Abstract
Nitrocellulose membranes, one of the most important and oldest cellulose derivatives, are commonly used for nucleic acid and protein detection in research and diagnostic applications. However, a limited number of studies have explored whether they can act as scaffolds for cell growth. In this study, we investigated this polymeric material for its ability to support the growth of human cells. Eight established cell lines were examined for adherence, growth, spread, and survival on nitrocellulose membranes by optical microscopy after hematoxylin and eosin and/or immunocytochemical staining and by scanning electron microscopy. Apoptosis and leakage of lactate dehydrogenase (LDH) were also assessed. All cells readily adhered to and spread on the surface of nitrocellulose membranes as well as coverslips, and the cells maintained the expression of digestive system-specific genes. No significant change was detected in apoptosis or leakage of LDH from cells grown on nitrocellulose membranes. These results suggested that nitrocellulose membranes have a suitable cytocompatibility towards human cells and that they might be used for tissue-engineering scaffolds. Moreover, we demonstrate an additional and underused property of nitrocellulose of specific relevance to microscopic imaging, as it can be rendered virtually transparent, thus the cells growing on such membranes can be observed directly under an optical microscope after staining.
Keywords: Polymer membrane, Nitrocellulose membrane, Cell growth, Adherence, Cytocompatibility
Introduction
Nitrocellulose membranes, derived from wood pulp, are one of the oldest cellulose derivatives with over a century of history (Auer et al. 2005). They have been widely used as a solid-phase immobilization support for DNA, RNA, and proteins in traditional molecular biology assays, such as Southern, Northern, and Western blots, as well as atomic force microscopy (Kreplak et al. 2007). These membranes are also used to detect coliforms in water in conjunction with a filtration device (Barry et al. 2007).
Nitrocellulose membranes are characterized has having good hydrophilia and flexibility as well as stable physical and chemical properties. In the biomedical fields, these porous membranes have been used as osmosis, ultrafiltration, and microporous membranes as well as electrophoresis films. However, little is known about whether these membranes can act as scaffolds in cell culture. Previous study has shown that slices of neonatal rat or adult human liver, 100–400 μm thick, can be cultured on nitrocellulose inserts at the air/fluid interface, and these hepatocytes express albumin for up to 10 days and are viable for up to 21 days, during which time new structures appear (Verrill et al. 2002). Similar results have been observed when these membranes were applied to the cultivation of vagus nerves and ovarian cortical slices (Chandra et al. 2005; Madhavan et al. 2002; Reimer et al. 1999; Peng et al. 2010). Furthermore, bacteria can attach to and grow on sterilized nitrocellulose sheets on the surface of agar plates (Fang et al. 2009; Bolaños et al. 2004).
Coverslips are one of the most common support platforms used in conventional cell culture, especially for staining and morphological observations; however, their rigid and fragile nature make them inconvenient for further dynamic observations, such as cell-cycle analysis, transfection, and evaluation of therapeutic efficacy (i.e., radiotherapy or anticancer drugs). In this study, we first tested eight established cell lines for their ability to adhere and spread on nitrocellulose membranes. We then performed hematoxylin and eosin (HE) and immunocytochemical staining to observe the cells under an optical microscope and compared them with cells growing on coverslips.
Materials and methods
Cell lines and cell culture
The information regarding the cell lines used in this study is shown in Table 1. Cells were maintained in Dubelcco’s modified Eagle’s medium (DMEM) or RMPI 1640 supplemented with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA). Cells were incubated in a 37 °C incubator supplemented with 5 % CO2 until 100 % confluency and split following 0.05 % trypsin digestion.
Table 1.
Origin, cell type, and culture conditions of the cell lines added to nitrocellulose membranes
| Cell line | Cell type | Obtained from | Basal medium |
|---|---|---|---|
| BGC823 | Epithelial, gastric carcinoma | SIBCB TCHu 11 | RPMI 1640 |
| HT29 | Epithelial, colorectal adenocarcinoma | ATCC HTB-38 | RPMI 1640 |
| HCT116 | Epithelial, colorectal carcinoma | ATCC CCL-247 | DMEM |
| HCT8 | Epithelial, ileocecal colorectal adenocarcinoma | ATCC CCL-244 | RPMI 1640 |
| SW1116 | Epithelial, colon adenocarcinoma | ATCC CCL-233 | DMEM |
| BxPC-3 | Epithelial, pancreatic carcinoma | SIBCB TCHu 12 | DMEM |
| Panc-1 | Epithelial, epithelioid carcinoma | ATCC CRL-1469 | DMEM |
| HepG2 | Epithelial, hepatocellular carcinoma | ATCC HB-8065 | DMEM |
ATCC American type culture collection (Manassas, Virginia, USA), SIBCB Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China)
Preparation of nitrocellulose membranes for cell culture
Nitrocellulose membranes (0.45 μm pore size; Millipore, Billerica, MA, USA) were cut into small round pieces, autoclaved for 15 min, and rinsed with phosphate buffered saline (PBS). The individual membrane pieces were then placed into 6-well/12-well cell culture plates, and a small amount of culture medium was added to retain moisture until the cells were seeded. Cells were directly placed onto the membrane at 3–10 × 104 cells/cm2. As a parallel control, cells were also plated without a membrane.
Scanning electron microscopy
After 2 or 4 days of culture, the membranes and coverslips with cells were washed 2–3 times with PBS, fixed overnight with 2.5 % glutaraldehyde in potassium-free PBS, and dehydrated sequentially in 50, 75, 85, and 95 % ethanol. The membrane and coverslip samples were then immersed in 100 % t-butanol for 30 min three times and freeze-dried at 13.3 Pa at 5 °C. The dried membranes and coverslips were gold-sputtered (100A) and observed under a scanning electron microscope (SEM; S-3000 N; Hitachi, Tokyo, Japan) at an electron beam radiation of 20 kV. The membranes without cells were directly observed by SEM after gold coating.
HE staining
Nitrocellulose membranes are opaque and white and cannot be directly observed under an optical microscope. We removed the nitrocellulose membranes with cells from the culture medium after culturing in 6-well plates for 48–72 h, washed them 2–3 times with PBS, and then fixed them for 30 min at room temperature with 4 % paraformaldehyde. For each sample, the membrane with cells was stained with HE, dehydrated with increasing concentrations of ethanol, rinsed twice with absolute ethanol, and dried at room temperature. In the final step, the membrane became transparent following xylene treatment and images were taken under a microscope (BX51; Olympus, Tokyo, Japan). Cells plated on glass coverslips were stained with HE using standard procedures.
Immunocytochemistry analysis
For immunocytochemistry analysis, paraformaldehyde-fixed cells on nitrocellulose membranes and coverslips were first treated for 15 min in 3 % hydrogen peroxide to inhibit endogenous peroxidase activity. They were then incubated overnight at 4 °C with primary antibodies against CK18 (1:50; N-16, sc-31700; Santa Cruz Biotechnology, CA, USA), CEA (1:50; ZM-0062; Zhongshan Goldenbridge Biotechnology, Beijing, China), and CA1-99 (1:50; ZM-0021; Zhongshan Goldenbridge Biotechnology, Beijing, China), followed by a 30 min incubation with an HRP-conjugated secondary antibody at room temperature. The immunostaining was visualized using a standard 3, 3′-diaminobenzidine protocol, and the cells were counterstained lightly with hematoxyline. Transparent treatment and image photographs were processed similar to the HE analysis. All immunostained sections were examined and evaluated by the same senior pathologist (Y.Z.).
Apoptosis assays and LDH leakage measurements
A total of 3–20 × 104 cells from membrane-based or regular culture were labeled with FITC-conjugated Annexin V (V13245; Invitrogen Life Technologies, Carlsbad, CA, USA) and propidium iodide (PI) for 15 min at room temperature and then analyzed by two-color flow cytometry using Cell Quest Analysis software (Becton–Dickinson, Mountain View, CA, USA). A minimum of 10,000 events were counted per sample. The percentage of Annexin V-positive cells was reported to reflect the relative proportion of apoptotic cells in the early and late stages of apoptosis (Anthony et al. 1998). For cells undergoing necrosis, Annexin V and PI-positive cells were expected, whereas the presence of FITC-Annexin V-positive but PI-negative cells was indicative of apoptotic rather than necrotic cell death. Cell culture supernatants were collected every 24 h for 7 days from both membrane-based and regular cell culture. LDH activity, a marker of cell deterioration, was measured using a biochemical auto-analyzer (AU5421; Olympus, Tokyo, Japan).
Statistical analysis
For LDH leakage analysis, differences between groups were analyzed using analysis of variance (ANOVA) for repeated measures followed by a Newman-Keuls test. For apoptosis analysis, the statistical significance of the differences between experimental variables and their reference group was determined using the Student’s t test. The level of significance was defined as p < 0.05. The values shown represent the mean ± SD for triplicate experiments. All statistical tests were performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
The properties of nitrocellulose membranes as scaffolds
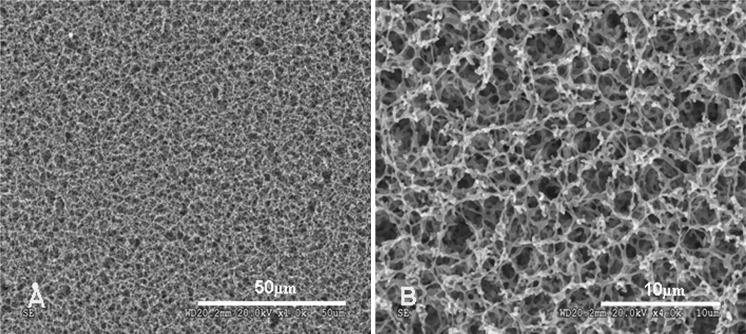
The physical characteristics of nitrocellulose membranes, such as high tensile strength, provided excellent handling and ensured that the membrane did not rip, tear, or crack during treatment. Nitrocellulose membranes were also easy to cut with scalpels or razor blades. Autoclaving under conventional conditions did not cause notable changes. Figure 1a and b shows the morphology of nitrocellulose membranes as characterized by SEM. Nitrocellulose membranes demonstrated the characteristics of a typical homogenous micro-filtration membrane, a highly porous and rough surface. The surface pores of this membrane were relatively uniform in diameter.
Fig. 1.
SEM images of nitrocellulose membranes. Low (a, ×1,000) and high (b, ×4,000) magnification images showing a sponge-like, three-dimensional structure with a highly porous and rough surface
Growth of cells on nitrocellulose membranes
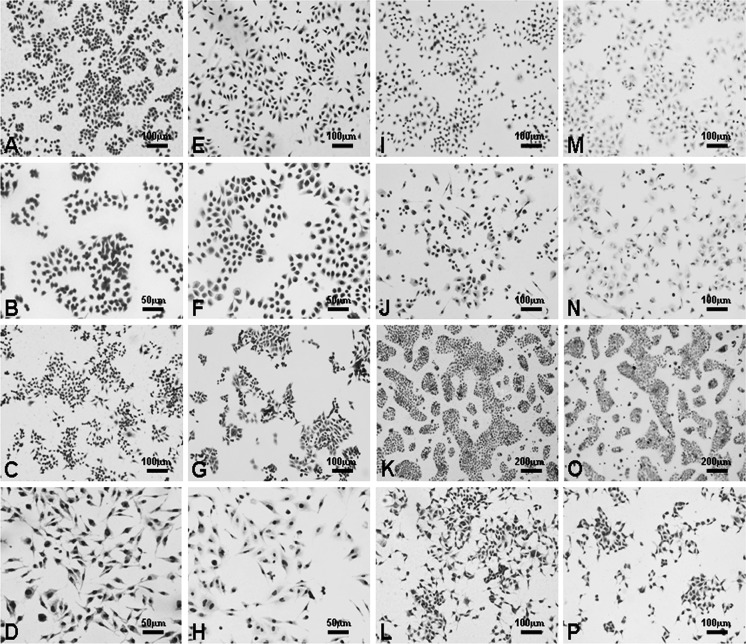
Cell morphology and proliferation were observed following HE staining and transparent treatment of the membranes of both the membrane and coverslip cultures. All of the cell lines listed in Table 1 showed high levels of attachment on both the membranes and coverslips. In all cases, the cells gradually proliferated and usually reached 100 % confluency within 1 week. Staining of the cells and observation with an optical microscope were the same for both the cells grown on membranes and on coverslips. Background staining with HE was observed to be lighter for the cells grown on membranes, and the cell profiles were clear (Fig. 2).
Fig. 2.
Human cell cultures on nitrocellulose membranes stained with HE. Cells were seeded on nitrocellulose membranes and cultured for 48–72 h: (a, ×200) BGC-823, (b, ×400) HT29, (c, ×200) HCT116, (d, ×400) HCT8, (i, ×200) SW1116, (j, ×200) PANC-1, (k, ×100) BxPC-3, and (l, ×200) HepG2. Human cells were seeded on coverslips and cultured for 48–72 h as controls: (e, ×200) BGC-823, (f, ×400) HT29, (g, ×200) HCT116, (h, ×400) HCT8, (m, ×200) SW1116, (n, ×200) PANC-1, (o, ×100) BxPC-3, and (p, ×200) HepG2. The imagings of stained cells on the nitrocellulose membranes were comparable to those on conventional coverslips
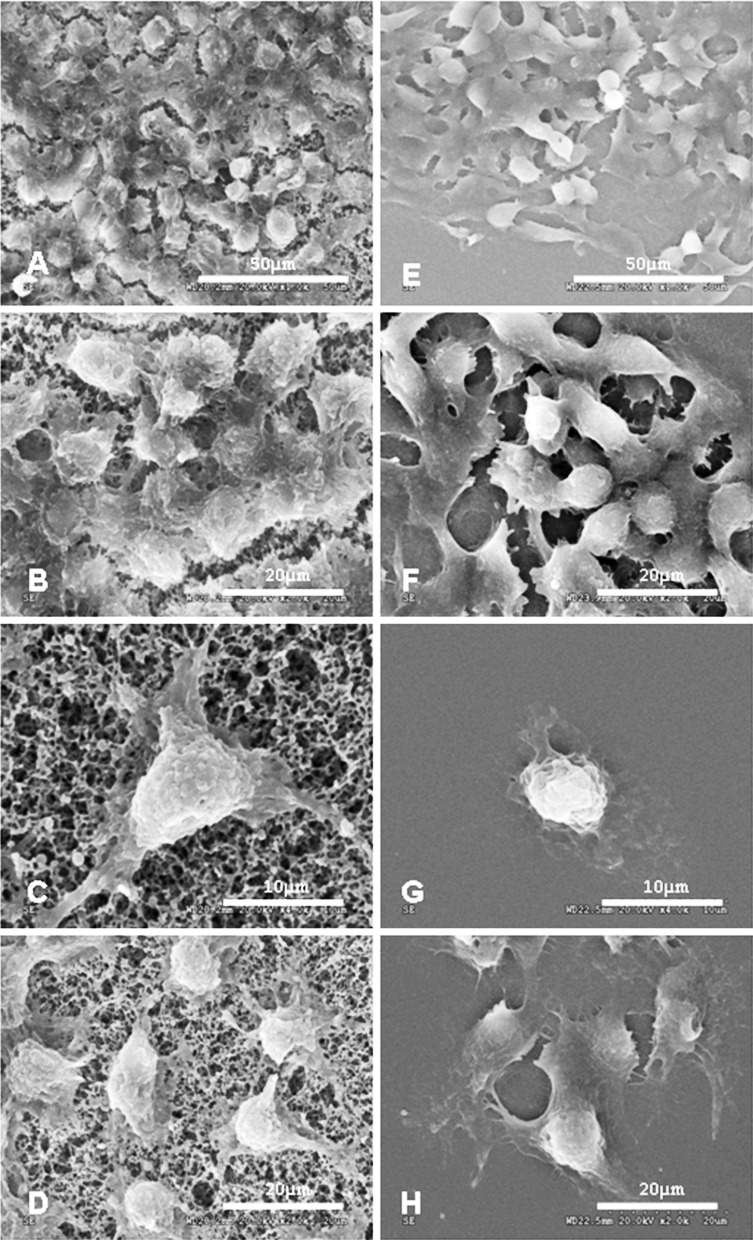
After 2 days of culture, HepG2 cells proliferated well and were evenly distributed on the membrane (Fig. 3a–d) as the same as coverslips (Fig. 3e–h), as observed via SEM. The cells were irregular in shape with sharp borders (Fig. 3d). Subsequently, the cells grew more densely and aggregated to cover the membrane surface (Fig. 3a, b). Cells were relatively flat on the nitrocellulose membranes, and various pseudopod-like structure formations were seen on the side of the membrane through which cells formed junctions with the nitrocellulose membrane (Fig. 3c). No obvious pseudopod -like structures formed between cells and coverslips (Fig. 3g).
Fig. 3.
SEM images of HepG2 cells. Cells cultured on nitrocellulose membranes at 2 days (d, ×2,000) and 4 days (a, ×1,000; b, ×2,000) after seeding. Various pseudopod-like structures formed on the side of the membrane by which cells had formed a tight bond with the porous membrane (c, ×4,000). Cells cultured on coverslips at the same time as the cells cultured on membranes after seeding as controls: 2 days (h, ×2,000) and 4 days (e, ×1,000; f, ×2,000) after seeding. No obvious pseudopod -like structures formed between cells and coverslips (g, ×4,000)
Immunocytochemistry analysis
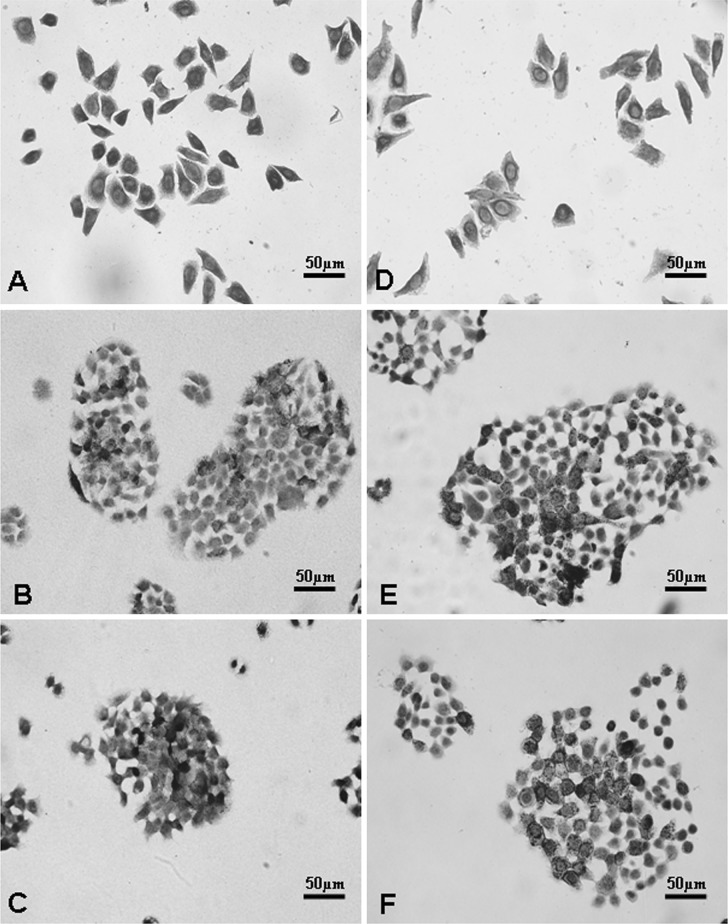
Immunocytochemistry analysis revealed that CK18, a representative marker of digestive system tumor cells originated from epithelial cells, was easily detected in the cytoplasm of the SW1116 colorectal cancer cell line (Fig. 4a). Immunoreactivity of CEA and CA 1–99, digestive system tumor biomakers, were also found on the cytomembrane of the BxPC-3 cell line (Fig. 4b, c). These results showed that membrane-supported cell culture can undergo immunochemistry without any technical issues and that the biologic features of cells growing on membranes are similar to those growing on coverslips (Fig. 4d–f).
Fig. 4.
Detection of cellular biological characteristics based on immunocytochemistry. CK18 was significantly detected in the SW1116 colorectal cancer cell line cultured on nitrocellulose membranes (a) and coverslips (d). CEA (b, e) and CA 1-99 (c, f), as digestive system tumor makers, were also observed in the cytoplasm and the cytomembrane of BxPC-3 cells cultured on nitrocellulose membranes (b, c) and coverslips (e, f). Original magnification: ×400
LDH leakage and apoptosis
We selected LDH leakage as an indicator of cytotoxicity. The leakage of LDH from BGC-823, HT29, HCT116, HCT8, SW1116, PANC-1, BxPC-3, and HepG2 cells was examined every 24 h for 7 days of culture. As shown in Table 2, the concentrations of LDH from membrane-supported HCT8 cells were lower than that observed with regular culture on plates (p < 0.05), while the LDH from other cell lines did not show any significant differences between the two different cell culture platforms (p > 0.05).
Table 2.
The concentrations of LDH from human cells cultured on nitrocellulose membranes and plastic culture plates
| Cell lines | Type of scaffolds | Number of wells | Culture time | F | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day1 | Day2 | Day3 | Day4 | Day 5 | Day 6 | Day 7 | |||||
| BGC-823 | Ma | 6 | 47.83 ± 7.25 | 69.33 ± 15.69 | 80.17 ± 10.38 | 85.50 ± 10.46 | 72.00 ± 9.96 | 120.00 ± 7.56 | 123.17 ± 6.65 | 0.320 | 0.596 |
| Pb | 6 | 56.00 ± 6.26 | 59.00 ± 7.01 | 80.00 ± 6.72 | 84.83 ± 6.43 | 68.67 ± 10.71 | 113.67 ± 3.27 | 130.67 ± 4.68 | |||
| HT29 | M | 6 | 13.83 ± 2.40 | 8.00 ± 2.61 | 13.00 ± 3.03 | 14.00 ± 4.34 | 34.67 ± 2.73 | 71.33 ± 3.50 | 71.00 ± 1.55 | 1.305 | 0.305 |
| P | 6 | 12.17 ± 3.66 | 10.50 ± 3.99 | 13.83 ± 1.47 | 22.17 ± 3.06 | 32.33 ± 4.63 | 72.50 ± 1.64 | 69.50 ± 1.76 | |||
| HCT116 | M | 6 | 39.00 ± 6.66 | 51.83 ± 11.99 | 57.00 ± 11.49 | 77.83 ± 5.42 | 52.33 ± 13.47 | 83.67 ± 1.51 | 60.17 ± 3.06 | 1.030 | 0.357 |
| P | 6 | 40.67 ± 8.38 | 48.83 ± 5.98 | 61.67 ± 5.99 | 70.17 ± 6.56 | 55.33 ± 10.93 | 82.00 ± 2.28 | 52.00 ± 1.41 | |||
| HCT8 | M | 6 | 27.50 ± 1.87 | 16.83 ± 4.82 | 16.67 ± 5.32 | 29.33 ± 3.14 | 29.50 ± 3.02 | 23.67 ± 3.33 | 37.50 ± 6.06 | 23.405 | 0.005 |
| P | 6 | 33.50 ± 1.87 | 16.67 ± 2.66 | 14.17 ± 1.94 | 25.33 ± 6.41 | 34.17 ± 2.93 | 27.83 ± 2.14 | 43.67 ± 2.16 | |||
| SW1116 | M | 6 | 19.17 ± 3.13 | 22.67 ± 4.27 | 31.17 ± 6.15 | 27.83 ± 2.99 | 38.67 ± 17.83 | 34.00 ± 4.38 | 25.67 ± 3.67 | 2.295 | 0.190 |
| P | 6 | 20.33 ± 3.27 | 14.33 ± 4.55 | 33.17 ± 6.62 | 28.17 ± 1.72 | 26.67 ± 6.41 | 37.33 ± 17.28 | 36.67 ± 10.56 | |||
| BxPC-3 | M | 6 | 29.33 ± 5.54 | 17.33 ± 2.94 | 24.33 ± 4.41 | 36.00 ± 6.51 | 48.33 ± 16.51 | 85.17 ± 1.60 | 89.00 ± 4.69 | 0.594 | 0.476 |
| P | 6 | 24.00 ± 4.47 | 21.17 ± 3.37 | 15.67 ± 4.27 | 37.17 ± 2.23 | 59.67 ± 19.61 | 90.67 ± 6.06 | 95.50 ± 7.34 | |||
| PANC-1 | M | 6 | 23.67 ± 5.61 | 37.67 ± 5.43 | 24.83 ± 1.17 | 38.67 ± 8.36 | 31.00 ± 3.74 | 15.00 ± 5.76 | 34.00 ± 7.48 | 0.021 | 0.891 |
| P | 6 | 23.67 ± 7.74 | 42.50 ± 3.15 | 31.67 ± 5.85 | 32.00 ± 3.95 | 19.00 ± 3.74 | 12.33 ± 7.94 | 58.00 ± 7.48 | |||
| HepG2 | M | 6 | 21.17 ± 1.94 | 25.67 ± 1.51 | 21.50 ± 3.94 | 33.50 ± 7.89 | 29.17 ± 2.86 | 26.17 ± 2.93 | 31.17 ± 4.88 | 0.858 | 0.397 |
| P | 6 | 21.33 ± 4.37 | 27.00 ± 2.19 | 24.17 ± 2.86 | 29.17 ± 6.68 | 27.83 ± 3.06 | 23.50 ± 4.23 | 27.83 ± 5.78 | |||
aNitrocellulose membranes, b Plastic culture plates as control
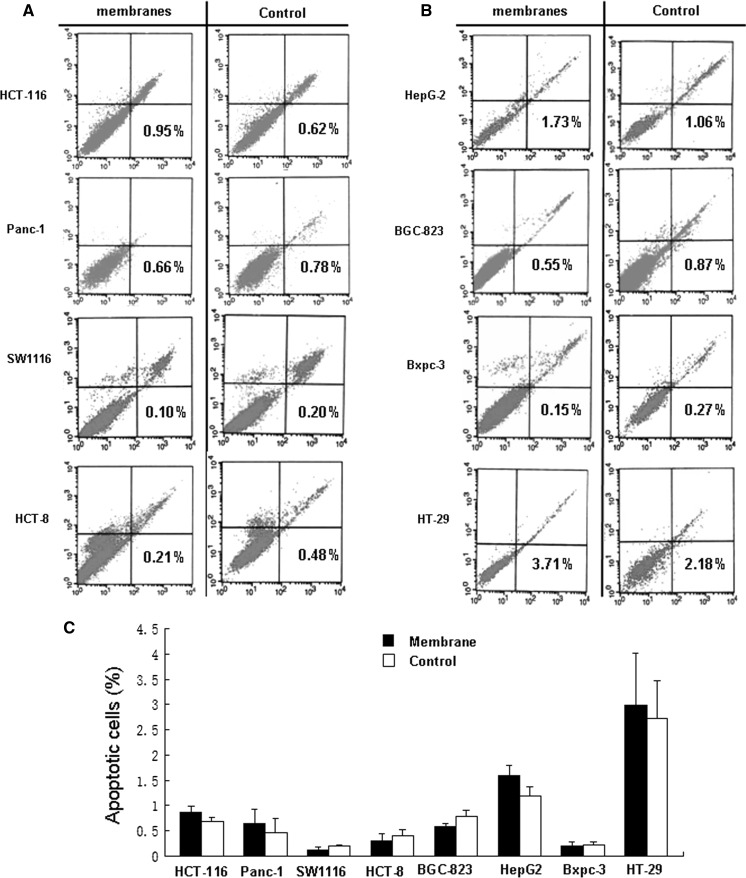
The presence of FITC-Annexin V-positive but PI-negative cells is indicative of apoptosis. Figure 5 shows that there were no significant differences between the two culturing platforms in regards to Annexin V/PI staining (p > 0.05), suggesting that nitrocellulose membranes can act as effective supports during cell culture.
Fig. 5.
Nitrocellulose membranes did not induce apoptosis in human cells. a, b, eight established human cell lines were cultured in the absence or presence of nitrocellulose membranes for 72 h. Cells grown in the absence of membranes were used as the controls. Cells were harvested and double-stained with Annexin V/PI and then subjected to FACScan analysis. The percentages of Annexin V-positive cells, indicative of apoptosis, are shown. c, there was no significant difference between the cells cultured with nitrocellulose membranes and the control cells. The data shown are representative of three experiments in total
Discussion
In this study, we demonstrated that nitrocellulose membranes can be used as efficient supports for cell growth. These membranes could be autoclaved and showed high flexibility and tenacity with the eight cancer cell lines of the digestive system tested. The morphology and function of the cells cultured on the membranes was similar to that of cells growing in conventional culture for both morphology and function. The cytotoxicity of the nitrocellulose membranes was very low, as no significant increase in apoptosis or LDH leakage was detected. We expanded the application of this membrane by directly observing the cells growing on the membranes under an optical microscope after a simple treatment that rendered the membranes transparent.
The opaque nature of nitrocellulose membranes makes them unable to be observed directly under a light microscope (Szabo et al. 2002; Yamasato and Satoh 2001). Recently, Barry et al. (2007) applied a new method for in situ microscopic observation of fungal morphology on nitrocellulose membranes with high-power magnification after treating the membranes with microscope immersion oil. In our study, we successfully processed the opaque white membrane to become transparent by simply staining the membrane before dipping it into xylene. Finally, we directly observed the cell morphology and in situ gene expression under a microscope. The observed background was comparable to conventional coverslips when using this method. This property of nitrocellulose indicated that it was a suitable alternative to glass slides for pathologic staining. Certainly, a disadvantage of nitrocellulose membranes is that they are opaque and cannot be directly observed live under an optical microscope without treatment. However, the membrane can sustain repeated washes without significant loss of cells (Thirumalapura et al. 2006), and optimal sizes and shapes of nitrocellulose can be readily obtained using sterile scissors.
Nitrocellulose membranes, which demonstrate good hydrophilia and adsorption properties, are one of the most common materials used in molecular biology assays (Tonkinson and Stillman 2002). DNA, RNA, and protein bind to the membranes in a noncovalent but irreversible manner by a mechanism that is not fully understood. It is believed that a dominant hydrophobic interaction and as well as electrostatic interactions may occur between cellular surface proteins and this membrane (Fang et al. 2009; Cretich et al. 2010), which allow the freshly dissected tissue samples to be adsorb to the membrane (Kreplak et al. 2007). Similarly, the adhesion of cells to the nitrocellulose membrane mainly takes advantage of the nitrocellulose’s affinity to membrane proteins.
The porous nature of these membranes also plays an important role in cell attachment and proliferation. The unique sponge-like three-dimensional microstructure of nitrocellulose membranes is favorable to cell nutrient transfusion, uptake, and metabolism, especially for cells that have special needs for space, such as hepatocytes. Mizuguchi et al. (2000) found that a special kind of “paper” material could satisfy the demands for hepatocytes culture based on the unique three-dimensional surface structure of the paper. Hence, the three-dimensional nature of nitrocellulose membranes provides a much greater binding capacity than the two-dimensional surfaces of conventional culture plates.
HepG2 is a cell line that is highly differentiated and specific to the liver, and it serves as a cell material for the construction of bioartificial livers (Kataoka et al. 2005). Our observations indicated that nitrocellulose membranes supported HepG2 cell attachment, morphology, proliferation, and function, which were similar to the results reported in other studies (Kataoka et al. 2005; Risbud et al. 2003; Kasoju et al. 2009; She et al. 2008), suggesting that we should further explore the application of nitrocellulose membranes in membrane bioreactors. Recently, several new membrane materials have been used in bioreactors, such as cuprophane, cellulose acetate, polysulfone, and polypropylene membranes. However some issues still need to be address, including the simulation construction of the hepatic lobule which is the smallest constitutional unit of liver cells and optimization of the cell culture microenvironment (Hashemi et al. 2009). Indeed, the performance of nitrocellulose membranes as a novel scaffold for construction of liver-assist reactors still needs to be examined in detail.
Conclusions
The nitrocellulose membrane is an excellent scaffold for the growth of human cells, and it may be potentially useful in cell-culture related assays and tissue engineering. Moreover, cells cultured on nitrocellulose membranes can be observed directly under an optical microscope after HE and/or immunocytochemistry, which offers an alternative to glass slides in pathology staining applications.
Acknowledgments
This work was supported by the Hi-Tech Research and Development (863) Program of China (2006AA02A140), the Cooperation Project in Industry, Education, and Research of Guangdong province and Ministry of Education of China (2011B090400018), and the Science and Technology Project of Guangdong Province (2009B060300020). We thank Dr. Jinshui Fan for critical review and editing of the manuscript.
Conflict of interest
There is absolutely no conflict of interest in this manuscript
Footnotes
Aimin Li and Yadong Wang contributed equally to this work.
Contributor Information
Side Liu, Phone: +86-020-61641537, FAX: +86-020-87280770, Email: liuside2011@163.com.
Yali Zhang, Phone: +86-020-62787933, FAX: +86-020-87280770, Email: yalizhang2012@163.com.
References
- Anthony RS, McKelvie ND, Cunningham AJ, Craig JI, Rogers SY, Parker AC. Flow cytometry using Annexin V can detect early apoptosis in peripheral blood stem cell harvests from patients with leukaemia and lymphoma. Bone Marrow Transplant. 1998;21:441–446. doi: 10.1038/sj.bmt.1701134. [DOI] [PubMed] [Google Scholar]
- Auer N, Hedger JN, Evans CS. Degradation of nitrocellulose by fungi. Biodegradation. 2005;16:229–236. doi: 10.1007/s10532-004-0896-9. [DOI] [PubMed] [Google Scholar]
- Barry DJ, Chan C, Williams GA. Nitrocellulose as a general tool for fungal slide mounts. J Clin Microbiol. 2007;45:1074–1075. doi: 10.1128/JCM.01609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños L, Redondo-Nieto M, Rivilla R, Brewin NJ, Bonilla I. Cell surface interactions of Rhizobium bacteroids and other bacterial strains with symbiosomal and peribacteroid membrane components from pea nodules. Mol Plant Microbe Interact. 2004;17:216–223. doi: 10.1094/MPMI.2004.17.2.216. [DOI] [PubMed] [Google Scholar]
- Chandra A, Maurya OP, Reddy B, Kumar G, Pandey K, Bhaduri G. Amniotic membrane transplantation in ocular surface disorders. J Indian Med Assoc. 2005;103:364–366, 368. [PubMed] [Google Scholar]
- Cretich M, Sedini V, Damin F, Pelliccia M, Sola L, Chiari M. Coating of nitrocellulose for colorimetric DNA microarrays. Anal Biochem. 2010;397:84–88. doi: 10.1016/j.ab.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Fang SB, Tseng WY, Lee HC, Tsai CK, Huang JT, Hou SY. Identification of Salmonella using colony-print and detection with antibody-coated gold nanoparticles. J Microbiol Methods. 2009;77:225–228. doi: 10.1016/j.mimet.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Salehnia M, Kamali M, Beigi Boroujeni M. The histological characteristics of cultured oral epithelium in different culture conditions. Iran Biomed J. 2009;13:109–115. [PubMed] [Google Scholar]
- Kasoju N, Bhonde RR, Bora U. Preparation and characterization of Antheraea assama silk fibroin based novel non-woven scaffold for tissue engineering applications. J Tissue Eng Regen Med. 2009;3:539–552. doi: 10.1002/term.196. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Nagao Y, Nukui T, Akiyama I, Tsuru K, Hayakawa S, Osaka A, Huh NH. An organic-inorganic hybrid scaffold for the culture of HepG2 cells in a bioreactor. Biomaterials. 2005;26:2509–2516. doi: 10.1016/j.biomaterials.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Kreplak L, Wang H, Aebi U, Kong XP. Atomic force microscopy of Mammalian urothelial surface. J Mol Biol. 2007;374:365–373. doi: 10.1016/j.jmb.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan HN, Priya K, Malathi J, Joseph PR. Preparation of amniotic membrane for ocular surface reconstruction Indian. J Ophthalmol. 2002;50:227–231. [PubMed] [Google Scholar]
- Mizuguchi T, Mitaka T, Sato F, Mochizuki Y, Hirata K. Paper is a compatible bed for rat hepatocytes. Artif Organs. 2000;24:271–277. doi: 10.1046/j.1525-1594.2000.06506.x. [DOI] [PubMed] [Google Scholar]
- Peng X, Yang M, Wang L, Tong C, Guo Z. In vitro culture of sheep lamb ovarian cortical tissue in a sequential culture medium. J Assist Reprod Genet. 2010;27:247–257. doi: 10.1007/s10815-010-9415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer M, Moller K, Sundler F, Hannibal J, Fahrenkrug J, Kanje M. Increased expression, axonal transport and release of pituitary adenylate cyclase-activating polypeptide in the cultured rat vagus nerve. Neuroscience. 1999;88:213–222. doi: 10.1016/S0306-4522(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Karamuk E, Schlosser V, Mayer J. Hydrogel-coated textile scaffolds as candidate in liver tissue engineering: II. Evaluation of spheroid formation and viability of hepatocytes. J Biomater Sci Polym Ed. 2003;14:719–731. doi: 10.1163/156856203322274969. [DOI] [PubMed] [Google Scholar]
- She Z, Jin C, Huang Z, Zhang B, Feng Q, Xu Y. Silk fibroin/chitosan scaffold: preparation, characterization, and culture with HepG2 cell. J Mater Sci Mater Med. 2008;19:3545–3553. doi: 10.1007/s10856-008-3526-y. [DOI] [PubMed] [Google Scholar]
- Szabo E, Nemeskeri A, Heinzlmann A, Suzuki N, Arimura A, Koves K. Cell immunoblot assay study demonstrating the release of PACAP from individual anterior pituitary cells of rats and the effect of PACAP on LH release. Regul Pept. 2002;109:75–81. doi: 10.1016/S0167-0115(02)00186-6. [DOI] [PubMed] [Google Scholar]
- Thirumalapura NR, Ramachandran A, Morton RJ, Malayer JR. Bacterial cell microarrays for the detection and characterization of antibodies against surface antigens. J Immunol Methods. 2006;309:48–54. doi: 10.1016/j.jim.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Tonkinson JL, Stillman BA. Nitrocellulose: a tried and true polymer finds utility as a post-genomic substrate. Front Biosci. 2002;7:c1–12. doi: 10.2741/tonkins. [DOI] [PubMed] [Google Scholar]
- Verrill C, Davies J, Millward-Sadler H, Sundstrom L, Sheron N. Organotypic liver culture in a fluid-air interface using slices of neonatal rat and adult human tissue–a model of fibrosis in vitro. J Pharmacol Toxicol Methods. 2002;48:103–110. doi: 10.1016/S1056-8719(03)00042-X. [DOI] [PubMed] [Google Scholar]
- Yamasato A, Satoh K. The establishment of conditions to efficiently screen photosynthesis-deficient mutants of Synechocystis sp. PCC 6803 by nitrofurantoin treatment. Plant Cell Physiol. 2001;42:414–418. doi: 10.1093/pcp/pce051. [DOI] [PubMed] [Google Scholar]