Abstract
l-alanyl-l-glutamine (AlaGln) is dipeptide that has better solubility and stability than Glutamine (Gln). In this study, we evaluated the utility of this dipeptide during culture of POTELLIGENT™ Chinese hamster ovary (CHO) cells expressing anti-CD20 chimeric antibody. Although AlaGln in the culture medium lowered the specific growth rate, the MAb titer was maximized when Gln was completely replaced by AlaGln in both the basal and feed media. Moreover, AlaGln augmented production of antibody not only at flask scale but also at spinner scale, although the extent of this effect was dependent on the cell clone. To explore the mechanism responsible for the effect of AlaGln on cell growth, we measured apoptosis in the early phase of cell culture on days 8, 9, and 10. The apoptotic ratio was reduced in medium containing AlaGln. Ammonia was generated in medium containing Gln when it was maintained at 37 °C, which impeded the growth and productivity of the cells. In contrast, AlaGln produced less ammonia under these conditions, which may have been one of the properties associated with its beneficial effects. We conclude that certain dipeptides can serve as superior alternative sources of amino acids in cell culture and antibody production.
Keywords: Cell culture, CHO, Defucosylated antibody, Dipeptide, l-alanyl-l-glutamine, Medium
Introduction
Because of their efficacy and good side-effect profiles, monoclonal antibodies (MAbs) represent a new generation of therapeutic agents (Hale 2006). Numerous therapeutic antibodies have been licensed so far and their use is now well established clinically (Walsh 2006). Generally speaking, most antibodies are produced by culturing mammalian cells to make best use of well-developed gene expression systems and higher glycosylation capabilities. However, these methods can be expensive, and to reduce the overall cost of therapeutic MAbs, several approaches have been tried to upgrade the efficacy per unit dose. For example, antibodies lacking fucose can be produced in α-1,6-fucosyltransferase (FUT8) knockout Chinese hamster ovary (CHO) cell lines (POTELLIGENT™ technology) and have been reported to show higher antibody-dependent cellular cytotoxicity (ADCC) (Mori et al. 2007; Shields et al. 2002; Shinkawa et al. 2003). In addition, use of larger scale production facilities can reduce the cost per unit weight, and tanks are now available that contain more than 10,000 L of culture medium (Birch and Racher 2006; Brian 2009). Finally, optimization of the composition of the culture medium to enhance production titers has been an area of active research. Among the approaches that have been investigated, attention has been focused on modifying the ratios of each component or adding extra material as a supplement. The statistical design method, such as “design of experiments” (DOE), can be used to determine effective concentrations of each ingredient and better combinations among them with a minimized set of experiments (Parampalli et al. 2007), and this approach has been widely used to develop commercial media. On the other hand, novel components can be found by screening libraries of natural extracts or rare materials. Usually, these tend to be a mixture of several components or materials that was synthesized de novo. Examples of such supplements that have been adopted for use in culture media include sericin (Terada et al. 2005) and coenzyme Q10 (Konno et al. 2011; Sun et al. 1995).
Glutamine (Gln) is a key component of culture medium because it is an essential amino acid, especially for those mammalian cells that are deficient in the gene for glutamine (Gln) synthesis. However, Gln degrades in culture medium and produces the toxic product ammonium. We have focused our attention on exploring ways to improve the stability of Gln and prevent the production of ammonium. Because certain dipeptides are more soluble and stable in aqueous solution than their constituent amino acids, we evaluated the ability of l-alanyl-l-glutamine (AlaGln), a dipeptide composed of alanine and Gln, to replace Gln. Formerly, such dipeptides were manufactured by chemical synthesis and were too expensive to add culture medium. However, novel fermentation methods have been discovered recently (Tabata et al. 2005, 2007) that can economically produce some dipeptides, enabling their use as an additive to culture medium. This technology can be used to produce AlaGln and other novel dipeptides.
We hypothesized that given the ability to economically produce these dipeptides, they might overcome the problems of stability and solubility and become advantageous supplements in culture medium for antibody production. Here, we evaluated the feasibility and practicality of the dipeptide used as culture supplements during growth of CHO cells expressing anti-CD20 chimeric antibody under conditions that mimic large-scale manufacture, and determined the impact of substituting AlaGln for Gln on cell growth, apoptosis, and antibody production.
Materials and methods
Cells lines
Two CHO cell lines expressing anti-CD20 chimeric antibody were investigated in this study. Both CHO cell lines are derived from Ms704 which is FUT8 knock out CHO/DG44 cells. Construction method of 1A7-15 clone was described elsewhere (Kanda et al. 2006). 12C-5 clone was another cell line screened from same host cell pool as 1A7-15 clone.
Materials
Erlenmeyer flasks (250 mL) were purchased from Corning (Corning, Corning, NY). Culture medium powder, EXCELL 302, and Iscove’s modified Dulbecco’s medium (IMDM) were obtained from SAFC (SAFC Biosciences, St. Louis, MO). The box shaker BR-40LF was from TAITEC (Taitec, Saitama, Japan).
Serum-free fed-batch culture without control of pH and dissolved oxygen (DO)
Fed-batch cultures in 250-mL Erlenmeyer flasks (Corning) were carried out at 35.0 °C in 5 % CO2 (v/v) with 100-rpm agitation for 14 days; pH and DO were not controlled during the culture period. CHO cells were inoculated at 3.0 × 105 cells/mL in 40 mL EXCELL 302 (SAFC) supplemented with either 6 mmol/L Gln (Wako Pure Chemical Industries, Ltd., Osaka, Japan) or 6 mmol/L AlaGln (Kyowa Hakko Bio Co., Ltd., Tokyo, Japan). 2.5 mL IMDM-based medium was fed on days 4, 6, 9, and 11. Either 50 mmol/L Gln or 50 mmol/L AlaGln was added to the medium before use. Culture aliquots were taken on days 4, 6, 9, 11, 13, and 14. Viable cell density was measured with a Cedex analyzer (Innovatis AG, Bielefeld, Germany) by trypan blue exclusion. As a model recombinant antibody product, we used anti-human CD20 chimeric IgG1. To evaluate the potential of AlaGln, basal culture medium containing either AlaGln or Gln and feed medium containing either AlaGln or Gln were prepared. The cell culture conditions were designed to combine these different basal and feed media as follows: 1) Gln in the basal medium and Gln in the feed medium (condition Gln–Gln), 2) Gln in the basal medium and AlaGln in the feed medium (condition Gln-AlaGln), and 3) AlaGln in the basal medium and AlaGln in the feed medium (condition AlaGln–AlaGln).
Serum-free fed-batch culture with control of pH and DO
A 1-L scale bioreactor (ABLE, Tokyo, Japan) was employed for serum-free fed-batch culture under the culture conditions of 35.0 °C, pH 7.1, and 50 % DO. Cell culture media were the same as those used for the Erlenmeyer flask cultures. Each clone was inoculated at 3.0 × 105 cells/mL in 800 mL culture medium to commence the cell culture. 40 mL feed medium was supplied on days 3, 5, 7, 9, and 11, while glucose (Wako) was maintained constant at approximately 4.0 g/L throughout the culture. Culture samples were withdrawn daily and analyzed.
Analysis of early apoptosis
The ratio of apoptotic cells to living cells was measured with a Guava EasyCyte (Millipore, Billerica, MA, USA). Cultured cells were dyed with the Nexin kit (Millipore), and the difference between viable cells and cells undergoing early apoptosis was visualized. The cells (10 × 105) taken from the 1 L-scale bioreactor on days 8, 9, and 10 were suspended in 100 μL of phosphate buffed saline (PBS) (Invitrogen, Grand Island, NY, USA). The resulting suspension was then centrifuged for 5 min at 450 × g and the supernatant was discarded. Packed cells were suspended again in 150 μL of dye reagent from the kit and incubated for 20 min at room temperature. The resulting sample was measured with the Guava EasyCyte to determine the apoptotic ratio.
HPLC analyses
Amino acids and dipeptides were derivatized with 9-fluorenylmethoxycarbonyl-Cl (FMOC-Cl) and measured by HPLC according to the following procedure: a sample of cell culture supernatant (30 μL) was mixed with 270 μL of 0.1 M sodium borate buffer (pH 9.0) and 300 μL of a solution of 1.5 mg/mL FMOC-Cl in acetone was added. After the mixture was incubated for 40 min at room temperature, 600 μL of 25 % (vol/vol) CH3CN in 0.25 M borate buffer (pH 5.5) was added. A sample (50 μL) of the solution was injected into the HPLC system (LC-10 system; Shimadzu, Kyoto, Japan) equipped with a Develosil ODS-HG-5 column (Nomura Kagaku, Tokyo, Japan). The separation was performed with a gradient of solvent A (20 mM (NH4)H2PO4 (pH 6.5):CH3OH (85:15)) to solvent B (CH3CN:H2O (9:1)). The flow rate was maintained at 1.0 mL/min and the column temperature was kept at 35 °C. Fluorescence was monitored at 254 and 630 nm for excitation and emission, respectively.
Results
Serum-free fed-batch culture in Erlenmeyer flasks
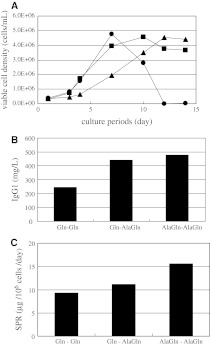
Cell cultures were carried out without control of pH and DO, and the resulting cell growth is shown in Fig. 1a. Cell culture profiles were significantly different depending on the source of the supplement and the combination. Cells cultured with a combination of Gln–Gln grew the most rapidly, but the viability declined and the yield of cells was less than or equal to 47.68 × 105 cells/mL. Cells cultured in Gln-AlaGln did not grow as rapidly as those in Gln–Gln, but the number of viable cells maintained nearly a maximum value of 45.83 × 105 cells/mL. Under the AlaGln–AlaGln condition, the cells grew most slowly and showed the lowest viable cell density among the three conditions.
Fig. 1.
Effect of AlaGln on 1A7-15 cell line in Erlenmeyer flask culture. a Comparison of cell growth. Combination of the supplements in basal and feed media was (1) Gln–Gln (black circle), (2) Gln–AlaGln (black square), (3) AlaGln–AlaGln (black triangle). b Comparison of MAb titer at the end of cell culture. c Comparison of SPR. Three other independent experiments with similar conditions exhibited similar results
Monoclonal antibody concentrations at the end of cell culture were 246 mg/L for Gln–Gln, 441 mg/L for Gln-AlaGln, and 478 mg/L for AlaGln–AlaGln (Fig. 1b). The productivities per cell (i.e. specific production rate: SPR) were calculated on the basis of these data (Fig. 1c), and the highest productivity was obtained under the AlaGln–AlaGln condition.
Serum-free fed-batch culture in a bioreactor
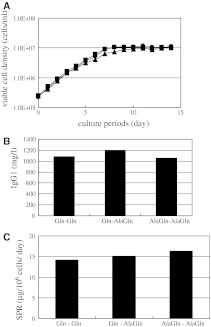
To examine the feasibility of supplementing with AlaGln in large-scale culture, we performed fed-batch culture of two clones in a 1-L bioreactor as a scaled-down model. The test conditions were the same as those for the Erlenmeyer flask cultures. The first clone, 1A7-15, which had been used for the Erlenmeyer flask cultures, showed similar cell culture profiles independent of the glutamine source in the basal and/or feed medium (Fig. 2a). When cultured under the AlaGln–AlaGln condition, the cells grew slowlier and reached a lower maximum cell density, as was the case in the Erlenmeyer flask cultures. In addition, AlaGln attenuated the decline in viability. AlaGln in the feed medium provided 100 mg/L higher titer (Fig. 2b). However, the MAb titer obtained with AlaGln in the basal medium was almost the same as that obtained with Gln–Gln. Among the three conditions, AlaGln–AlaGln showed the highest SPR value followed by Gln-AlaGln, then Gln–Gln (Fig. 2c).
Fig. 2.
Effect of AlaGln on 1A7-15 cell line in 1 L bioreactor culture. a Comparison of cell growth. Combination of the supplements in basal and feed media was (1) starting culture with Gln and feeding medium with Gln (black circle), (2) starting culture with Gln and feeding medium with AlaGln (black square) and (3) starting culture with AlaGln and feeding medium with AlaGln (black triangle). b Comparison of MAb titer at the end of cell culture. c Comparison of SPR. Three other independent experiments with similar conditions exhibited similar results
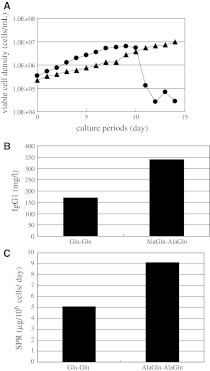
The other cell line, 12C-5, was also investigated under two conditions in which either Gln or AlaGln was used for both the basal and feed media (Fig. 3a). The culture prolife was significantly different between the two conditions. The density of viable cells was significantly reduced under the Gln–Gln condition but not under the AlaGln–AlaGln condition. The cells produced 171 mg/L MAb in absence of AlaGln but 341 mg/L, or twice the titer, in the presence of AlaGln (Fig. 3b). The productivity (SPR) under the AlaGln–AlaGln condition was also almost double that under Gln–Gln (Fig. 3c).
Fig. 3.
Effect of AlaGln on 12C-5 cell line in 1 L bioreactor culture. a Comparison of cell growth. Combination of the supplements in basal and feed media was (1) starting culture with Gln and feeding medium with Gln (black circle) and (2) starting culture with AlaGln and feeding medium with AlaGln (black triangle). b Comparison of MAb titer at the end of cell culture. c Comparison of SPR. Three other independent experiments with similar conditions exhibited similar results
Time course of change in AlaGln concentration during cell culture
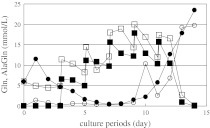
The change in concentration of AlaGln over time was measured during cell culture of 1A7-15 (Fig. 4). The consumption of AlaGln during cell culture with Gln-AlaGln was compared with that with AlaGln–AlaGln. In the case of the Gln-AlaGln condition, the concentration of Gln decreased as culture proceeded and it was consumed during the first 5 days. From days 5 to 10, the concentration of Gln was almost 0 mM. In contrast, the concentration of AlaGln was reduced to 0 mmol/L during the first 3 days under the Gln-AlaGln condition. Between days 4 and 10, the concentration of AlaGln was increased by the addition of the feed medium and began to decrease thereafter. The concentration of Gln increased in response to the decline in AlaGln.
Fig. 4.
Time course change of Gln and AlaGln through the 1 L bioreactor culture of 1A7-15 cell line. Gln concentration of Gln-AlaGln condition (black circle), AlaGln concentration of Gln-AlaGln condition (black square), Gln concentration of AlaGln–AlaGln condition (circle), AlaGln concentration of AlaGln–AlaGln condition (square) were monitored
Under the AlaGln–AlaGln condition, the time course of the changes in concentrations of Gln and AlaGln were almost the same as those under Gln-AlaGln except for the absence of Gln in the initial 3 days of cell culture. The initial AlaGln concentration was set at 5 mmol/L, the concentration increased by degrees until day 12, after which it decreased.
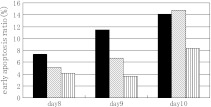
Analysis of early apoptosis
To examine the mechanism of the effects of Gln and AlaGln on cell growth and MAb production, the early apoptotic ratio was measured by Guava EasyCyte. The strain 1A7-15 was cultured in a bioreactor under the culture conditions of Gln–Gln, Gln-AlaGln, and AlaGln–AlaGln, and samples were drawn on days 8, 9, and 10. Harvested cells were stained with the nexin kit and the early apoptosis ratio was measured. The percentage of the cultured cells displaying apoptosis on day 8 is shown in Fig. 5.
Fig. 5.
Comparison of early apoptosis ratio: Gln–Gln condition (black), Gln-AlaGln condiiton (hatched) and AlaGln–AlaGln (vertical)
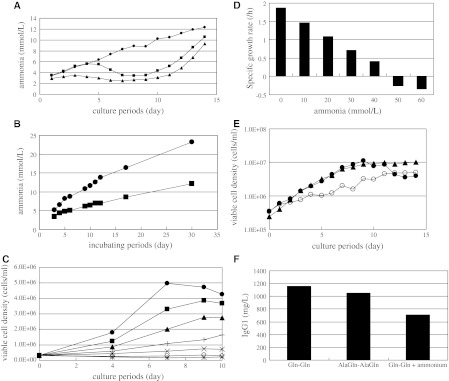
The cells from the AlaGln–AlaGln condition showed the lowest ratio of apoptosis, while those from Gln–Gln maintained the highest value. Cells from the Gln-AlaGln condition showed relatively little apoptosis on days 8 and 9. To determine whether the time course of apoptosis corresponded to that of ammonium production, we measured the ammonium concentrations over time (Fig. 6a). Ammonia in the cell culture medium under the Gln–Gln condition increased as culture proceeded; however, under the conditions in which Gln was replaced with AlaGln, the ammonia declined between days 6 and 10.
Fig. 6.
a Time course change of ammonia during cell culture with 1 L bioreactor of 1A7-15 (1) starting culture with Gln and feeding medium with Gln (black circle), (2) starting culture with Gln and feeding medium with AlaGln (black square) and (3) starting culture with AlaGln and feeding medium with AlaGln (black triangle). b Time course change of ammonia in culture medium including either 12 mmol/L Gln (black circle) or 12 mmol/L AlaGln (black square). c Ammonia effect on cell culture with Erlenmeyer flask of 1A7-15: ammonia 0 mmol/L (black circle), ammonia 10 mmol/L (black square), ammonia 20 mmol/L (black triangle), ammonia 30 mmol/L (plus symbols), ammonia 40 mmol/L (times symbols), ammonia 50 mmol/L (circle), ammonia 60 mmol/L (asterisk). d Specific cell growth rate of c between days 0 and 4. e Ammonia effect on 1A7-15 cell growth in 1 L bioreactor culture: Gln–Gln (black circle), AlaGln–AlaGln (black triangle) and Gln–Gln + 24 mmol/L ammonia (circle). f Comparison of MAb titer at the end of cell culture (e). Two other independent experiments with similar conditions exhibited similar results
Effect of ammonia on cell culture
To determine the physicochemical stability of Gln and AlaGln, the changes in ammonia level were measured for the basal medium during storage. Basal culture medium containing either 12 mmol/L Gln or 12 mmol/L AlaGln was incubated at 37.0 °C for 30 days. As shown in Fig. 6b, an increase in ammonia level was correlated with the duration of storage. The rates of degradation were different between Gln and AlaGln, and Gln generated ammonia more rapidly after 30 days of storage.
To test the extent to which ammonia damages cell growth, cell culture was carried out in Erlenmeyer flasks in the presence of 0, 10, 20, 30, 40, 50, and 60 mmol/L ammonia (Fig. 6c). As the concentration of ammonia increased, cell growth was dramatically inhibited, especially between days 4 and 7. Specific cell growth ratios between days 0 and 4 were calculated for each condition (Fig. 6d). When ammonia was added during culture in the 1-L bioreactor (Fig. 6e), cell growth was again clearly inhibited by the presence of ammonia. The production titer of MAb was also inhibited when ammonia was intentionally added to the basal medium (Fig. 6f). At the end of cell culture, the density of viable cells under the Gln–Gln condition decreased to same level as that observed when ammonia was added.
Discussion
Regarding the optimization of dipeptide content in culture medium, little research has been conducted to date, although there have been some reports on mammalian cell culture media containing dipeptides (Butler and Christie 1994; Christie and Butler 1994; Minamoto et al. 1991). In this paper, we describe the advantages of dipeptides as supplements to cell culture medium. In flask culture, a radical decrease in the density of viable cells at the late stage was found when Gln was used in the basal and feed media (the Gln–Gln condition). In contrast, little decrease in viable cell density was observed under the Gln-AlaGln condition, and no decline in the density of viable cells was observed under the AlaGln–AlaGln condition, although the maximum cell density was lower than that in the other two conditions. While the use of AlaGln as the source of Gln in the culture medium lowered the specific growth rate, the MAb titer was maximized when Gln was completely replaced by AlaGln. When productivities per cell were calculated from the MAb titers at the end of culture and the final cumulative cell density, the productivity of the cells was highest under the AlaGln–AlaGln condition, despite the observation that the cumulative cell density was not necessarily the highest.
Cell culture in a 1-L bioreactor produced two different results depending on the cell clone. For the 1A7-15 cell line, AlaGln in the feed medium delayed the decline in viability and enhanced the MAb titer by 100 mg/L, although the difference in titer was not as striking as that in the Erlenmeyer flask cultures. For the 12C-5cell line, productivity was greatly improved, and complete replacement of Gln with AlaGln in the basal and in the feed media produced a two-fold higher titer compared to the condition without AlaGln.
To characterize the effect of AlaGln during cell culture, the concentration profiles of AlaGln and Gln were evaluated during cell culture in the 1-L bioreactor. Although Gln and AlaGln are both nitrogen sources, Gln was consumed more readily than AlaGln. From days 5 to 10, the Gln concentration was maintained at almost 0 mM whereas the AlaGln concentration was increased by the addition of the feed culture. During this period, AlaGln was thought to serve as the source of Gln for the cells, since Gln did not exist in the culture medium, yet the density of viable cells continued to increase. In addition, the cell line used in this study has not glutamine synthase activity, and Gln is required for cell growth; therefore, as Gln was depleted, other components (in this case, AlaGln) must have served as the Gln source. After day 10, the AlaGln concentration gradually decreased and that of Gln increased. Perhaps, these changes were caused by peptidase released from dead cells. To confirm this hypothesis, the peptidase activity of the cultured broth at day 12 was purified by membrane filter and tested. Addition of AlaGln to the supernatant and incubation for 24 h increased the Gln concentration, whereas the broth to which AlaGln was not added did not show increased Gln (data not shown). This result supports the existence of a peptidase in the culture supernatant that degrades AlaGln to Gln. Considering the delay in cell growth observed under the AlaGln–AlaGln condition, we assume that AlaGln is degraded into Ala and Gln to be consumed as a nitrogen source instead of being used in other uptake systems such as transporters. In other words, peptidase digestion plays a dominant role when AlaGln is provided as a source of nutrition.
To explore the mechanism responsible for the effect of AlaGln on cell growth, apoptosis in the early phase of cell culture on days 8, 9, and 10 was measured. The results demonstrated that the apoptotic ratio was reduced under the AlaGln conditions. This may be attributed to the difference in stability between AlaGln and Gln in culture medium, resulting in inducing apoptosis (Buzanska et al. 2000; Suzuki et al. 2002) Gln undergoes autolysis and produces ammonia, while AlaGln is stable and produces less ammonia. This may represent a practical advantage of AlaGln for use as a supplement to culture medium because culture medium is generally stored in stainless steel tanks at ambient temperature for large-scale manufacturing. Our experiment demonstrated that Gln in medium maintained at 37 °C produced more ammonia than medium with AlaGln. Since ammonia quantity produced from Gln is equivalent to Gln quantity in the medium, Fig. 6b results contained ammonia generated from other than Gln. Peptides present in soy hydrolysate in the medium are a possible source of ammonia, as other components are all chemically defined element. Judging from ammonia concentration time course change, AlaGln suppressed ammonia accumulation compared to Gln. Furthermore, the results of Erlenmeyer flask culture clearly showed that accumulation of ammonia impaired cell growth. To extend these observations to conditions that mimicked those of manufacturing, the direct effect of ammonia was demonstrated on cells cultured in a 1-L bioreactor. When ammonia was added, both the cell growth and the MAb titer declined. These data suggest that substitution of AlaGln for Gln and/or combinations of AlaGln with Gln could improve cell culture profiles.
In summary, this article describes the efficacy of a dipeptide, AlaGln, on cell culture profiles. Addition of AlaGln to the culture medium enhanced MAb titers, with the degree of change in productivity depending on the cell line and culture conditions. The stability of AlaGln led to a decrease in accumulated ammonia during culture storage compared to that seen with Gln, and this improved cell culture performance (i.e., cell growth and MAb titer), especially under manufacturing conditions. From these data, we conclude that certain dipeptides can serve as superior alternative sources of amino acids in cell culture.
Abbreviations
- MAb
Monoclonal antibody
- ADCC
Antibody-dependent cellular cytotoxicity
- SPR
Specific mab production rate
References
- Birch JR, Racher AJ. Antibody production. Adv Drug Deliv Rev. 2006;58:671–685. doi: 10.1016/j.addr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Brian K. Industrialization of MAb production technology. MAbs. 2009;1:443–452. doi: 10.4161/mabs.1.5.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M, Christie A. Adaptation of mammalian cells to non-ammoniagenic media. Cytotechnology. 1994;15:87–94. doi: 10.1007/BF00762382. [DOI] [PubMed] [Google Scholar]
- Buzanska L, Zablocka B, Dybel A, Domanska-Janik K, Albrecht J. Delayed induction ob apoptosis by ammonia in C6 Glioma cells. Am J Physiol Gastrointest Liver Physiol. 2000;283:G986–G995. doi: 10.1016/s0197-0186(00)00030-9. [DOI] [PubMed] [Google Scholar]
- Christie A, Butler M. Glutamine-based dipeptides are utilized in mammalian cell culture by extracellular hydrolysis catalyzed by specific peptidase. J Biotechnol. 1994;37:277–290. doi: 10.1016/0168-1656(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Dong J, Mandenius CF, Lubberstedt M, Urbaniak T, Nussler AKN, Knobeloch D, Gerlach JC, Zeilinger K. Evaluation and optimization of hepatocyte culture media factors by design of experiments (DoE) methodology. Cytotechnology. 2008;57:251–261. doi: 10.1007/s10616-008-9168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G. Therapeutic antibodies-delivering the promise? Adv Drug Deliv Rev. 2006;58:633–639. doi: 10.1016/j.addr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konnno Y, Yano K, Shitara K, Hosoi S, Satoh M. Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng. 2006;94:680–688. doi: 10.1002/bit.20880. [DOI] [PubMed] [Google Scholar]
- Konno Y, Aoki M, Takagishi M, Sakai N, Koike M, Wakamatsu K, Hosoi S. Enhancement of antibody production by the addition of Coenzyme-Q10. Cytotechnology. 2011;63:163–170. doi: 10.1007/s10616-010-9330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto Y, Ogawa K, Abe H, Iochi Y, Mitsugi K. Development of a serum-free and heat-sterilizable medium and continuous high-density cell culture. Cytotechnology. 1991;5:S35–S51. doi: 10.1007/BF00573879. [DOI] [PubMed] [Google Scholar]
- Mori K, Iida S, Yamane-Ohnuki N, Kanda Y, Kuni-Kamochi R, Nakano R, Imai-Nishiya H, Okazaki A, Shinkawa T, Natsume A, Niwa R, Shitara K, Satoh M. Nonfucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology. 2007;55:109–114. doi: 10.1007/s10616-007-9103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parampalli A, Eskridge K, Smith L, Meagher MM, Mowry MC, Subramanian A. Developement of serum-free medium in CHO-DG44 cells using a central composite statistical design. Cytotechnology. 2007;54:57–68. doi: 10.1007/s10616-007-9074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Sun IL, Sun EE, Crane FL. Comparison of growth stimulation of HeLa cells, HL-60 cells, and mouse fibroblasts by coenzyme Q10. Protoplasma. 1995;184:214–219. doi: 10.1007/BF01276923. [DOI] [Google Scholar]
- Suzuki H, Yanaka A, Shibahara T, Matsui H, Nakahara A, Tanaka N, Muto H, Momoi T, Uchiyama Y. Ammonia-induced apoptosis is accelerated at higher pH is gastric surface mucous cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G986–G995. doi: 10.1152/ajpgi.00482.2001. [DOI] [PubMed] [Google Scholar]
- Tabata K, Hashimoto S. Fermentative production of l-Alanyl-l-Glutamine by a metabolically engineered Escherichia coli strain expressing l-Amino Acid -Ligase. Appl Environ Microbiolo. 2007;2007:6378–6385. doi: 10.1128/AEM.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata K, Ikeda H, Hashimoto S. ywfE in Bacillus subtilis Codes for a Novel Enzyme, l-Amino Acid Ligase. J Bacteriol Aug. 2005;2005:5195–5202. doi: 10.1128/JB.187.15.5195-5202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Sasaki M, Yanagihara K, Yamada H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J Biosci Bioeng. 2005;100:667–671. doi: 10.1263/jbb.100.667. [DOI] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks 2006. Nat Biotech. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]