Abstract
Ionizing radiation is classified as a potent carcinogen, and its injury to living cells, in particular to DNA, is due to oxidative stress enhancing apoptotic cell death. Our present study aimed to characterize and semi-quantify the radiation-induced apoptosis in CNS and the activity of Mentha extracts as neuron-protective agent. Our results through flow cytometry exhibited the significant disturbance and arrest in cell cycle in % of M1: SubG1 phase, M2: G0/1 phase of diploid cycle, M3: S phase and M4: G2/M phase of cell cycle in brain tissue (p < 0.05). Significant increase in % of apoptosis and P53 protein expression as apoptotic biomarkers were coincided with significant decrease in Bcl2 as an anti-apoptotic marker. The biochemical analysis recorded a significant decrease in the levels of reduced glutathione, superoxide dismutase, deoxyribonucleic acid (DNA) and ribonucleic acid contents. Moreover, numerous histopathological alterations were detected in brain tissues of gamma irradiated mice such as signs of chromatolysis in pyramidal cells of cortex, nuclear vacuolation, numerous apoptotic cell, and neural degeneration. On the other hand, gamma irradiated mice pretreated with Mentha extract showed largely an improvement in all the above tested parameters through a homeostatic state for the content of brain apoptosis and stabilization of DNA cycle with a distinct improvement in cell cycle analysis and antioxidant defense system. Furthermore, the aforementioned effects of Mentha extracts through down-regulation of P53 expression and up-regulation of Bcl2 domain protected brain structure from extensive damage. Therefore, Mentha extract seems to have a significant role to ameliorate the neuronal injury induced by gamma irradiation.
Keywords: Gamma irradiation, Mentha piperita, DNA cycle, Apoptosis
Introduction
Humans exposed to ionizing radiations during diagnostic, therapeutic and industrial purposes are more susceptible to neural injury and synapses disturbances. Apart from this, humans also get exposed to ionizing radiations during air and space travel, background radiation, nuclear accidents, and use of electronic devices (UNSCEAR 2000). Ionizing radiation (gamma irradiation) induces various pathological changes such as demyelization, cell cycle disturbance and widespread oxidative damage with increased apoptotic rate. Ionizing radiation passage through living tissues, generates reactive free radicals such as hydroxyl radicals (·OH) and superoxides. These free radicals can interact with critical macromolecules, such as DNA, proteins or membranes inducing cell damage, cell dysfunction and death (Karbownik and Reiter 2000). Radiation induces mitotic cell death in dividing cells and activates pathways that lead to death by apoptosis in interphase cells and differentiated cells in form of reproductive/mitotic cell death (Haimovitz-Friedman et al. 1994; Haimovitz-Friedman 1998). Radiation-induced changes in the cellular oxidative status may play a role in proliferation, differentiation and the initiation of irreversible cell injury (Fuks et al. 1994; Przedborski et al. 1996). Several defense systems are involved in protection from oxidative stress, among which reduced glutathione (GSH) occupies a prominent position as the main water-soluble non-enzymatic antioxidant (Dringen et al. 2000). Radioprotective agents are those administered before exposure to ionizing radiation to reduce the damaging effects for normal cells, including radiation-induced lethality (Parihar et al. 2007). Many synthetic as well as natural agents have the efficacy as a protector against radiation injuries (Nair et al. 2001). Plant extracts such as Mentha (Samarth et al. 2002) have been found to have radioprotective effects in mammals. Mentha piperita (Linn.), commonly called peppermint (Family: Labiatae), is considered aromatic, stimulant, diuretic, anesthetic (local), antiseptic, digestive, tonic and carminative (Sokovic et al. 2009). The ancient Romans believed that the smell of Menthe stirred the mind (Yu et al. 2004). Mentha leaf extract (eugonol, caffeic acid, rosmarinic acid, and alpha tocopherol) has been shown to have antioxidant, antiperoxidant and antimutagenic properties (Samarth. 2007). Moreover, recent studies reported that Mentha leaf extract provided protection against radiation-induced cellular injury through delaying or preventing the onset of radiation-induced oxidative stress (Jajetia 2007; Samarth 2007).
Therefore, our study branched into two main objectives; to investigate the deleterious effects of gamma ionizing radiation on the mechanisms of apoptosis and cell cycle phases of the brain of Swiss albino mice and to evaluate the activity of Mentha leaves extract as a natural neuro-radioprotective agent.
Materials and methods
Experimental animals
Adult male Swiss albino mice (Mus musculus, 6–8 weeks old, weighing 22–25 g) were used in the present work. They were housed in cages with free access to drinking water and diet and maintained in the animal care facility of the Experimental Animal Care Center throughout the duration of the experiment. The animals were kept at 22–24 °C with the 12 h light/dark cycle. All animals received human care and our study complies with the guidelines of the institution. The local committee approved the design of the experiments, and the protocol conformed to the guidelines of the National Institutes of Health (NIH).
Whole body gamma-irradiation
Animals were placed in a specially designed well-ventilated acrylic container; the whole body of the animals was exposed to gamma irradiation for 10 min with a dose rate of 0.6 Gy/min to attain radiation dose level of 6 Gy (Abou-Seif et al. 2003; Samarth and Kumar 2003). The exposure was at a distance of 77.5 cm from the biological gamma cell-3500 irradiator, Cobalt-60 source (Atomic Energy Agency, Canada) belonging to Middle Eastern Regional Radioisotopes Center for the Arab Countries (Dokki, Giza, Egypt).
Mentha extracts (ME)
Fresh leaves of Mentha piperita Linn plants were collected, washed, air dried, powdered and extracted with double distilled water (DDW) by refluxing for 36 h (12 h × 3) at 80 °C as described by Samarth and Kumar (2003) and was given to each mouse by oral gavages at a dose of 1 g/kg b.wt./day (Samarth 2007).
Experimental design
Mice from inbred colony were divided into four groups (six mice each). Group-II and -IV received aquous Mentha leaf extract by oral gavages (1 g/kg b.wt./day) for seven consecutive days before irradiation. While control groups (group-I and -III) received double distilled water (ddH2O) equal to the dose of Mentha extract. On the 7th day, the mice of group-III and -IV were exposed to 6 Gy gamma irradiation as previously described in “Whole body gamma-irradiation”. All mice of the four groups were provided with their regular food and drinking water ad libitum throughout the time of the experiment.
Collection of samples
Animals were sacrificed on the 7th day after treatment or irradiation (12 h after overnight fasting). Brain tissues were quickly dissected, rinsed with isotonic saline, dried by blotting between two pieces of filter paper, and stored at −20 °C in plastic vials for further analysis. Frozen tissue samples were subjected for antioxidant GSH estimation, DNA content, and flow cytometric analysis to investigate cell cycle, DNA analysis, percentage of apoptotic cells, Bcl2 and P53 expression. Other samples of the brain tissue were stored in neutral formalin for histological studies.
Flow cytometric analysis
The flow cytometry analysis was performed in the Mansoura University Hospital using FACS (flow activated cell sorter) Calibur Flow Cytometer (Becton Dickinson, Sunnyvale, CA, USA) equipped with a compact air-cooled low power 15 mW Argon ion laser beam (488 nm). The average number of evaluated nuclei per specimen was 20.000 and the number of nuclei scanned was 120/s. DNA histogram derived from flow cytometry was obtained with a computer program for Dean and Jett mathematical analysis (Dean and Jett 1974). Data analysis was conducted using DNA analysis program MODFIT (Verity Software House, Inc., PO Box 247, Topsham, ME, 04086 USA, version: 2.0 powers Mac with 131,072 kb Registration No: 42000960827-16193213 Date made: 16-September, 1996). This software calculated the CV (coefficient of variation) around the G0/G1 peak and the percentage of cells in each phase (G0/G1, S and G2/M) of the DNA cell cycle for each sample. The analysis of apoptotic cell death was performed by measuring the DNA contents using FACS Calibur Flow Cytometer (Nicoletti et al. 2001). Flow cytometry P53 and Bcl2 protein expression was performed using fluorescence threshold using FACS Calibur Flow Cytometer (Becton Dickinson, Sunnyvale, CA, USA) as described by Brotherick et al. (1995).
Quantitative estimation of nucleic acids (DNA and RNA)
Nucleic acids of brain homogenate were extracted according to Melmed et al. (1976). Quantitative determination of DNA content was assayed in nucleic acid extract through the reaction of its sugar components with diphenylamine reagent, while RNA content was determined by a method depending on the reaction of its sugar component with orcinol reagent (Burton 1956).
Reduced GSH and SOD estimation
Reduced glutathione (GSH) content was assayed according to the method of Beutler et al. (1963). The method utilized metaphosphoric acid for protein precipitation and 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) for color development measured at 412 nm. Superoxide dismutase (SOD) was assayed according to Misra and Fridovich (1972). The assay procedure depends upon the inhibition of epinephrine auto-oxidation in an alkaline medium (pH 10.2) to adrenochrome, which is markedly inhibited by the presence of SOD. Epinephrine was added to the assay mixture, containing tissue supernatant and the change in extinction coefficient was measured at 480 nm.
Histopathological studies
Brain tissues were carefully fixed in neutral formalin solution (10 %), dehydrated in ascending grades of ethanol, cleared in xylene, embedded in a paraffin wax, sectioned at 5–7 μm and stained with hematoxylin and eosin. The stained sections were examined and photographed under a light microscope to detect histopathological changes (Drury et al. 1976).
Statistical analysis
All data were statistically analyzed by one way ANOVA (analysis of variance) test and post comparison was carried out with Waller–Duncan k ratio (Waller and Duncan 1969) using SPSS program (Statistical Package for Social Science) version 11. The results are presented as mean ± SE. The values of p ≤ 0.05 were considered statistically significant based on least significant difference (LSD) probability.
Results
Flow cytometry analysis
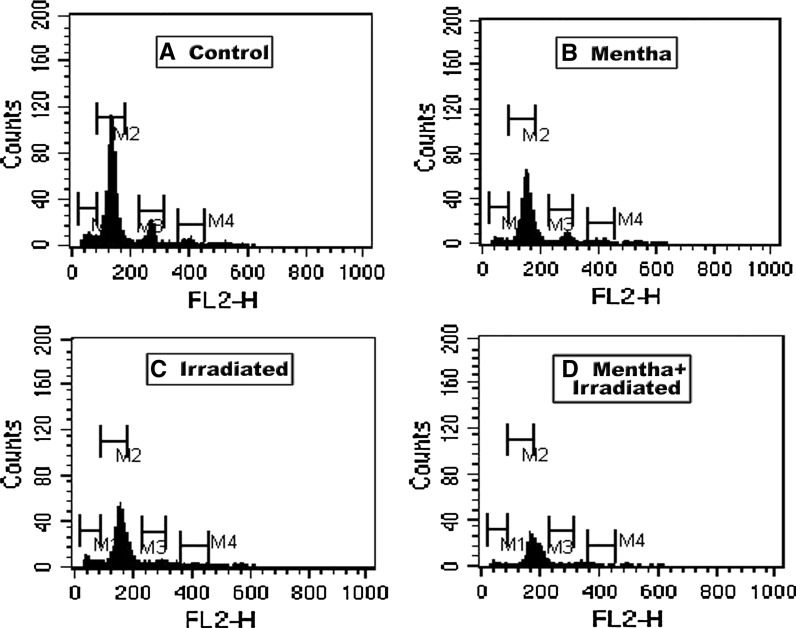
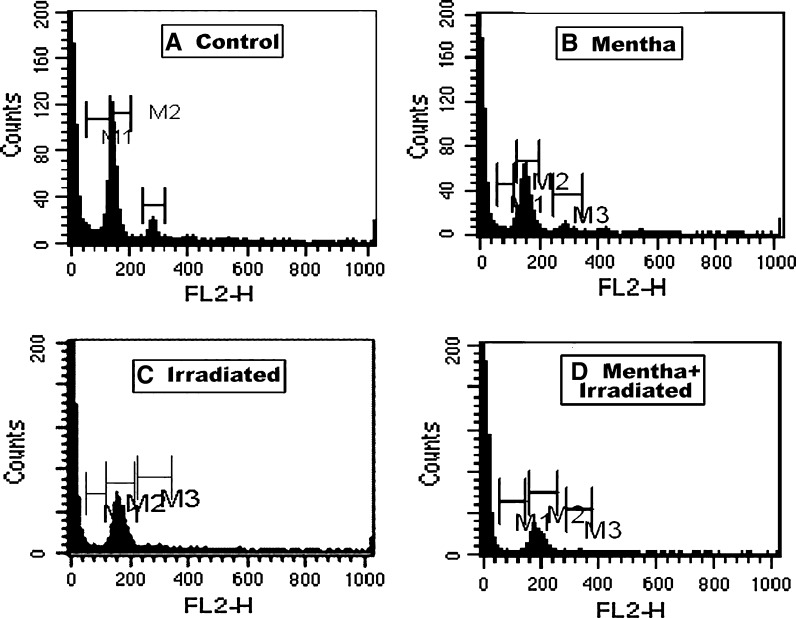
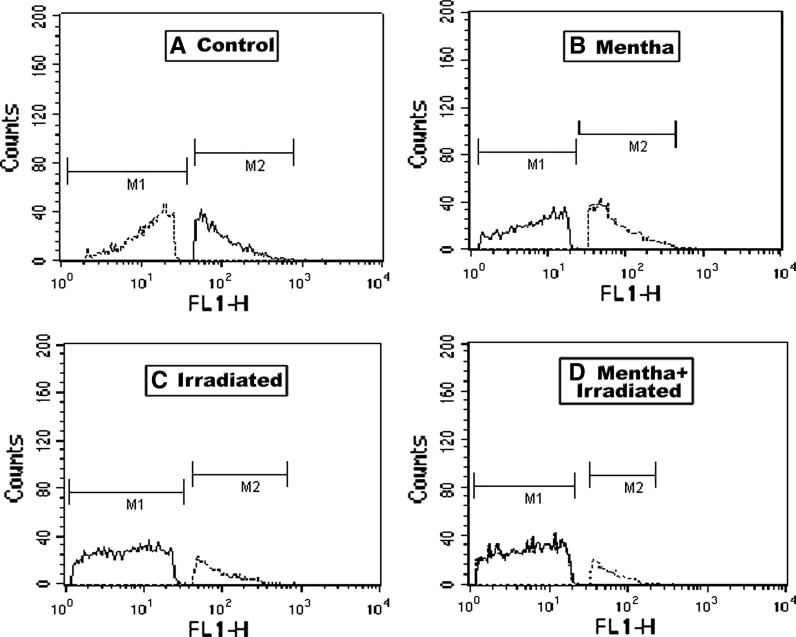
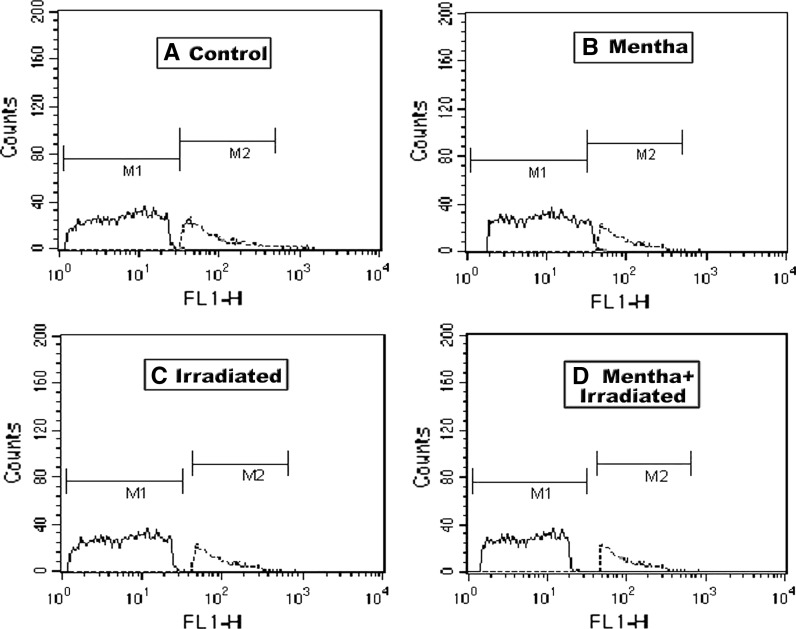
As shown in Tables 1, 2 and 3 as well as in Figs. 1, 2, 3 and 4, the oral administration of Mentha extract (1 g/kg b.wt.) alone for 7 days showed significant changes in the cell cycle by flow cytometry analysis (SubG1 apoptosis %, G0/1 phase of diploid cycle %, S phase %, and G2/M phase of diploid cycle %) but did not induce pronounced changes in the other estimated parameters for which the values were found near normal levels. Gamma-irradiation of normal mice group at 6 Gy with a single whole body dose exposure exhibited a significant increase in the G0/1 phase of the cell cycle and S phase (%) and a significant decline in G2/M phase of cell cycle (%) compared to control (Table 1; Fig. 1). The percentage of apoptotic cells and P53 protein expression (%) was highly increased, whereas the level of Bcl2, as an inhibitor gene for apoptosis, was decreased in the irradiated group compared to the control mice group (Table 2; Figs. 2, 3, 4). A marked decrease in brain DNA, RNA and GSH contents, however, and a significant increase in brain SOD activity were observed in the only gamma-irradiated group compared with that of the control mice group (Table 3). On the other side, an improvement in the above-mentioned parameters was observed in the Mentha treated irradiated mice group compared to the irradiated mice group without Mentha treatment.
Table 1.
Brain DNA cell cycle flow cytometry analysis in control and different treatment groups of mice
| Parameters | Animal groups | |||
|---|---|---|---|---|
| Control | Mentha | Irradiated | Mentha + irradiated | |
| G0/G1 (%) | 90.6 ± 0.08a | 93.3 ± 0.1b | 93.5 ± 0.04b | 91.2 ± 0.5a |
| G2/M (%) | 9.4 ± 0.07a | 2.8 ± 0.1b | 3.4 ± 0.2c | 1.6 ± 0.07d |
| Total S-phase (DNA content) | 0.0 ± 0.0a | 3.9 ± 0.2b | 3.1 ± 0.3c | 7.2 ± 0.4d |
| ANOVA | p < 0.05 | |||
Data are expressed as mean ± SE of 6 mice. Within each row, means with different superscript (a, b, c or d) were significantly different at p < 0.05. Where means superscripts with the same letters mean that there is no significant difference (p > 0.05). Values in italics represent significantly the highest values
Table 2.
Brain apoptotic cells and apoptotic regulators (P53 and Bcl2 protein expression) in control and different treatment groups of mice
| Parameters | Animal groups | |||
|---|---|---|---|---|
| Control | Mentha | Irradiated | Mentha + irradiated | |
| Apoptosis (%) | 4.2 ± 0.3a | 3.9 ± 0.3a | 23.3 ± 0.6b | 13.7 ± 0.4c |
| P53 (%) | 12.7 ± 0.1a | 11.98 ± 0.3a | 18.08 ± 0.2b | 13.95 ± 0.3c |
| Bcl2 (%) | 15.3 ± 0.2a | 14.6 ± 0.1a | 7.3 ± 0.2b | 10.5 ± 0.1c |
| ANOVA | p < 0.05 | |||
Data are expressed as mean ± SE of 6 mice. Within each row, means with different superscript (a, b, c or d) were significantly different at p < 0.05. Where means superscripts with the same letters mean that there is no significant difference (p > 0.05). Values in italics represent significantly the highest values
Table 3.
Brain DNA and RNA contents and antioxidant parameters level in control and different treatment groups of mice
| Parameters | Animal groups | |||
|---|---|---|---|---|
| Control | Mentha | Irradiated | Mentha + irradiated | |
| Brain DNA mg/g) | 22.3 ± 1.2a | 23.1 ± 1.3a | 15.4 ± 1.1b | 18.5 ± 1.2c |
| Brain RNA mg/g) | 4.6 ± 0.3a | 5.0 ± 0.09a | 2.3 ± 0.2b | 3.5 ± 0.1c |
| GSH (mg/g) | 5.6 ± 0.1a | 6.0 ± 0.1a | 3.3 ± 0.1b | 4.5 ± 0.2c |
| SOD (U/g) | 151.3 ± 1.5a | 152.3 ± 1.1a | 116.7 ± 1.9b | 139.2 ± 0.6c |
| ANOVA | p < 0.05 | |||
Data are expressed as mean ± SE of 6 mice. Within each row, means with different superscript (a, b, c or d) were significantly different at p < 0.05. Where means superscripts with the same letters mean that there is no significant difference (p > 0.05). Values in italics represent significantly the highest values
Fig. 1.
Flow cytometric analysis of DNA cell cycle of brain in control and different treatment groups of mice. M1 subG1 apoptsis %, M2 G0/1 phase of cell cycle %, M3 S phase %, M4 G2/M phase of cell cycle %. The percentages for the different phases are shown in the Tables 1 and 2
Fig. 2.
Flow cytometric analysis of apoptosis % of brain in control and different treatment groups of mice. M1 subG1 phase, M2 G0/1 phase of cell cycle, M3 S phase
Fig. 3.
Flow cytometric analysis of P53 protein expression of brain control and different treatment groups of mice. M1 subG1 phase, M2 G0/1 phase of cell cycle
Fig. 4.
Flow cytometric analysis of Bcl2 as an anti-apoptotic marker of brain in control and different treated mice groups. M1 subG1 phase, M2 G0/1 phase of cell cycle
Histopathological studies
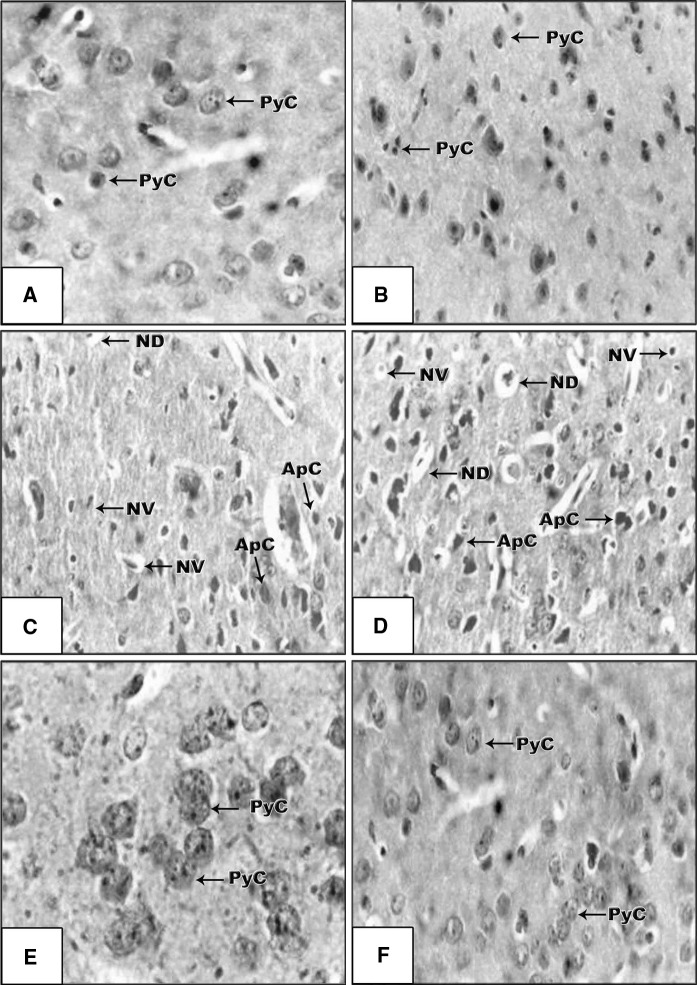
As shown in Fig. 5, no notable histopathological changes were observed in the cortex of Mentha extract treated mice which displayed normal structure of pyramidal cells (PyC) with normal chromatin (Fig. 5b) compared with control mice (Fig. 5a). Whereas, numerous histopathological alterations in brain tissues of mice exposed to gamma-irradiation are shown in Fig. 5c, d, characterized by chromatolysis in PyC of the cortex, nuclear vacuolation (NV), numerous apoptotic cells (ApC) and neural degeneration (ND). Moreover, the mice exposed to irradiation and pre-treated with Mentha extract showed reasonable protection with few apoptotic cells and displayed nearly normal structure with normal PyC as shown in Fig. 5e, f. These changes were in accordance with the observed flow cytometric data in the present study.
Fig. 5.
a A brain section of control mice with normal pyramidal cells (PyC-arrows) with clearly displayed nucleolus. Each cell exhibited large oval darkly stained nuclei and a thin peripheral cytoplasmic coat (H&E 9100). b A brain section of Mentha treated mice with normal neuronal structures as seen of the control (H&E ×100). c, d A brain section of gamma irradiated mice showing some histopathological changes of cerebral cortex (arrows) such as neural degeneration (ND), apoptotic cells (ApC) and nuclear vacuolation (NV) (H&E ×100). e, f A brain section of gamma irradiated mice previously treated with Mentha extract displaying nearly normal structure with normal pyramidal cells (PyC-arrows) and mild apoptotic cells (H&E ×100)
Discussion
Gamma-irradiation induces apoptosis in many cell types depending upon the regulation of the signaling pathways of the tumor suppressor gene P53 (Clarke et al. 1993; Komarova et al. 1997) controlling cell proliferation and induction of apoptosis (White et al. 1994; White 1995). γ-irradiation induces DNA damage and triggers P53 activity up-regulation (Allday et al. 1995; MacFarlane et al. 1996; Loft and Poulsen 1996). Gamma-irradiation causes a variety of lesions in DNA including single and double-strand breaks, DNA-protein cross-links, oxidized bases and basic sites (Morgan et al. 1996; Cadet et al. 1999; UNSCEAR 2000; Schmidt-Ullrich et al. 2000).
In the present study, mice exposed to gammair-radiation had remarkable alterations in cell cycle analysis (SubG1 apoptosis %, G0/1 phase of cell cycle, S phase % and G2/M phase of cell cycle) indicating apoptosis in brain tissue. These observations are in accordance with the study of Strasser-Wozak et al. (1998) who suggested that irradiation induces G2/M cell cycle arrest and apoptosis in P53-deficient lymphoblastic leukemia cells. Apoptosis is a gene-driven cell death process widely distributed under both normal and pathological conditions (Gobé et al. 1999). It is characterized by chromatin condensation and fragmentation, formation of apoptotic bodies with nuclear fragments and the display of markers of phagocytosis on the cell surface. Mitotic markers appear in neurons at risk for death in a variety of neurodegenerative conditions, in the mouse and in humans. Previous studies showed that cell cycle disturbances in a mature neuron may lead to cell death rather than cell division, and blocking cell-cycle initiation can prevent many types of neuronal cell death. Cell cycle is a highly regulated process with numerous checks and balances that ensures a homeostatic balance between cell proliferation and cell death in the presence of appropriate environmental signals. So, the arrest in cell cycle in our results was explained as killing of cells by radiation via apoptotic pathways and mitosis linked death in the G1 phase in which cells are more sensitive.
There are many expressed proteins involved in the activation and inhibition of apoptosis including the protein P53 and Bcl2 (Strasser et al. 2000; Leist and Jäättelä 2001). These results may be due to increased lipid peroxidation and DNA damage in the brain tissue. Oxidative stress might subsequently increase the frequency of transformation of cells to a malignant phenotype leading to apoptosis (Gorbunov et al. 2000). Ionizing radiation exerted its deleterious effect by interacting with water in cells and producing hydrogen peroxide and hydroxyl radicals abstracting hydrogen atom from sugar residues, causing base release and strand break of DNA or RNA producing impaired gene transcription (Cadet et al. 2004; Wang et al. 2010). Moreover, interaction of H2O2 with Fe2+ and Cu+ generates hydroxyl radicals (OH) which are highly damaging factors to mtDNA (Mattson 2004). Furthermore, the change in DNA may be triggered by the production of the hydroxyl radicals reacting with membrane lipids and induces their peroxidation by producing toxic 4-hydroxynonenal aldehyde forming adducts with bases of DNA damaging DNA in brain tissues and enhances aging and neurodegenerative disorders (Keller and Mattson 1998; Luczaj and Skrzydlewska 2003; Petersen and Doorn 2004). The present observations are also consistent with a large body of data demonstrating radiation effects on CNS cells. Peña et al. (2000) stated that 4 h after the exposure to radiation caused P53-independent cell death in a dose and time-dependent fashion. At low to intermediate levels of irradiation, apoptosis preceded by accumulation of cells in the G2/M phase of the cell cycle and arrest in the G1 (RNA and Protein synthesis)/S (DNA synthesis) phases. Apoptotic signaling transduction within the cell is mainly via two molecular pathways; the death receptor pathway (the extrinsic pathway) and the mitochondrial pathway (the intrinsic pathway). Both of them activate a variety of proteases, mainly caspases (cysteinyl aspartate-specific proteases), and endonucleases, which finally degrade cellular components (Guicciardi and Gores 2005). The Bcl2 family is the best characterized protein family involved in the regulation of apoptotic cell death. It includes both anti-apoptotic members (Bcl2) and pro-apoptotic members (Bax), thus the Bcl2 family acts as critical life-death decision point makers in the common pathway of apoptosis (Zhao et al. 2001; Schattenberg et al. 2006).
Additionally, through the present monitoring levels of the key apoptotic regulators (Bcl2 and P53), our observations showed a significant decrease of Bcl2 expression joined by a significant increase in P53 expression in brain of mice exposed to gamma-irradiation. These data give a clear evidence for disrupted apoptotic signals in brain tissue of mice exposed to gamma-irradiation in favor of anti-apoptotic Bcl2 over the proapototic P53. These findings may be attributed to three mechanisms by which Bcl2 may mediate endothelial cell cytoprotection independently of cytochrome c release: (1) increased survivin expression, (2) inhibition of P53 accumulation, and (3) inhibition of p38; RAF-mitogen-activated protein kinase (MAPK) (Kumar et al. 2007).
Moreover, we have reported the association among radiation-induced neurotoxicity and decreased endogenous antioxidant system GSH and SOD and their reduced ability in scavenging high levels of ROS produced in the brain. The significant changes in total SOD (MnSOD and CuZnSOD) can be explained by the deficiency of serum levels of Zn, Mn and Cu (being essential for regulating cellular redox state) which may be related to the reduction of SOD activity in brains of irradiated mice exerting mitochondrial impairments and dysfunction (Radjicic et al. 1999).
Furthermore, the recorded related impairments of radiation, in the current study, are more pronounced in histological examination. There were a variety of histological abnormalities in the brain of mice exposed to gamma radiation such as signs of chromatolysis in PyC of cortex. In addition to NV, numerous ApC and ND were detected. This finding of brain damage is due to the free radicals generation associated with radiation-induced oxidative stress. This is explained by another study of the authors through the increased levels of TBARS, NO and H2O2 in the brain of gamma-irradiated mice, indicating high level of oxidative stress and free radicals generation (Hassan et al. 2012). Moreover, various studies reported that the exposure to radiation induced oxidative damage in several organs (Bhatia and Manda 2004; Hassan et al. 2010, Wang et al. 2010) and mitochondrial membranes (Kamat et al. 2000). Moreover, the oxidative stress linked to dopaminergic neurons damage might be owned to lipid peroxidation products 4-hydroxynonenal (4HNE) and malondialdehyde (MDA) which inhibit the aldehyde biotransformation step of DA catabolism to 3,4-dihydroxyphenylacetic acid (DOPAC) yielding elevated levels of the endogenous neurotoxin 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is more toxic to dopaminergic neurons than DA quinones (Jinsmaa et al. 2009).
Antioxidant supplementation during radiation therapy poses a conundrum for the radiation oncologist, as antioxidants protect normal cells from reactive oxygen species. A wide range of antioxidant phytochemicals, including flavonoids, polyphenols, carotenoids, and organosulfur compounds, are antioxidants with high radioprotective ability in experimental systems (Weiss and Landauer 2003).
In the present study, the pretreatment of mice with Mentha leaf extract have been assigned as neuro-radio-protective mediators from radiation induced brain injury through management of the cell cycle arrest and apoptosis. These findings are evidenced by the observed improvement of most of the examined biomarkers, including endogenous antioxidants system, DNA and RNA contents, DNA cell cycle, the apoptotic regulators (Bcl2 and P53), and histopathological changes. Mentha has been shown to have antioxidant properties because of the presence of eugenol, caffeic acid, rosmarinic acid, and α-tocopherol (Krishnaswamy and Raghuramulu 1998; Al-Sereiti et al. 1999). Furthermore, it could enhance error-free repair of damage and hence, could be antimutagenic (Vokovic-Gacic and Simic 1993). This is also supported by the finding of Adamama-Moraitou et al. (2004) who suggested that the reduction in lipid peroxidation and up-regulation of the endogenous antioxidant enzymes, such as catalase, GST and SOD, resulted in the expression of genes involved in DNA repair. The current radio-protective Mentha extract may be assigned to its different functional constituent like antioxidants flavonoids, caffeic acid, eugenol, polyphenols and α-tocopherol (Kanatt et al. 2007; Sokovic et al. 2009). Polyphenols regulate mRNA levels of endogenous antioxidant enzymes, such as catalase, GST, SOD, and DNA repair system genes and thus counteract oxidative stress and DNA damage complications induced by ionizing radiation (Samarth et al. 2006, Hassan et al. 2012). Additionally, the fundamental role of endogenous antioxidants appeared in form of glutathione (GSH) with a significant increase after Mentha administration. Its increased activity in the removal of deleterious reactive oxygen species and maintenance of the protein thiol redox state, glutathione reductase (GR) is crucial for the cell’s antioxidant defense mechanism and maintenance of enzyme activities and protein functions (Chaudiere and Ferrari-Iliou 1999). The glutathione-dependent antioxidant system protects cells from oxidative stress-induced dysfunction and cell injury through 3 major pathways. GSH serves as an electron donor for glutathione peroxidase, GSH also contributes to the detoxification and elimination of toxic electrophilic metabolites and xenobiotics (Commandeur et al. 1995). GSH selectively recycles oxidized glutaredoxin to the reduced enzyme, generating GSSG, which in turn is reduced to GSH by GR (Srinivasan et al. 1997). Furthermore, the beneficial effect of pretreatment with Mentha on radiation induced tissue injury, cell cycle arrest, and apoptosis may be related to the effectiveness of Mentha against chromosomal damage and/or may be through improvement of DNA content by the antimutagenic properties of Mentha piperita (Samman et al. 1998).
Conclusion
In conclusion, Mentha extract offered sufficient protection against gamma-irradiation-induced brain injury and apoptosis in mice, probably by exerting a protective effect against free radical scavenging, antioxidant activity and membrane stabilizing by mechanisms that include up-regulation of the key apoptotic regulators (Bcl2 and P53). Mentha fractionation guided evaluation may result in the development of ideal radioprotections, which modulates the cytotoxic effects of radiation and better radiation therapy in the near future.
Acknowledgments
The authors of this manuscript express their deepest gratitude and appreciation to Dr. Ahmed El-Mansy, Zoology Department, Faculty of Science, Mansoura University, Mansoura, Egypt for his financial assistance in histopathological examination in the present work.
Abbreviations
- DNA
Deoxyribonucleic acid
- RNA
Ribonucleic acid
- GSH
Reduced glutathione (GSH)
- SOD
Superoxide dismutase
- GST
Glutathione-S-transferase (GST)
- DNA
Deoxyribonucleic acid
- Gy
Gray
- M1
SubG1 apoptosis %
- M2
G0/1 phase of diploid cycle %
- M3
S phase %
- M4
G2/M phase of diploid cycle %
- PyC
Pyramidal cells of cortex
- NV
Nuclear vacuolation
- ApC
Numerous apoptotic cell
- ND
Neural degeneration
- H
Means one marker for each phase formed by the soft ware program
- FACS
Flow activated cell sorter
- CV
Variation coefficient
- FL2-H
Fluorescent-2-height means the fluorescent-2-height for measuring the fluorescence of the used red stain (propidium iodide) at wave length 588 nm
References
- Abou-Seif MAM, El-Naggar MM, El-Far M, Ramadan M, Salah BN. Amelioration of radiation-induced oxidative stress and biochemical alteration by SOD model compounds in pre-treated g-irradiated rats. Clin Chim Acta. 2003;337:23–33. doi: 10.1016/S0009-8981(03)00192-X. [DOI] [PubMed] [Google Scholar]
- Adamama-Moraitou KK, Rallis TS, Papazoglou LG, Papasteriadis A, Roubies N, Kaldrimidou H, Leontides LS. Liver biochemical and histopathological findings in dogs with experimentally induced exocrine pancreatic insufficiency. Can J Vet Res. 2004;68:56–61. [PMC free article] [PubMed] [Google Scholar]
- Allday MJ, Inman GJ, Crawford DH, Farrell PJ. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 1995;14:4994–5005. doi: 10.1002/j.1460-2075.1995.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn. and its therapeutic potential. Ind J Exp Biol. 1999;37:124–130. [PubMed] [Google Scholar]
- Beutler E, Duron O, Kelly BM. An improved method for the detection of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bhatia AL, Manda K. Study on pre-treatment of melatonin against radiation-induced oxidative stress in mice. Environ Toxicol Pharmacol. 2004;18:13–20. doi: 10.1016/j.etap.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Brotherick I, Shenton BK, Cowan WK, Angus B, Horne CHW, Higgs MJ, Lennard TWJ. p53 expression measured by flow cytometry. A comparison of three monoclonal antibodies and the relationship with grade and DNA ploidy in breast cancer. Cancer Immunol Immunother. 1995;41:146–150. doi: 10.1007/BF01521339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–319. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9–21. doi: 10.1016/S0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Cadet J, Bellon S, Douki T, Frelon S, Gasparutto D, Muller E, Pouget JP, Ravanat JL. Radiation-induced DNA damage: formation, measurement, and biochemical features. J Environ Pathol Toxicol Oncol. 2004;23:33–43. doi: 10.1615/JEnvPathToxOncol.v23.i1.30. [DOI] [PubMed] [Google Scholar]
- Chaudiere J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–962. doi: 10.1016/S0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Commandeur JN, Stijntjes GJ, Vermeulen NP. Enzymes and transport systems involved in the formation and disposition of glutathione S-conjugates. Role in bioactivation and detoxication mechanisms of xenobiotics. Pharmacol Rev. 1995;47:271–330. [PubMed] [Google Scholar]
- Dean PN, Jett JH. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974;60:523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Drury RA, Wallington EA, Cancerson R. Carlton’s histopathological techniques. 4. Oxford: Oxford University Press; 1976. [Google Scholar]
- Fuks Z, Persaud R, Alfieri A, McLoughlin M, Schwartz JL, Cordon-Cardo C, Seddon AP, Haimovitz-Friedman A. Basic fibroblast factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- Gobé GC, Harmon B, Leighton J, Allan J. Radiation-induced apoptosis and gene expression in neonatal kidney and testis with and without protein synthesis inhibition. Int J Radiat Biol. 1999;75:973–983. doi: 10.1080/095530099139737. [DOI] [PubMed] [Google Scholar]
- Gorbunov NV, Pogue-Geile KL, Epperly MW, Bigbee WL, Draviam R, Day BW, Wald N, Watkins SC, Greenberger JS. Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Radiat Res. 2000;154:73–86. doi: 10.1667/0033-7587(2000)154[0073:AOTNOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovitz-Friedman A. Radiation-induced signal transduction and stress response. Radiat Res. 1998;150:S102–S108. doi: 10.2307/3579812. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan C-C, Ehleiter D, Persaud R, McLaughlin M, Fuks Z. Ionizing radiation acts directly on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HA, Edrees GM, Salama OS, El-sayede EA. Radioprotective effect of Mentha piperita (Linn.) extract against gamma irradiation induced-haematopoietic disorders in swiss albino mice. J Egypt Soc Toxicol. 2010;43:53–62. [Google Scholar]
- Hassan HA, Hafez HS, Isa AM. Neuroprotective effect of Mentha extract against oxidative stress and biochemical alterations in pre-treated gamma irradiated Swiss albino mice. J Med Plant Res. 2012;6:2995–3002. [Google Scholar]
- Jajetia GC. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr. 2007;40:74–81. doi: 10.3164/jcbn.40.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol. 2009;22:835–841. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat JP, Boloor KK, Devasagayam TPA, Venkatachalam SR. Antioxidant properties of Asparagus racemosus against damage induced by gamma-radiation in rat liver mitochondria. J Ethnopharmacol. 2000;71:425–435. doi: 10.1016/S0378-8741(00)00176-8. [DOI] [PubMed] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007;100:451–458. doi: 10.1016/j.foodchem.2005.09.066. [DOI] [Google Scholar]
- Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1046/j.1525-1373.2000.22502.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mattson MP. Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction, and death in the nervous system. Rev Neurosci. 1998;9:105–116. doi: 10.1515/revneuro.1998.9.2.105. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Chernov MV, Franks R, Wang KH, Armin G, Zelnick CR, Chin DM, Bacus SS, Stark GR, Gudkov AV. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during development and determines radiation and drug sensitivity in vivo. EMBO J. 1997;16:1391–1400. doi: 10.1093/emboj/16.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy K, Raghuramulu N. Bioactive phytochemicals with emphasis on dietary practices. Ind J Med Res. 1998;108:167–181. [PubMed] [Google Scholar]
- Kumar P, Coltas IK, Kumar B, Chepeha DB, Bradford CR, Polverini PJ. Bcl-2 protects endothelial cells against gamma-radiation via a Raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res. 2007;67:1193–1202. doi: 10.1158/0008-5472.CAN-06-2265. [DOI] [PubMed] [Google Scholar]
- Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- Luczaj W, Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol Biol Lett. 2003;8:391–413. [PubMed] [Google Scholar]
- MacFarlane M, Jones NA, Dive C, Cohen GM. DNA-damaging agents induce both p53-dependent and p53-independent apoptosis in immature thymocytes. Mol Pharmacol. 1996;50:900–911. [PubMed] [Google Scholar]
- Mattson MP. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann NY Acad Sci. 2004;1012:37–50. doi: 10.1196/annals.1306.004. [DOI] [PubMed] [Google Scholar]
- Melmed RN, El-Aser AA, Holt SJ. Hypertrophy and hyperplasia of the neonatal rat. Exocrine pancreas induced by orally administered soybean trypsin inhibitor. Biochem Biophys Acta. 1976;421:280–288. doi: 10.1016/0304-4165(76)90294-4. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Morgan WF, Day JP, Kaplan MI, McGhee EM, Limoli CL. Genomic instability induced by ionizing radiation. Radiat Res. 1996;146:247–258. doi: 10.2307/3579454. [DOI] [PubMed] [Google Scholar]
- Nair CKK, Parida DK, Nomura T. Radio-protectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- Nicoletti I, Mannucci R, Migloirati G, Riccardi C, Grignani F. Common method for measuring apoptotic cell death by flow cytometry. Purdue Cytom CD-ROM Ser. 2001;3:1–9. [Google Scholar]
- Parihar VK, Dhawan J, Kumar S, Manjula SN, Subramanian G, Unnikrishnan MK, Rao CM. Free radical and radioprotective activity of dehydrozingerone against whole body gamma irradiation in Swiss albino mice. Chem Biol Interact. 2007;170:49–58. doi: 10.1016/j.cbi.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Peña LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TD. Role of neuronal nitric oxide in l-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjicic R, Cvijic G, Djokic I, Davidovic V. Effect of propranolol and cold exposure on the activities of antioxidant enzymes in the brain of rats adapted to different ambient temperatures. J Therm Biol. 1999;24:433–437. doi: 10.1016/S0306-4565(99)00076-5. [DOI] [Google Scholar]
- Samarth RM. Protection against radiation induced hematopoietic damage in bone marrow of Swiss albino mice by Mentha piperita (Linn) J Radiat Res. 2007;48:523–528. doi: 10.1269/jrr.07052. [DOI] [PubMed] [Google Scholar]
- Samarth RM, Kumar A. Radioprotection of Swiss albino mice by plant extract Mentha piperita (Linn.) J Radiat Res. 2003;44:101–109. doi: 10.1269/jrr.44.101. [DOI] [PubMed] [Google Scholar]
- Samarth RM, Goyal PK, Kumar A. Modulation of serum phosphatases activity in Swiss albino mice against gamma irradiation by Mentha piperita (Linn.) Phytothér Res. 2002;16:586–589. doi: 10.1002/ptr.984. [DOI] [PubMed] [Google Scholar]
- Samarth RM, Panwar M, Kumar M, Kumar A. Protective effects of Mentha piperita Linn on benzo[a]pyrene-induced lung carcinogenicity and mutagenicity in Swiss albino mice. Mutagenesis. 2006;21:61–66. doi: 10.1093/mutage/gei075. [DOI] [PubMed] [Google Scholar]
- Samman MA, Bowen ID, Taiba K, Antonius J, Hannan MA. Mint prevents shamma-induced carcinogenesis in hamster cheek pouch. Carcinogenesis. 1998;19:1795–1801. doi: 10.1093/carcin/19.10.1795. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liv Int. 2006;26:904–911. doi: 10.1111/j.1478-3231.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153:245–257. doi: 10.1667/0033-7587(2000)153[0245:STACRR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, Leo JL, Griensven DV. Chemical composition of essential oils of thymus and Mentha species and their anti-fungal activities. Molecules. 2009;14:238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan U, Mieyal PA, Mieyal JJ. pH profiles indicative of rate-limiting nucleophilic displacement in thioltransferase catalysis. Biochemistry. 1997;36:3199–3206. doi: 10.1021/bi962017t. [DOI] [PubMed] [Google Scholar]
- Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Strasser-Wozak EMC, Hartmann BL, Geley S, Sgonc R, Böck G, Dos Santos OAJ, Hattmannstorfer R, Wolf H, Pavelka M, Kofler R. Irradiation induces G2/M cell cycle arrest and apoptosis in p53-deficient lymphoblastic leukemia cells without affecting Bcl-2 and Bax expression. Cell Death Differ. 1998;5:687–693. doi: 10.1038/sj.cdd.4400402. [DOI] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) (2000) Sources and effects of ionizing radiation. Report to the General Assembly, with scientific annexes. United Nations Sales Publication E.00.IX.3, United Nations, New York
- Vokovic-Gacic B, Simic D. Identification of natural antimutagens with modulating effects on DNA repair. Basic Life Sci. 1993;61:269–277. doi: 10.1007/978-1-4615-2984-2_25. [DOI] [PubMed] [Google Scholar]
- Waller RA, Duncan DB. A Bayes rule for the symmetric multiple comparison problems. J Am Stat Assoc. 1969;64:1484–1503. [Google Scholar]
- Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/S0300-483X(03)00149-5. [DOI] [PubMed] [Google Scholar]
- White E. Regulation of p53-dependent apoptosis by E1A and E1B. Curr Top Microbiol Immunol. 1995;199:33–58. doi: 10.1007/978-3-642-79586-2_3. [DOI] [PubMed] [Google Scholar]
- White E, Chiou SK, Rao L, Sabbatini P, Lin H-J. Control of p53-dependent apoptosis by E1B, Bcl-2, and Ha-ras proteins. Cold Spring Harbor Symp Quant Biol. 1994;59:395–402. doi: 10.1101/SQB.1994.059.01.044. [DOI] [PubMed] [Google Scholar]
- Yu TW, Xu M, Dashwood RH. Antimutagenic activity of spearmint. Environ Mol Mutagen. 2004;44:387–393. doi: 10.1002/em.20063. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]