibiotics were of cell culture grade unless otherwise indicated, and purchased from Sigma Chemicals Co., (St. Louis, MO, USA). The plastic ware was from Nunc (Roskilde, Denmark), and membrane filters were from Millipore Corp., (Bedford, MA, USA). Fetal bovine serum (FBS) was from Life Technologies, India Pvt. Ltd. (Bangalore, India). Cell culture media and FBS were from the same batch throughout the study, and were reconstituted freshly as per manufacturers’ instructions and filter-sterilized (0.22 μm) prior to use.
Tissue collection and cell cultures
Gravid bubaline uteri of first trimester were collected from abattoir and brought to laboratory within 4–5 h of slaughtering. The uterus was washed using sterile saline and uterine wall was incised under aseptic conditions to expose fetal membranes. Amnion was separated from chorion by peeling (Yadav et al. 2011). The tissue was disintegrated by partial trypsinization (0.25 % trypsin–EDTA solution, 25–30 min. at 37 °C). The cell clumps were allowed to settle for 5 min. at room temperature. The cells in suspension were transferred into another tube and cell pellet was obtained by centrifugation at 450×g for 10 min. The cells were washed thrice with PBS and re-suspended in culture medium (DMEM containing 10 % FBS, 1 % non-essential amino acids, 1 % vitamins, 1 % penicillin/steptomycin/ampicillin). Cells were seeded at a density of 1 × 106 cells/ml in 25 cm2 cell culture flasks under humidified conditions at 38.5 ± 0.5 °C in 5 % CO2. The confluent cell monolayer was trypisinized partially (0.25 % trypsin–EDTA at 37 °C, 5 min.), washed to remove trypsin and sub-cultured for further propagation studies.
Cryopreservation of cells
The cells were cryopreserved at an interval of five passages. The cryopreservation of cells was performed as per protocol used in our lab (Yadav et al. 2011). Briefly, the confluent cultures were trypsinised as above and washed thrice by centrifugation using cell culture medium. The cell pellet obtained was resuspended in pre-cooled (4 °C) cryopreservation medium (DMEM containing 10 % DMSO and 20 % FBS) in 1 ml cryovials. The cryovials were held at −40 °C for ~24 h before transferring into liquid nitrogen (−196 °C). After 7 days of cryopreservation, the cryovials were taken out and warmed by placing them in a water bath (37 °C) for ~15 s. The cell suspension was washed to remove residual cryopreservation medium. The cells (at a density of 1 × 106 cells/ml) were recultured in 25 cm2 cell culture flask. A fraction of cell volume (~50–100 μl) was used to evaluate the cell survival rate using trypan blue (0.4 %) staining and counting live (unstained)/dead (blue stained) cells.
Expression of pluripotency markers
Cells exhibiting fibroblast-like phenotype were cultured in six well culture plate in CO2 incubator (5 % CO2) at 38.5 ± 0.5 °C under saturated humidified conditions and allowed to reach at least 70 % confluency. Alkaline phosphatase (AP) staining was carried out in monolayer cells using staining kit as per instructions of the manufacturer (Sigma, St. Louis, MO, USA). Expression of AP and other pluripotency genes, viz., OCT-4, NANOG and SOX-2 were studied in primary tissue and at passages 2–10. The inner cell mass (ICM) cells of in vitro produced blastocysts were used as positive control to monitor AP expression (Yadav et al. 2011).
Reverse transcription PCR (RT-PCR) for pluripotency gene expression
Expression of pluripotency genes was analyzed by RT-PCR as reported earlier (Yadav et al. 2011). Total RNA was isolated from primary tissue and cells at different passages using Gen Elute Mammalian Total RNA miniprep kit (Sigma, RTN70). RNA concentration was measured at 260 nm using spectrophotometer (Picodrop, UK). DNase-I treated RNA served as a template for RT. The RT-PCR was performed using one step RT-PCR kit (Life Technologies, India Pvt. Ltd.) and oligo dT primers. The RT reaction was initiated by taking 3–5 μg of total RNA in RNase-free water, random primers (100 μM) and oligo dT (50 μM), incubated at 65 °C for 5 min., and then immediately kept on ice. The heat-treated RNA was incorporated into RT reaction mixture containing Super Script® III reverse transcriptase (200 U/μl), 5X RT buffer, di-thiothretol (DTT, 0.1 M), and dNTPs (10 mM). The RT-PCR cycles comprised of heating the PCR mixture at 65 °C for 5 min, incubation on ice for 1 min, at 25 °C for 5 min., and again at 50 °C for 50 min., followed by inactivation of reaction mixture at 80 °C for 15 min. The first complementary DNA (cDNA) strand was further amplified using gene-specific primers (Table 1). 25 μl of PCR reaction mixture was prepared by using cDNA, 10X PCR buffer, 25 mM MgCl2, 10 mM dNTP mix, 10 μM forward and reverse primers each, and Taq DNA polymerase (5 U/μl). The PCR amplification cycles included 36 cycles, each comprising of denaturation at 94 °C for 30 s, annealing at touch down of 56–54 °C for 30 s, elongation at 72 °C for 30 s, and final extension at 72 °C for 5 min. A set of reaction recipe without template cDNA was used as −ve control. β-actin was amplified at each stage as a house keeping marker gene.
Table 1.
Details of primers used for expression of pluripotency genes
| Sr no. | Gene | Primer sequence | Annealing temp (°C) | Size (bp) | Source | Accession no. |
|---|---|---|---|---|---|---|
| 1. | OCT-4 | GTTCTCTTTGGAAAGGTGTTC (F) ACACTCGGACCACGTCTTTC (R) |
55 | 341 | Bos Taurus | AF487022.1 |
| 2. | NANOG | GGGAAGGGTAATGAGTCCAA (F) AGCCTCCCTATCCCAGAAAA (R) |
56 | 211 | Bubalus bubalis | DQ487022.1 |
| 3. | SOX-2 | ACACCAATCCCATCCACACT (F) TTTCTGCAAAGCTCCTACCG (R) |
56–54 | 220 | Bos Taurus | AM774325.1 |
| 4. | β-actin | CTCTTCCAGCCTTCCTTCCT (F) GGGCAGTGATCTCTTTCTGC (R) |
55 | 178 | Bubalus bubalis | DQ661647.1 |
The amplified DNA fragments were resolved on 2 % (w/v) agarose gel containing ethidium bromide (0.5 μg/ml) and visualized under gel documentation system (Alpha Imager, Alpha Innotech Corp., San Leandro, CA, USA). The gene-specific bands were excised from the agarose gel and purified using AuPrep gel extraction kit (Life Technologies India Pvt. Ltd.) for further analysis.
Karyotyping
The karyotyping of cultured cells was analyzed using standard protocol as described earlier (Yadav et al. 2011). Briefly, actively proliferating cells were incubated with colchicines (0.1 μg/ml) for 4 h at 37 °C. The cells were washed twice with DPBS, trypsinized, suspended in a chilled hypotonic solution (68 mM KCl) and incubated for 20 min at 37 °C and then fixed for 10 min in chilled fixative (methanol and glacial acetic acid, 3:1). The pellets were suspended in 5 ml of chilled fixative for another 10 min. The metaphase spreads were prepared by dropping the cell suspension onto ice cold glass slides. The air-dried cell spreads were stained with 2 % Giemsa stain for 5–6 min. and then observed under oil immersion (1,000×) for chromosomal analysis.
Induced in vitro differentiation of cells
For differentiation, the AM cells in culture were dissociated by partial trypsinization, washed as above and then cultured in lineage-specific differentiation medium. For osteogenic differentiation, the medium comprised of DMEM containing 10 % FBS, 10−7 M dexamethasone, 50 μM ascorbic acid and 10 mM β-glycerol phosphate. The differentiation of cells was assessed morphologically and by Alizarin red stain (as an indicator of calcium mineralization).
Alizarin red staining
Culture medium was aspirated from culture wells and the cells were fixed in 4 % paraformaldehyde for 1 h, and washed twice with water. Alizarin red solution was added to overlay the cells followed by incubation at room temperature for 30 min. The stain was aspirated and cells were washed 4 times with water and visualized under phase contrast microscope.
Results
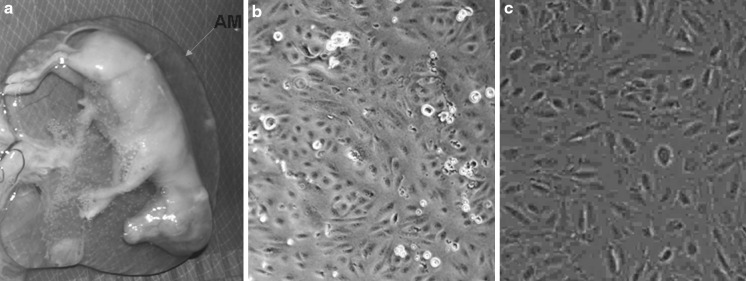
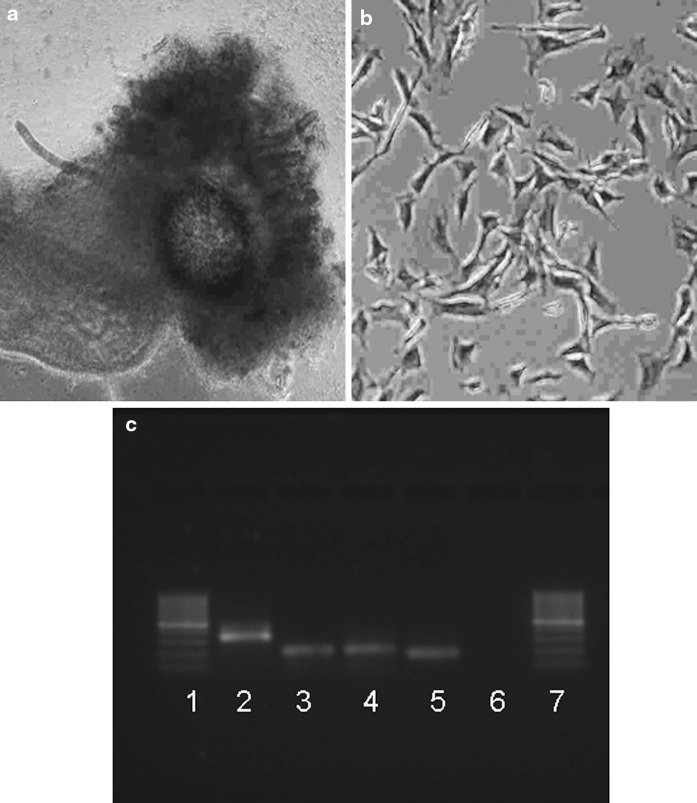
The AM is a thin avascular membrane (Fig. 1a) derived from fetus, composed of an inner epithelial layer and an outer layer of connective tissue which contiguous over the umbilical cord with the fetal skin. The cells from AM started emerging and anchoring to cell culture flasks within 24 h after initiation of culture. Majority of the cells exhibited anchorage dependent growth and a fibroblast-like morphology (Fig. 1b) resulting in establishing primary cultures and become confluent (Fig. 1c) within 2–3 days. In initial cultures, the average population doubling time up to 10 passages was 3 days which increased gradually in later passages, being up to 4 days per passage. The amniotic cells were cultured continuously for over 21 passages in 75 days with average passage time of 3.57 days. The viability of pre-freezing and frozen-thawed cells was observed to be 96 and 79.6 %, respectively. The frozen-thawed cells were normal in morphology, passage time and chromosomal profile. The expression of AP taken as initial marker of pluripotency was detected in primary tissue (Fig. 2a) and the cells at different passages (Fig. 2b). Besides, a normal chromosomal profile (2n = 50) was observed, indicating that genomic integrity of the cells was maintained. Although RT-PCR analysis showed that amniotic tissue and the cells at P2, P5, P10, expressed OCT-4, NANOG, SOX-2 and β-actin (Fig. 2c). Positive expression during various passages i.e. at P2, P5, P10, were also confirmed by sequencing of the PCR products. The bubaline gene sequences obtained were aligned with published sequences from other species and analyzed using the Basic Local Alignment Search Tool (BLAST).
Fig. 1.
Isolation and culture of amnion cells from amniotic membrane. a A thin-walled amniotic membrane that surrounds the fetus, b Amnion cells resemble to fibroblasts like morphology (×100), c confluent culture (70–80 %) of amnion cells in primary culture (×100)
Fig. 2.
Characterization of buffalo amnion cells at different passages of culture. a Expression of AP in primary tissue (×400), b at passage 5 exhibition of strong positive staining (red) in buffalo amnion cells (×100), b RT-PCR analysis of gene expression in amnion cells, where Lane1 100 bp ladder 2 OCT-4 3 NANOG 4 SOX-2 5 β-actin 6 Negative control 7 100 bp ladder
Induced in vitro differentiation of cells
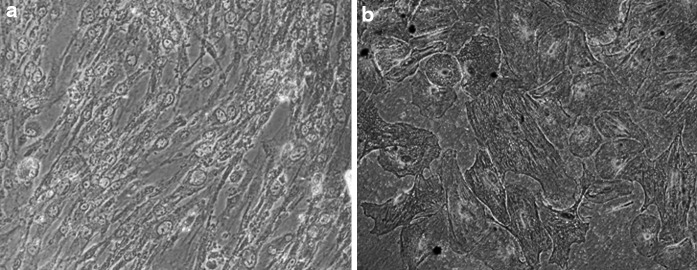
When AM cells were cultured in osteogenic differentiation medium, their morphology started altering towards osteocytes (Fig. 3a) after 7 days of culture, and it was observed till 21st day of culture. On day 14 and 21 of culture, Alizarin red stained positive depicted the calcium deposits in differentiated cells (Fig. 3b).
Fig. 3.
In vitro induced differentiation of amniotic membrane derived amnion cells. a osteocytes (×100), b osteogenic differentiated cells depicting Alizarin red (×200)
Discussion
Recently, it has been shown that MS cells can be found in almost any post-natal organs and tissues including adipose tissue (da Silva et al. 2006; Mimeault and Batra 2008). This knowledge has enormous applications in veterinary medicine and tissue engineering for acute injuries and chronic disorders. Experimental and clinical studies have demonstrated that AM cell transplantation promotes re-epithelialisation, decreases inflammation and fibrosis and modulates angiogenesis. The cells obtained from AM can differentiate into osteogenic, adipogenic, chondrogenic and myogenic lineages in vitro (In‘t Anker et al. 2003; Portmann-Lanz et al. 2006), which might be potential source of clinical biomaterial for engraftment (Bailo et al. 2004). Samandari et al. (2011) used human AM cells as healing accelerator and bone induction in dogs and indicated that AM decreased fibrin-leukocytic exudates and inflammation and observed a suitable cover for experimentally induced injuries. The AM contains collagen, laminin and fibronectin which provide an appropriate substrate for bone induction. This substrate-promoted bone induction might contribute to induction of progenitor cells and/or stem cells in the niche area where surgery had been undertaken.
The livestock somatic and stem cells have a much broader therapeutic and developmental potential than previously assumed (Kues et al. 2005; Talbot and Blomberg 2008). Primordial germ cells and ICM-derived embryonic stem (ES) cell-like cells from cattle (Yadav et al. 2005) and buffalo (Verma et al. 2007), which can produce chimeric animals, albeit without germline contribution have already been documented (Shim et al. 1997; Cibelli et al. 1998).
Further, fetuses from abattoir can be used as sources of different organs and extra embryonic tissue to isolate various types of fetal stem cell-like cells (Yadav et al. 2011, 2012). In this study, the pluripotent stem cell-like cells have been isolated from abattoir-derived bubaline amnion. The cells were found to express molecular markers that are specific to pluripotent ES cells. The fetal and AF-derived cells in buffaloes have already been found to express key pluripotency molecular markers (Yadav et al. 2011; Dev et al. 2011). In this study in long term cultures cells were able to proliferate up to 75 days and their proliferation rate was more up to 10 passages then decreased later may be due to accumulations of more non-dividing cells, was confirmed by earlier study conducted by Gupta et al. (2007) in continuous culture of goat fibroblasts cells.
The ability of the cells to grow in vitro for a prolonged period and maintaining chromosomal integrity may enhance their applications in bubaline assisted reproduction and veterinary regenerative applications. In the present study bubaline amnion cells were cryopreserved through a simplified method, which was succesfully used for fetal fibroblast cells (Yadav et al. 2012). This shows the possibilities of using cryopreservation protocols for establishing somatic cell banking in buffaloes for conservation of valuable genotypes and germplasm. Whether amnion-derived pluripotent cells will prove suitable for improving the efficiency of chimera generation or nuclear transfer cloning in buffaloes has yet to be confirmed conclusively.
The ES-like cells have shown expression of some pluripotency markers, stem cell-specific cell surface antigens and ability of cells to differentiate into other cell types in buffaloes (Verma et al. 2007; Anand et al. 2011; Kumar et al. 2011). Yadav et al. (2011) have shown expression of key pluripotency genes in the AF-derived stem cell-like cells in buffalo. In the present study, the amnion-derived stem cells show comparable expression of key pluripotency genes, namely, NANOG, OCT-4 and SOX-2. Similar kinds of results were obtained from amniotic epithelial cells isolated from human placenta expressing the pluripotent stem cell-specific transcription factors Oct-4 and Nanog (Miki et al. 2005).
MS cells from adult tissue and organs are in the focus of attention in veterinary medicine and associated basic research disciplines. Differentiation of MS and ES cells into other cell lineages has been reported in many species. In our study, the osteogenic differentiation of cells showing mineralization was confirmed by the calcium specific stain, Alizarin red. Our results validate the expression of typical markers for osteogenesis as reported earlier (Dragoo et al. 2005). However, to the best of our knowledge this is first ever report for osteogenic differentiation of amnion cells in buffalo. Further investigation will be required in order to determine whether the stem cell marker-positive cells are remnants of the pluripotent cells from the bubaline fetus or if amniotic cells maintain their stem cell nature for a separate, specific function which is yet to be determined.
If placental stem cells are maintained throughout the pregnancy, the mechanism and the functional implications of this will be the basis of future exploration. In addition, amniotic epithelial cells are known to have unique characteristics, such as low level expression of major histocompatibility complex antigens, and a less restricted differentiation potential. The differentiation of the AE cells to the neural lineage is well documented (Miki and Strom 2006).
The present study, in conjunction with previous studies, inferred that Bubaline AM cells have MS cell characters. The conclusions are based on: 1. mRNA expression of pluripotency-related markers (OCT-4, NANOG, and SOX-2), 2. the differentiation potential of the cells and their ability to retain chromosomal stability. Finally, this finding and information could be very useful for the further applications in livestock assisted reproduction, veterinary tissue engineering and regenerative medical therapies.
Acknowledgments
The authors wish to fully acknowledge Department of Biotechnology, Government of India, for financial support.
Conflict of interest
The authors declare no conflict of interest.
References
- Anand T, Kumar D, Singh MK, Shah RA, Chauhan MS, Manik RS, Singla SK, Palta P. Buffalo (Bubalus bubalis) embryonic stem cell-like cells and preimplantation embryos exhibit comparable expression of pluripotency-related antigens. Reprod Domest Anim. 2011;46:50–58. doi: 10.1111/j.1439-0531.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, Albertini A, Zorzi F, Cavagnini A, Candotti F, Wengler GS, Parolini O. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.TP.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Stice SL, Golueke PL, Kane JJ, Jerry J, Blackwell C, Ponce de Leon FA, Robl JM. Transgenic bovine chimeric offspring produced from somatic cell-derived stem like cells. Nat Biotechnol. 1998;16:642–646. doi: 10.1038/nbt0798-642. [DOI] [PubMed] [Google Scholar]
- da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Dev K, Gautam SK, Giri SK, Kumar A, Yadav A, Verma V, Kumar P, Singh B. Isolation, culturing and characterization of feeder-independent amniotic fluid stem cells in buffalo (Bubalus bubalis) Res Vet Sci. 2011;93:743–748. doi: 10.1016/j.rvsc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Lieberman JR, Lee RS, Deugarte DA, Lee Y, Zuk PA, Hedrick MH, Benhaim P. Tissue engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665–1673. doi: 10.1097/01.PRS.0000161459.90856.AB. [DOI] [PubMed] [Google Scholar]
- Gupta N, Taneja R, Pandey A, Mukesh M, Singh H, Gupta SC, Fibbe WE, Kanhai NH. Replicative senescence, telomere shortening and cell proliferation rate in Gaddi goat’s skin fibroblast cell line. Cell Biol Int. 2007;31:1257–1264. doi: 10.1016/j.cellbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- In‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai NH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- Kues WA, Carnwath JW, Niemann H. From fibroblasts to stem cells: implications for cell therapies and somatic cloning. Reprod Fertil Dev. 2005;17:125–134. doi: 10.1071/RD04118. [DOI] [PubMed] [Google Scholar]
- Kumar D, Anand T, Singh KP, Singh MK, Shah RA, Chauhan MS, Singla SK, Palta P, Manik RS. Derivation of buffalo embryonic stem-like cells from in vitro-produced blastocysts on homologous and heterologous feeder cells. J Assisted Reprod Gen. 2011;28:679–688. doi: 10.1007/s10815-011-9572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovati AB, Corradetti B, Lange Consiglio A, Recordati C, Bonacina E, Bizzaro D, Cremonesi F. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived proginator cells. Vet Res Commun. 2011;35:103–121. doi: 10.1007/s11259-010-9457-3. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Coyne TM, Black IB, Woodbury D. Fate of amnion-derived stem cells transplanted to the fetal rat brain: migration, survival and differentiation. J Cell Mol Med. 2008;12:1256–1264. doi: 10.1111/j.1582-4934.2008.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2:133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda AS, Nakao T. Role of buffalo in the socioeconomic development of rural Asia: current status and future prospectus. Anim Sci J. 2003;74:443–455. doi: 10.1046/j.1344-3941.2003.00138.x. [DOI] [Google Scholar]
- Nowak-Imialek M, Kues W, Carnwath JW, Niemann H. Pluripotent stem cells and reprogrammed cells in farm animals. Microsc Microanal. 2011;17:197–474. doi: 10.1017/S1431927611000080. [DOI] [PubMed] [Google Scholar]
- Parry S, Strauss JF., 3rd Pre-mature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–673. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bernasch G, Hengstschlager M. Oct-4 expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Samandari MH, Adibi S, Khoshzaban A, Aghazadeh S, Dihimi P, Torbaghan SS, Keshel SH, Shahabi Z. Human amniotic membrane, best healing accelerator, and the choice of bone induction for vestibuloplasty technique (an animal study) Transpl Res Risk Manag. 2011;3:1–8. [Google Scholar]
- Shim H, Gutierrez-Adan A, Chen LR, BonDurant RH, Behboodi E, Anderson GB. Isolation of pluripotent stem cells from cultured porcine primordial germ cells. Biol Reprod. 1997;57:1089–1095. doi: 10.1095/biolreprod57.5.1089. [DOI] [PubMed] [Google Scholar]
- Singh B, Chauhan MS, Singla SK, Gautam SK, Verma V, Singh AK, Manik RS, Sodhi M, Mukesh M. Reproductive biotechniques in buffalo: status, prospects and challenges. Reprod Fertil Dev. 2009;14:499–507. doi: 10.1071/RD08172. [DOI] [PubMed] [Google Scholar]
- Singh B, Gautam SK, Chauhan MS, Singla SK, Kumar S, Kumar V, Yadav PS. Cellular reprogramming-advances and opportunities for applications in veterinary and animal sciences. In: Berhardt Leon V., editor. Advances in medicine and biology. NY: Nova Science Publishers; 2011. pp. 194–214. [Google Scholar]
- Talbot NC, Blomberg LA. The pursuit of ES cell lines of domestic ungulates. Stem Cell Rev. 2008;4:235–254. doi: 10.1007/s12015-008-9026-0. [DOI] [PubMed] [Google Scholar]
- Verma V, Gautam SK, Singh B, Manik RS, Palta P, Singla SK, Goswami SL, Chauhan MS. Isolation and characterization of embryonic stem cell-like cells from in vitro-produced buffalo (Bubalus bubalis) embryos. Mol Reprod Dev. 2007;74:520–529. doi: 10.1002/mrd.20645. [DOI] [PubMed] [Google Scholar]
- Yadav PS, Kues WA, Herrmann D, Niemann H. Bovine ICM derived cells express the Oct4-ortholog. Mol Reprod Dev. 2005;72:182–190. doi: 10.1002/mrd.20343. [DOI] [PubMed] [Google Scholar]
- Yadav PS, Mann A, Singh V, Yashveer S, Sharma RK, Singh I. Expression of pluripotency genes in buffalo (Bubalus bubalis) amniotic fluid cells. Reprod Domest Anim. 2011;46:705–711. doi: 10.1111/j.1439-0531.2010.01733.x. [DOI] [PubMed] [Google Scholar]
- Yadav PS, Singh RK, Singh B. Fetal stem cells in farm animals—applications in health and production. Agric Res. 2012;1:67–77. doi: 10.1007/s40003-011-0001-7. [DOI] [Google Scholar]
- Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528–535. doi: 10.1097/01.TP.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Zhao HY, Zhao XE, Quan FS, Hau S, He XY, Liu J, He XN, Lin H. Development of cloned embryos from porcine neural stem cells and amniotic fluid derived stem cells transfected with enhanced green fluorescence protein gene. Reproduction. 2009;137:793–801. doi: 10.1530/REP-08-0469. [DOI] [PubMed] [Google Scholar]