Abstract
Filaminopathy is a subtype of myofibrillar myopathy caused by mutations in FLNC, the gene encoding filamin C, and histologically characterized by pathologic accumulation of several proteins within skeletal muscle fibers. With the aim to get new insights in aggregate composition, we collected aggregates and control tissue from skeletal muscle biopsies of six myofibrillar myopathy patients harboring three different FLNC mutations by laser microdissection and analyzed the samples by a label-free mass spectrometry approach. A total of 390 proteins were identified, and 31 of those showed significantly higher spectral indices in aggregates compared with patient controls with a ratio >1.8. These proteins included filamin C, other known myofibrillar myopathy associated proteins, and a striking number of filamin C binding partners. Across the patients the patterns were extremely homogeneous. Xin actin-binding repeat containing protein 2, heat shock protein 27, nebulin-related-anchoring protein, and Rab35 could be verified as new filaminopathy biomarker candidates. In addition, further experiments identified heat shock protein 27 and Xin actin-binding repeat containing protein 2 as novel filamin C interaction partners and we could show that Xin actin-binding repeat containing protein 2 and the known interaction partner Xin actin-binding repeat containing protein 1 simultaneously associate with filamin C. Ten proteins showed significant lower spectral indices in aggregate samples compared with patient controls (ratio <0.56) including M-band proteins myomesin-1 and myomesin-2. Proteomic findings were consistent with previous and novel immunolocalization data. Our findings suggest that aggregates in filaminopathy have a largely organized structure of proteins also interacting under physiological conditions. Different filamin C mutations seem to lead to almost identical aggregate compositions. The finding that filamin C was detected as highly abundant protein in aggregates in filaminopathy indicates that our proteomic approach may be suitable to identify new candidate genes among the many MFM patients with so far unknown mutation.
Myofibrillar myopathies (MFM)1 encompass a genetic and clinically heterogenous group of muscle disorders characterized by focal myofibrillar destruction and massive protein aggregation within skeletal muscle fibers (1). The mechanisms leading to aggregate formation are not well understood but an impairment of protein degradation systems seems to play an important role (2). Immunohistochemical studies identified various proteins accumulating in these aggregates (3) but the precise composition is unknown so far. The latter may give us important new insights in the pathogenesis of MFM.
Filaminopathy is a subtype of MFM caused by mutations in the rod domain of FLNC, the gene encoding FLNc (4–7). Filamins are a small group of large cytoskeletal proteins that crosslink F-actin filaments and act as scaffold for transmembrane receptors, signaling, and adapter proteins. FLNc is the striated muscle-specific isoform that cross-links actin at the Z-disc level and is important for the maintenance of myofibrillar integrity. Filaminopathy is leading to a progressive muscle weakness usually manifesting between the fourth and sixth decade of life. The pattern of severely and hardly affected muscles detected by magnetic resonance imaging is very homogeneous across patients with different FLNC mutations (8, 9, and unpublished data). In advanced stages of the disease, patients generally lose the ability to walk and show respiratory insufficiency because of weakness of respiratory muscles. A cardiac involvement is also frequent (4, 5).
Over the last years proteomics has developed to a promising tool for the analysis of the skeletal muscle proteome. Several studies including gel-based and mass spectrometric approaches have been performed that mainly aimed at the global cataloguing and biochemical characterization of the whole rodent muscle proteome or of cellular substructures under physiological and aging conditions (10,11, and for review see (12)). In aged muscles, for example, abundance changes have been detected for proteins involved in metabolism, contractile activity, myofibrillar remodeling, and stress response (12). In biomedical research global studies focused on the identification of novel panels of protein biomarker candidates for neuromuscular diseases: Analyses of muscle tissue of the dystrophin deficient mouse model of Duchenne muscular dystrophy (mdx mouse) identified altered levels of protein biomarkers involved in nucleotide metabolism, cellular stress response, energy metabolism, and ion handling (for review see (13)). Significant alterations of proteins playing an important role in various metabolic pathways were found in a mouse model for hypokalemic myopathy (14). In a 2-DE study of total muscle extracts from dysferlinopathy patients, 35 proteins were found to be differentially expressed (15). Metabolic and contractile proteins represented the majority of the changes suggesting an active process of muscle regeneration and a remodeling of fiber type as a result of dysferlin deficiency. In sporadic inclusion body myositis (sIBM), proteins associated with amyloidosis were up-regulated (16). A comparison of protein expression in sIBM to non-IBM inflammatory myopathies indicated an impairment of detoxification, energy metabolism, and protein folding in sIBM (16).
All of the above mentioned studies used total muscle protein extracts or soluble cytosolic protein fractions resulting in limitations because of high sample heterogeneity and complexity. Muscle tissue is composed of a complex mixture of different cell types from epi-, endo-, perimysium, muscle spindles, blood vessels etc. and changes in the protein abundance might be the result of differences in sample composition rather than disease-related effects. Additionally, the presence of a number of highly abundant and unusually large proteins (e.g. actin, myosin, titin) hampers the detection of potentially relevant low abundant disease-associated proteins. Those limitations could be bypassed by application of technologies allowing specific isolation of physiologically and pathophysiologically relevant muscular substructures before proteomic analysis, such as (sub) cellular microdissection. This can either be performed manually (17, 18) or laser-assisted, and provides unparalleled accuracy obtaining pure cell populations or even pure subcellular structures (for review see (19)). Laser microdissection (LMD) in combination with mass spectrometry has been successfully applied for reducing body myopathy (RBM) (20), a hereditary muscle disease histologically characterized by intracytoplasmic inclusions, so called reducing bodies. FHL1 (four and a half LIM domain 1) was identified as the most prominent component of isolated reducing bodies. Subsequently performed mutational analysis revealed different pathogenic FHL1 mutations in RBM patients. This was the first example of an essential contribution of a proteomic approach to the identification of the disease-causing mutation in a hereditary myopathy.
The aim of our study was to identify proteins that accumulate in protein aggregates within muscle fibers in MFM associated with FLNC mutations in order to get new insights in the pathomechanisms and to detect specific protein biomarker candidates for differential diagnosis. Skeletal muscle biopsies of six patients with three different FLNC mutations have been included in this study. LMD in combination with a newly established label-free mass spectrometry approach reproducibly identified and relatively quantified 390 proteins in total. We were able to detect new components of protein aggregates in filaminopathy and describe and characterize the so far unknown interaction between FLNc and Xin actin-binding repeat containing protein 2 (Xirp2).
EXPERIMENTAL PROCEDURES
Patients
Six filaminopathy patients with a MFM phenotype were included in this study. In each case, diagnosis was confirmed by genetic analysis and histological and immunofluorescence studies on skeletal muscle sections as described before (4). Three patients harbored the p.W2710X mutation located in the dimerization domain of FLNC (d24Δ1) that was first described by Vorgerd et al. in 2005 (7). One patient carried a second mutation in the dimerization domain (d24Δ2, manuscript in preparation) and two patients the p.V930_T933del mutation in Ig-like domain 7 of FLNC (d7Δ1) (6). All patients presented a proximally pronounced muscle weakness. Details of the patients including age at onset and biopsy are provided in Table I. Informed consent was obtained from all patients (with approval of the ethics committee of the Ruhr-University Bochum [#4078–11]).
Table I. Overview of patients studied in this work.
| ID | Gender | Age at onset [years] | Age at biopsy [years] | Muscle | FLNC mutation (domain) |
|---|---|---|---|---|---|
| 1 | Female | 50 | 52 | Medial gastrocnemius | Dimerization domain (d24Δ1) |
| 2 | Male | 39 | 42 | Vastus lateralis | Dimerization domain (d24Δ1) |
| 3 | Female | 42 | 46 | Vastus lateralis | Dimerization domain (d24Δ1) |
| 4 | Female | 36 | 46 | Medial gastrocnemius | Ig-like domain 7 |
| 5 | Male | 39 | 49 | Vastus lateralis | Ig-like domain 7 |
| 6 | Female | 37 | 41 | Medial gastrocnemius | Dimerization domain (d24Δ2) |
Muscle Biopsies
Skeletal muscle biopsies were obtained by surgical procedure. After biopsy the excised tissue was cut into pieces with a volume of about 0.5 cm3. The pieces were embedded into Tissue-Freezing Medium® (Leica Microsystems, Wetzlar, Germany) and frozen in liquid nitrogen.
Detection of Aggregates by Immunofluorescence Staining and Laser Microdissection
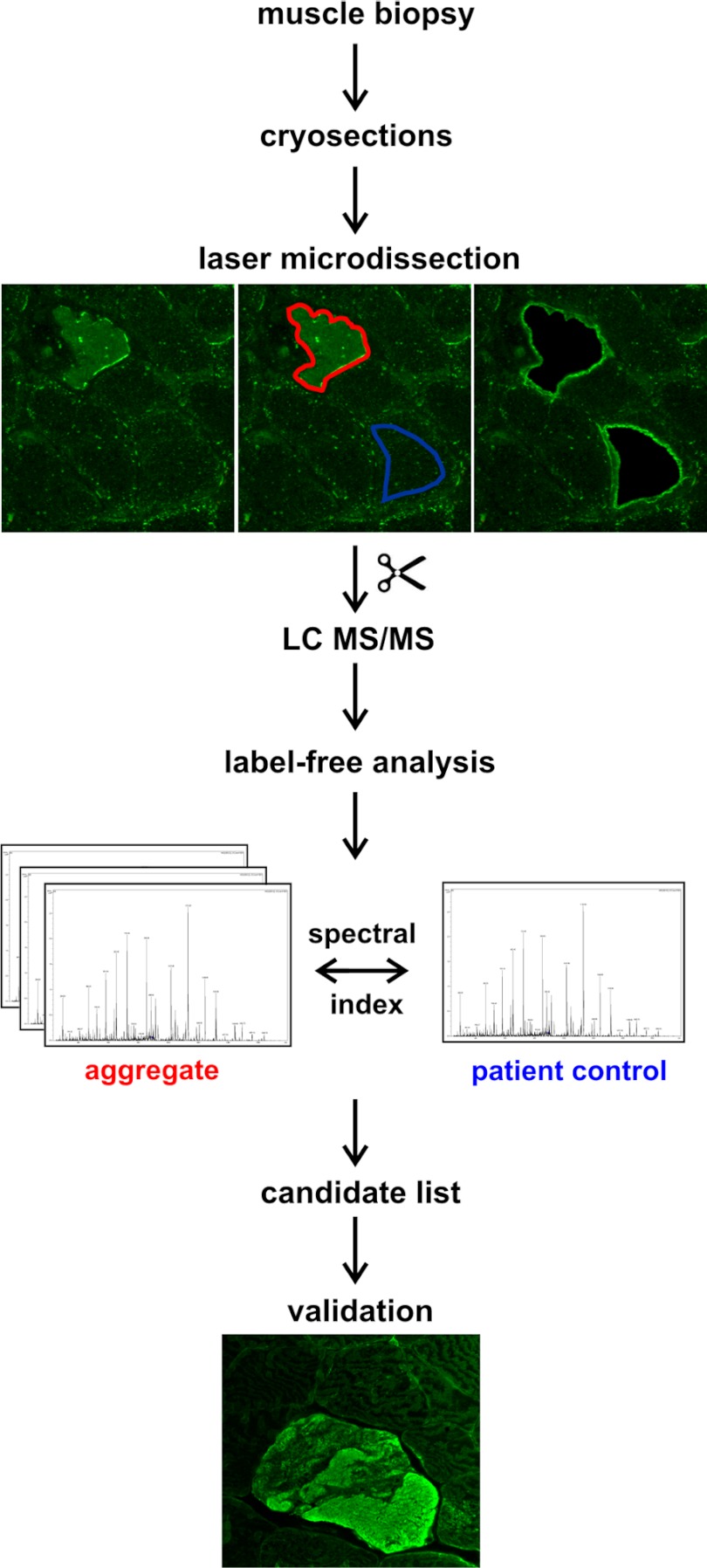
A workflow illustrating the steps of sample and data processing is provided in Fig. 1. Immunofluorescence staining was performed on 10 μm frozen skeletal muscle sections, placed on polyethylene terephthalate (PET) membranes (Leica Microsystems, Wetzlar, Germany). The sections were incubated with a primary antibody directed against myotilin (mouse monoclonal, clone RS034, Novocastra/Leica Microsystems, Wetzlar, Germany) in a dilution of 1:20 in PBS (0.1 m NaCl + 3 mm KCl + 1 mm KH2PO4 + 5 mm Na2HPO4, pH 7.4) for 1 h at room temperature (RT) and with a secondary goat anti-mouse IgG antibody conjugated with DyLight 488 (Dianova, Hamburg, Germany, 1:1000 in PBS) for 45 min at RT. After a second washing-step with PBS the tissue slides were air-dried and stored at 4 °C.
Fig. 1.
Workflow for the identification of MFM biomarkers by combining LMD and label-free MS analysis. Frozen sections of skeletal muscle biopsies were immunostained using an antibody directed against myotilin in order to identify protein aggregates in abnormal muscle fibers. Protein aggregates and surrounding muscle tissue (latter used as control) were collected by laser microdissection (LMD). After tryptic in-solution digestion of LMD-samples protein identification was performed by LC-MS/MS. Differential proteins in aggregates and surrounding muscle tissue were identified by a label-free approach based on spectral counts. For validation samples were immunostained using an antibody directed against the respective candidate protein from the label-free analysis.
For each patient, aggregates with a total area of 250,000 μm2 were collected in tubes with 40 μl formic acid (FA, 98–100%) by laser microdissection (LMD) (LMD 6500, Leica Microsystems, Wetzlar, Germany), in the following referred to as “aggregate samples.” In filaminopathy, this area corresponds to about 100 large aggregates. The respective area (250,000 μm2) of muscle fibers not harboring protein aggregates from the same patient served as control (see Fig. 1), in the following referred to as “patient controls.”
Sample Processing and Protein Digestion
After incubation for 1 h at RT followed by sonication for 5 min (RK31, BANDELIN electronic, Berlin, Germany) the samples were centrifuged (5 min, 10,000 rpm, RT) and directly frozen at −80 °C. For tryptic in solution-digestion FA was vaporized in vacuum (rotational-vacuum-concentrator RVC2–25CD plus, Martin Christ GmbH, Osterode am Harz, Germany). The collected tissue material was diluted in 50 mm ammonium bicarbonate (pH 7.8) to a final volume of 74.25 μl. Samples were reduced by adding 0.25 μl 2 m dithiotreitol and incubation for 20 min at 56 °C followed by alkylation with 0.55 m iodoacetamide for 15 min at RT. The pH was adjusted to 7.4 before adding 1 μl of 1% Trypsin Enhancer ProteaseMAX™ Surfactant (Promega, Mannheim, Germany) in 50 mm NH4HCO3 (pH 7.8). Subsequently, 1.8 μl of a trypsin (Serva Electrophoresis GmbH, Heidelberg, Germany) solution (1 μg/μl in 50 mm acetic acid) was added and proteins were digested overnight at 37 °C. The process was terminated by the addition of 5.25 μl 10% TFA. Samples were purified with OMIX C18 Tips (Varian, Agilent Technologies, Böblingen, Germany) followed by concentration for 5 min in vacuum. The final volume was adjusted to 63 μl with 1% TFA. Fifteen microliters were used for each mass spectrometric analysis.
Mass Spectrometry
Nano-HPLC-MS/MS was performed on an UltiMate 3000 RSLCnano LC system (Dionex, Idstein, Germany). First, samples were loaded on a trap column (Dionex, 75 μm × 2 cm, particle size 3 μm, pore size 100 Å) with 0.1% trifluoroacetic acid (flow rate 10 μl/min). After washing, the trap column was serially connected with an analytical C18 column (Dionex, 75 μm × 25 cm, particle size 2 μm, pore size 100 Å). The peptides were separated with a flow rate of 400 nl/min using the following solvent system: (A) 95% acetonitrile, 0.1% formic acid; (B) 80% acetonitrile, 0.1% formic acid. In a first step a gradient from 100% A to 40% B (95 min) was used, followed by a second gradient from 40% B to 95% B within 2 min and finally a gradient from 95% to 5% B.
The HPLC system was directly coupled to a nanoelectrospray ionization source (Thermo Fisher Scientific, Schwerte, Germany). ESI-MS/MS was performed on a LTQ Orbitrap Velos (Thermo Fisher Scientific). MS spectra were scanned between 300 and 2000 m/z with a resolution of 30,000 and a maximal acquisition time of 500 ms. The m/z values initiating MS/MS were set on a dynamic exclusion list for 35 s. Lock mass polydimethylcyclosiloxane (m/z 445.120) was used for internal recalibration. The 20 most intensive ions (charge>1) were selected for MS/MS-fragmentation in the ion trap. Fragments were generated by low-energy collision-induced dissociation (CID) on isolated ions with collision energy of 35% and maximal acquisition time of 50 ms.
The mass spectrometric raw data associated with this manuscript may be downloaded from ProteomeCommons.org Tranche using the following hash:
3Kxvps+jyBF2Sj0ROCApB+2ZNWFX6QkVZz4GWAP5sE8KFMZ58MrxkjMj6gIKMx6mIog2SRQhL6wAsnro3NfHu4NJzIcAAAAAAAAPSw = =.
The hash may be used to prove exactly what files were published as part of this manuscript's data set, and the hash may also be used to check that the data has not changed since publication.
Data Processing and Database Search
Raw files were transformed to MGF-files (ProteomDiscoverer 1.3, Thermo Scientific Fisher), imported in ProteinScape™ (version 2.1, Bruker Daltonics, Bremen, Germany), and analyzed using Mascot (Matrixscience, London, UK) with a peptide mass tolerance of 10 ppm and a fragment mass tolerance of 0.5 Da. Searches were performed allowing one missed cleavage site after tryptic digestion. Carbamidomethylation (C), oxidation (M), and phosphorylation (S,T,Y) were considered as variable modifications. All data were searched against a database created by DecoyDatabaseBuilder (21) containing the whole UniProt/Swissprot (release 2011/06, 529056 entries) with one additional shuffled decoy for each protein, resulting in a database containing 1,058,112 entries.
Protein Quantification
After peptide identification an algorithm using a given minimal peptide score (minPepScore) and minimal number of peptides per protein (minNrPeps) was applied. The algorithm performs the following steps:
Step 1) Score calculation for all protein groups by adding up the Mascot ion scores of the group's peptide spectrum matches (PSMs), which have a score of at least minPepScore. Here, a protein group is defined as the set of all entries in the database containing the same set of identified peptides. If two PSMs are equal except for the score, only the higher score is taken.
Step 2) Reporting the highest scoring protein group which has at least minNrPeps peptides consisting of PSMs not yet flagged as used up and flag all the PSMs of the reported protein group as used up.
Step 3) Repetition of step 2 until no more protein groups are reported with the given minPepScore and minNrPeps.
A local false discovery rate (FDR) was calculated for each protein group, regarding a group as decoy, if it consists of decoy proteins only. With this strategy the minPepScore was calculated, which yielded the list with the most target (opposed to decoy) groups beneath an FDR threshold of 5%. For the given data a minPepCount of 2 was used to exclude “one hit wonders”, which yielded a minPepScore of 22 for the longest list. Among the proteins in this list, every PSM was extracted.
These PSMs were further processed using the Pivot table function of Microsoft Excel resulting in a table representing spectral counts for every peptide belonging to a certain protein. A spectral index (SI) based on spectral and peptide counts was calculated as described previously (22, 23) and subsequently used as basis for label-free quantification. In brief, spectral index calculation was performed by summing up all spectral counts belonging to the respective protein. Because of protein sequence homologies some identified peptides could be assigned to more than one protein. Therefore, only peptides were considered that were unique within the complete data set. To identify differentially expressed proteins, the ratio between the averaged spectral indices of aggregates and controls was calculated and Student‘s t test was conducted for each protein. To control the FDR, the resulting p values were adjusted for multiple testing according to Benjamini and Hochberg (24) and compared with the significance level alpha = 0.05. A protein was accepted as differentially expressed with a ratio >1.8 in aggregates or controls and a p value adjusted <0.05.
Validation of Proteomic Findings by Immunofluorescence Studies
Selected proteins identified as differentially expressed by proteomic analyses were validated by immunofluorescence studies on 6 μm thick frozen serial skeletal muscle sections of filaminopathy patients. The following primary antibodies were used: mouse monoclonal antibodies against myomesin-1 (clone BB78, dilution 1:500,(25)), myomesin-2 (clone AA241, dilution 1:200,(25)), myotilin (clone RS034, dilution 1:20, Novocastra/Leica Microsystems, Wetzlar, Germany), heat-shock protein 27 (clone 2B4, dilution 1:500, Novocastra/Leica Microsystems, Wetzlar, Germany) and Xirp2 (clone Xirp2, dilution 1:10, (26)); polyclonal rabbit antibodies against FLNc Ig-like domains d16–20 and N-RAP (custom made by BioGenes, Berlin, Germany, dilution 1:1000) and Rab35 (Abcam plc, Cambridge, UK, dilution 1:500). Sections were incubated overnight at 4 °C with primary antibodies and washed three times with PBS for 5 min. Subsequently, the sections were incubated with isotype specific secondary antibodies conjugated with DyLight 488 (Dianova, Hamburg, Germany, dilution 1:1000) or Texas Red (Dianova, Hamburg, Germany, dilution 1:400) for 45 min at RT and then washed again three times with PBS for 5 min. A novel rabbit polyclonal antiserum (7700) was raised against a bacterially expressed recombinant mouse Xirp2 fragment encompassing aminoacids 335–667 (UniProt Q4U4S6–2, Biogenes, Berlin, Germany). To remove antibodies also recognizing Xin from the serum, these antibodies were absorbed using a recombinantly expressed human Xin fragment, rendering the serum highly specific for human Xirp2.
Design of cDNA Constructs and Expression and Purification of Recombinant Proteins
DNA cloning was performed using standard procedures (27). For recombinant expression in E. coli FLNc cDNA fragments containing Ig-like domains 18–19, 18–21, and 20–21 were cloned into pET23-EEF (28) whereas cDNA fragments encoding the carboxyterminus of Xirp2 (amino acids 2768–3327 and 2982–3327 according to Uniprot A4UGR9–2) were cloned in pET23-T7 or pET23myc, a newly constructed vector based on pET23-T7, with a c-myc tag instead of the T7 tag. The construct encoding the T7-tagged carboxyterminus of Xin encodes amino acids 1686 - 1812 (Uniprot Q702N8–1). Protein expression in, and purification from E. coli BL21(DE3)Codon Plus cells (Stratagene, La Jolla, CA) was performed as described previously (29). Inverse PCR was used to delete the cDNA encoding the FLNc-specific insertion from Ig-like domain 20. 5′-phosphorylated primers that hybridize to the regions on either side of the insertion (GTGAGGCCAGCTCTCAGGACATGACTGp and CTGGGATCTTGAGGTTGAGGTCACAGGp) were used to amplify the complete pET23-EEF vector containing Ig-like domains 18–20 using Phusion High-Fidelity DNA Polymerase (NEB, Frankfurt, Germany). The plasmid was recircularized by intramolecular ligation, transformed to and propagated in E. coli JM 109 bacteria, and sequenced to verify correct deletion.
Co-immunoprecipitation Assays
For co-immunoprecipitation experiments 1 μg of myc- or T7-tagged Xin or Xirp2 fragment was added to 10 μg EEF-tagged purified recombinant FLNc fragment in IP buffer (0.05% Triton X-100, 1% BSA, protease inhibitors [Roche mini complete, Roche Diagnostics, Mannheim, Germany] in PBS). After incubation at RT for 1 h, 10 μl of the myc-tag mAb (clone 9E10, tissue culture supernatant), or 0.75 μg T7-tag mAb (Novagen) was added. Subsequent to an incubation for 30 min, 25 μl Dynabeads protein G (Dynal Biotech, Hamburg, Germany) was added and the mixture was incubated at 4 °C for 2–3 h. The beads were washed three times with IP buffer without BSA and boiled in SDS sample buffer. Bead-associated proteins were separated by SDS-PAGE, transferred to nitrocellulose and immunodetected using antibodies directed against the c-myc-, T7- or EEF-tag (YL1/2,(30)).
Competition assays were performed by incubating 1 μm T7-tagged purified recombinant FLNc d18–21 with 10 μm of EEF-tagged Xin or myc-tagged Xirp2 fragment, respectively, in the presence of increasing amounts (0–50 μm) of the fragment of the other Xirp protein for 1 h at RT. Immunoprecipitation with the T7-tag antibody and analysis of the immunoprecipitated proteins was performed as described above.
RESULTS
Filaminopathy is characterized by massive protein aggregation in skeletal muscle fibers, but the composition of aggregates is largely unknown. To fill this gap we applied a workflow consisting of laser microdissection and label-free spectral count based proteomics for samples of filaminopathy patients (Fig. 1). In all patient muscle biopsies, the number of abnormal fibers harboring protein aggregates detected by our modified immunofluorescence protocol was sufficient to collect the defined aggregate area of 250,000 μm2. Within the complete collective, a total of 390 different proteins have been quantified. Using stringent data analysis parameters (adjusted p value <0.05 and regulation factor >1.8, if applicable) we identified 31 proteins with a significantly higher spectral index in aggregates from filaminopathy patients compared with patient controls (Table II). The proteins listed in Table II were ordered by the mean sum of peptides detected in protein aggregates of the patients normalized to the number of total counts estimating the relative amount of the different proteins in the aggregates. The ranking of proteins in aggregate samples of individual patients is given in Supplemental Table S1. Notably and as a proof of concept, already known marker proteins of filaminopathy aggregates like desmin, myotilin, αB-crystallin, Xin actin-binding repeat-containing protein 1 (Xin), collagen VI, and dysferlin (4) could be identified and quantified by our proteomic approach. Additionally, potential new marker proteins were detected and in part validated using immunostaining on patient's muscle biopsy sections.
Table II. Results of the label-free quantification. Listed are proteins with an adjusted p value <0.05 and regulation factor (ratio) >1.8 (if applicable). acc. = accession number, SC = sequence coverage (%), SI = spectral index,*number of different peptides, **t-Test p value, ***adjusted p value (correction for multiple testing).
| Acc. | Protein | SC | Pep.* | Aggregate (SI) | Control (SI) | Ratio | p value** | Corrected p value*** |
|---|---|---|---|---|---|---|---|---|
| Q14315 | Filamin-C | 55.3 | 101 | 207.7 | 29.5 | 7.0 | 8.3 × 10−7 | 5 × 10−5 |
| P17661 | Desmin | 60.0 | 24 | 116.4 | 29 | 4.0 | 5.2 × 10−7 | 4 × 10−5 |
| A4UGR9 | Xin actin-binding repeat-containing protein 2 (Xirp2) | 26 | 83 | 64.4 | 0 | n.a. | 1.9 × 10−6 | 8 × 10−5 |
| Q86VF7 | Nebulin-related-anchoring protein (N-RAP) | 21.7 | 56 | 49.8 | 0.8 | 63.0 | 6.0 × 10−9 | 1.2 × 10−6 |
| P02511 | αB-crystallin | 55.4 | 8 | 30.9 | 3.0 | 10.3 | 1.8 × 10−7 | 1.7 × 10−5 |
| Q5VST9 | Obscurin | 5.9 | 57 | 30.3 | 10.0 | 3.0 | 2.1 × 10−5 | 0.0006 |
| Q702N8 | Xin actin-binding repeat-containing protein 1 (Xin) | 21.9 | 37 | 30 | 1.14 | 25.9 | 5.4 × 10−8 | 7 × 10−6 |
| P48681 | Nestin | 21.7 | 32 | 26.8 | 0.4 | 67.6 | 7.0 × 10−7 | 4.5 × 10−5 |
| P12111 | Collagen alpha-3(VI) chain | 9.3 | 30 | 16.9 | 3.5 | 4.4 | 0.0001 | 0.003 |
| Q13203 | Myosin-binding protein H | 35.8 | 10 | 16.5 | 1.1 | 14.8 | 0.0009 | 0.01 |
| Q9UBF9 | Myotilin | 34.7 | 18 | 16 | 8.7 | 1.8 | 0.002 | 0.02 |
| O75923 | Dysferlin | 11.4 | 21 | 12.7 | 1.7 | 7.3 | 0.0001 | 0.003 |
| P04792 | Heat shock protein beta-1 (Hsp27/HspB1) | 38.5 | 8 | 12 | 2.1 | 5.8 | 0.0003 | 0.006 |
| P62805 | Histone H4 | 40.8 | 4 | 9.9 | 3.4 | 2.95 | 0.003 | 0.03 |
| P02545 | Lamin-A/C | 17.3 | 12 | 5.1 | 0.8 | 6.5 | 0.0009 | 0.01 |
| P08670 | Vimentin | 18.2 | 4 | 4.3 | 1.0 | 4.1 | 0.001 | 0.02 |
| P28074 | Proteasome subunit beta type-5 | 28.9 | 6 | 3.8 | 0.2 | 24.6 | 0.001 | 0.01 |
| Q86TC9 | Myopalladin | 5.2 | 8 | 3.3 | 0 | n.a. | 0.001 | 0.02 |
| Q0ZGT2 | Nexilin | 6.8 | 4 | 3 | 0.5 | 6.2 | 1.4 × 10−5 | 0.0004 |
| P13591 | Neural cell adhesion molecule 1 | 3.7 | 4 | 2.8 | 0 | n.a. | 9.6 × 10−5 | 0.002 |
| P21333 | Filamin-A | 2.0 | 1 | 2.6 | 0.3 | 9.2 | 7.1 × 10−6 | 0.0002 |
| Q9H7C4 | Syncoilin | 8.7 | 4 | 1.8 | 0 | n.a. | 0.003 | 0.02 |
| P05023 | Sodium/potassium-transporting ATPase subunit alpha-1 | 11.6 | 6 | 1.8 | 0 | n.a. | 0.0009 | 0.01 |
| P28289 | Tropomodulin-1 | 5.8 | 2 | 1.4 | 0.2 | 8.9 | 0.002 | 0.02 |
| P28070 | Proteasome subunit beta type-4 | 13.3 | 5 | 1.4 | 0 | n.a. | 0.004 | 0.04 |
| Q0D2I5 | Intermediate filament family orphan 1 | 6.6 | 3 | 1.3 | 0 | n.a. | 0.0002 | 0.003 |
| O75369 | Filamin-B | 3.7 | 1 | 1.3 | 0 | n.a. | 0.0001 | 0.003 |
| O43760 | Synaptogyrin-2 | 3.6 | 3 | 1.3 | 0 | n.a. | 8.7 × 10−5 | 0.002 |
| P05026 | Sodium/potassium-transporting ATPase subunit beta-1 | 14.2 | 2 | 1.1 | 0 | n.a. | 1.1 × 10−9 | 4.3 × 10−7 |
| O00487 | 26S proteasome non-ATPase regulatory subunit 14 | 6.5 | 2 | 1 | 0 | n.a. | 0.001 | 0.01 |
| Q15286 | Ras-related protein Rab35 | 13.9 | 1 | 0.9 | 0 | n.a. | 0.0007 | 0.01 |
Validation of Proteomic Data
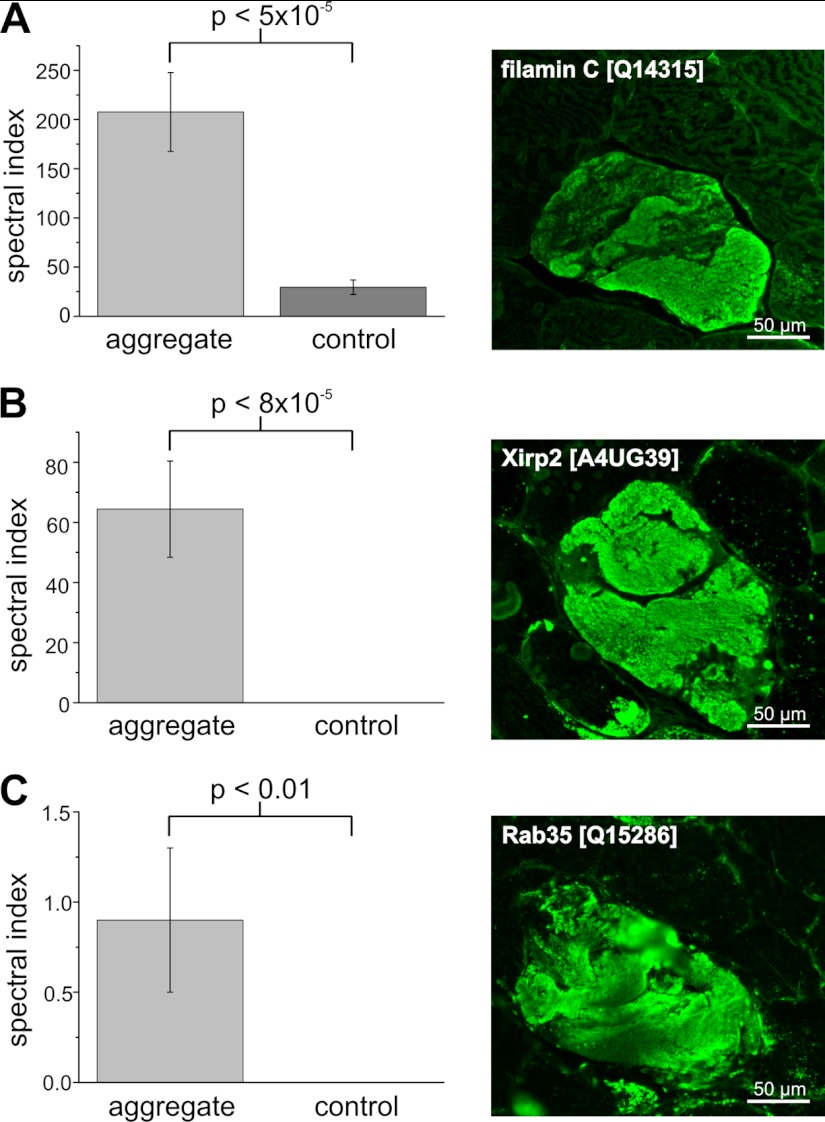
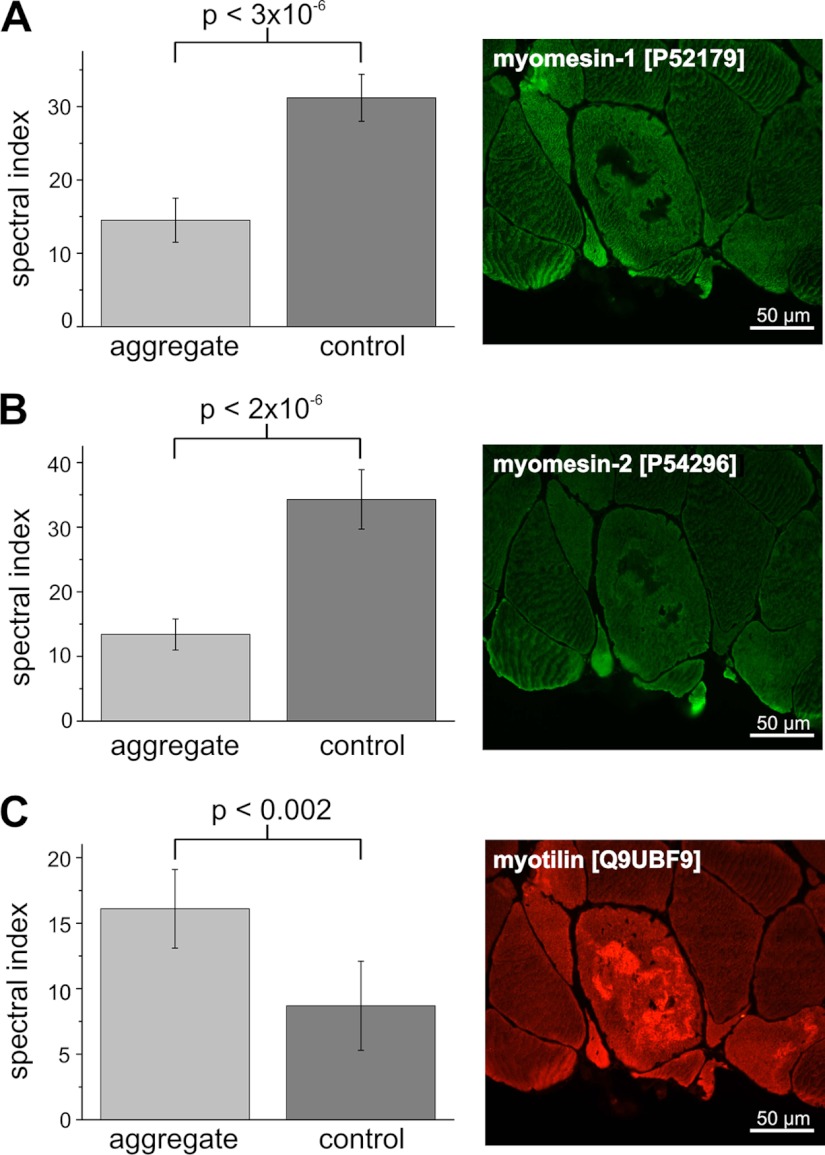
As expected, aggregates displayed a highly increased immunoreactivity for the disease causing protein FLNc, which was identified as a significantly enriched protein in the filaminopathy aggregates (adj. p <4 × 10−5, Fig. 2A). The 83 peptides of Xirp2 that was not known to be a component of aggregates in filaminopathy patients before, were detected solely in the aggregate samples using our proteomic pipeline, consistent with immunofluorescence findings (Fig. 2B). Similarly, ras-related protein 35 (Rab35), nebulin-related-anchoring protein (N-RAP), and Hsp27 peptides were identified in aggregate samples but not or barely in the respective patient controls. Consistently, immunofluorescence staining revealed markedly increased immunoreactivity for Rab35 (Fig. 2C), N-RAP and Hsp27 (not shown) only in aggregates within abnormal muscle fibers. Proteomic analysis also identified proteins with a significantly lower concentration in aggregates compared with patient controls (Table III). Exemplarily for this group of proteins we validated myomesin-1 and myomesin-2, two proteins normally located in the M-band of sarcomeres. Both revealed significantly lower spectral index values in the aggregate samples than in the patient controls (Table III). Immunofluorescence staining (Figs. 3A and 3B) revealed a significantly decreased immunoreactivity for myomesin-1 and myomesin-2 in the myotilin-positive aggregates (Fig. 3C) and thus confirmed these results. In contrast, surrounding muscle fibers showed normal staining for myomesin-1 and myomesin-2.
Fig. 2.
Validation of higher abundant candidate MFM biomarkers by immunofluorescence analyses. IF studies were performed on frozen serial sections of skeletal muscle biopsies from filaminopathy patients using antibodies directed against FLNc, Xirp2 and Rab35. Consistent with the results of the label-free approach, abnormal fibers with FLNc containing aggregates (A) also show a markedly increased immunoreactivity for Xirp2 (B), and Rab35 (C).
Table III. Results of the label-free quantification. Listed are proteins with an adjusted p value <0.05 and regulation factor (ratio) <0.56 (if applicable). acc. = accessionnumber, SC = sequence coverage (%), SI = spectral index,*number of different peptides, **t-Test p value, ***adjusted p value (correction for multiple testing).
| Acc. | Protein | SC | Pep.* | Aggregate (SI) | Control (SI) | Ratio | p value** | Corrected p value*** |
|---|---|---|---|---|---|---|---|---|
| P54296 | Myomesin-2 | 40.2 | 45 | 13.3 | 34.4 | 0.4 | 1.9 × 10−6 | 8.3 × 10−5 |
| P52179 | Myomesin-1 | 30.6 | 52 | 14.5 | 31.2 | 0.5 | 2.8 × 10−6 | 0.0001 |
| P31930 | Ubiquinol-cytochrome-c reductase complex core protein 1 | 27.7 | 9 | 0.9 | 3.8 | 0.2 | 0.001 | 0.02 |
| P35573 | Glycogen debranching enzyme | 45.6 | 6 | 0.8 | 3.6 | 0.2 | 0.003 | 0.02 |
| P00403 | Cytochrome c oxidase subunit 2 | 18.9 | 4 | 0.7 | 3.6 | 0.2 | 0.0004 | 0.008 |
| O75746 | Calcium-binding mitochondrial carrier protein Aralar1 | 17.0 | 11 | 0.7 | 3.7 | 0.2 | 0.002 | 0.02 |
| P24310 | Cytochrome c oxidase polypeptide VIIa-heart | 16.5 | 1 | 0.1 | 1.6 | 0.1 | 0.002 | 0.02 |
| P19404 | NADH dehydrogenase flavoprotein 2 | 18.5 | 5 | 0 | 1.8 | n.a. | 0.0003 | 0.005 |
| Q02880 | DNA topoisomerase 2-beta | 1.1 | 2 | 0 | 1.3 | n.a. | 0.0007 | 0.01 |
| P40926 | Malate dehydrogenase | 8.9 | 3 | 0 | 1.1 | n.a. | 0.004 | 0.04 |
Fig. 3.
Validation of the lower abundant candidate MFM biomarker myomesin-1 and -2 by immunofluorescence analysis. Immunofluorescence analyses were performed on frozen serial sections of skeletal muscle biopsies from filaminopathy patients. Corresponding to the results of the proteome study, abnormal fibers with an increased immunoreactivity for myotilin (C) revealed a decreased immunoreactivity for myomesin-1 (A) and myomesin-2 (B).
Identification of Novel FLNc Binding Partners
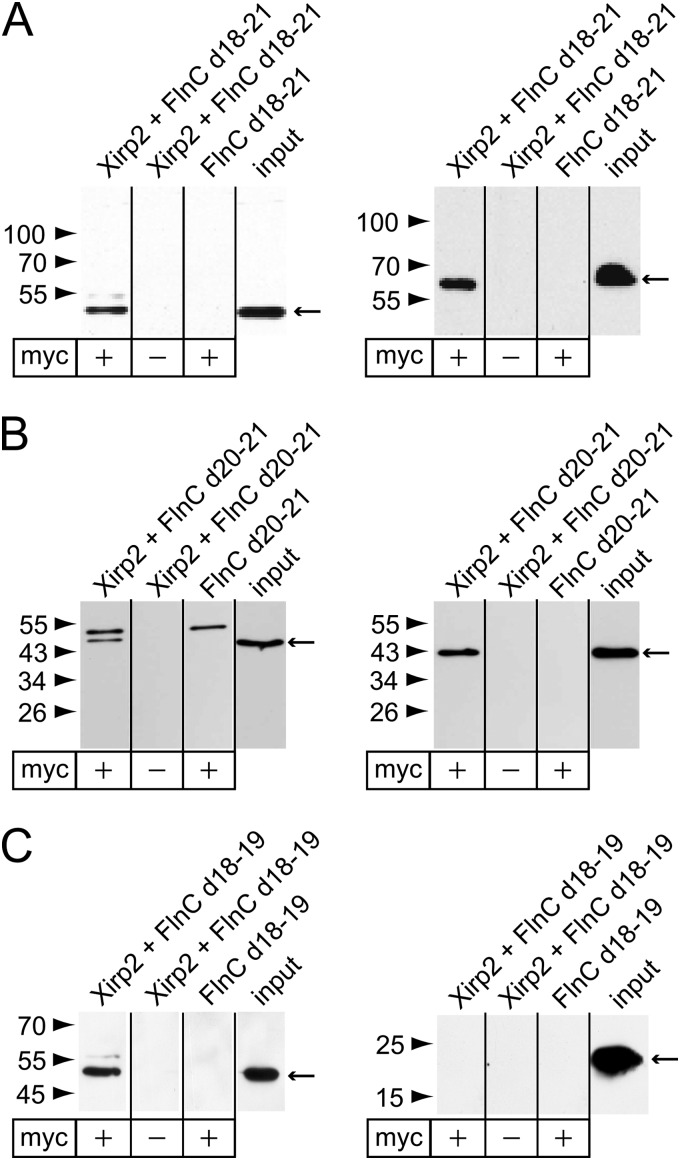
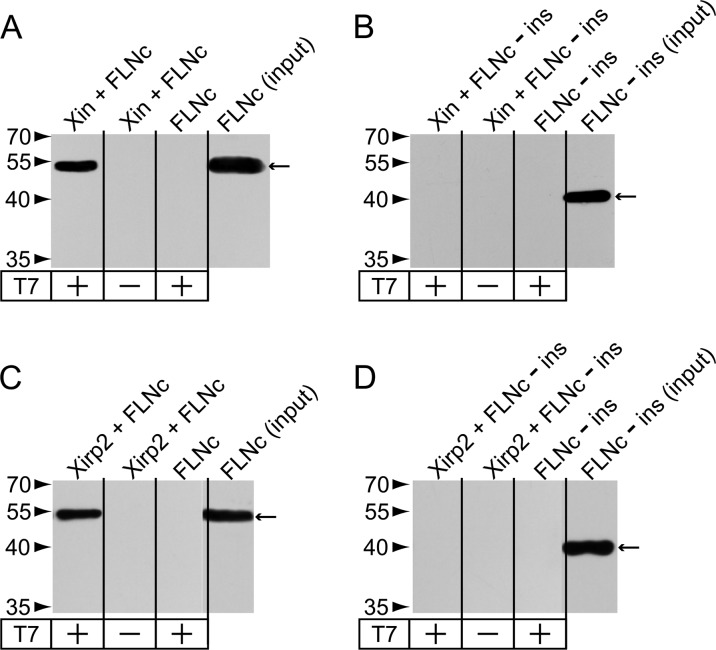
The fraction of proteins accumulating in aggregates with a high spectral index included not only FLNc but also many of its binding partners: N-RAP, obscurin, Xin, and myotilin were all identified as FLNc ligands (31, 32, 33). Apart from Xin, also the second member of the family of xin actin-binding repeat containing proteins, Xirp2, was detected in aggregates. Hence, we speculated that Xirp2 might also interact with FLNc. The filamin binding site in Xin is located at its C-terminus (31). Because Xin and Xirp2 share a similar domain layout, a C-terminal fragment of Xirp2 was tested for binding to fragments (d18–19, d18–21, d20–21) of human FLNc in co-immunoprecipitation assays. This approach identified d20–21, the domains that also bind Xin (31), as the binding site of Xirp2 because only fragments containing these domains could be coprecipitated (Fig. 4). None of the homologous fragments of filamin A and B (FLNa, FLNb) bound to Xirp2 (results not shown). Therefore, these results demonstrate that Xirp2 and Xin are both FLNc-specific binding partners.
Fig. 4.
Identification of Xirp2 as interaction partner of FLNc. Co-immunoprecipitation of the myc-tagged Xirp2 fragment (2982–3327) and EEF-tagged FLNc fragments. Xirp2 was precipitated using a c-myc-specific antibody (left panels). Coprecipitated EEF-tagged FLNc fragments were detected using an EEF-specific antibody (right panels). Precipitated proteins and molecular weight standards are indicated by arrows and arrowheads, respectively. Signals of the heavy chains of the c-myc antibody (∼55kDa), are visible in the left panels. Co-IP experiments with FLNc fragment d18–21 (A), d20–21 (B) and d18–19 (C) indicate that d18–21 and d20–21, but not d18–19 bind Xirp2.
Because Xin and Xirp2 do not bind FLNa and FLNb, we speculated that the unique insertion in FLNc Ig-like domain 20 is necessary for binding to both proteins. Indeed, deletion of the insertion from the fragment FLNc d18–21 abolished binding of both ligands (Fig. 5). This indicates that either the insertion directly binds Xin and Xirp2, or that the insertion is responsible for folding the domain in a way that enables binding of both Xirps. The Xin construct used in this experiment was a truncated variant of a fragment that was previously used to confirm binding of Xin to FLNc (31), now narrowing down the FLNc-binding site to amino acids 1686–1812.
Fig. 5.
Interaction of FLNc with Xin and Xirp2 depends on the unique insertion in Ig-like domain 20. Co-immunoprecipitation of T7-tagged Xin 1686–1812 (A, B) or Xirp2 2768–3327 (C, D) fragments and EEF-tagged FLNc fragment d18–21 with (A, C) or without (-ins, B, D) the insertion in Ig-like domain 20. Xin and Xirp2 were precipitated using a T7-tag-specific antibody. Coprecipitated EEF-tagged FLNc fragments were detected using an EEF-specific antibody. Precipitated proteins and molecular mass standards are indicated by arrows and arrowheads, respectively. Note that only FLNc d18–21 is co-precipitated, whereas d18–21-ins does not bind both Xirps.
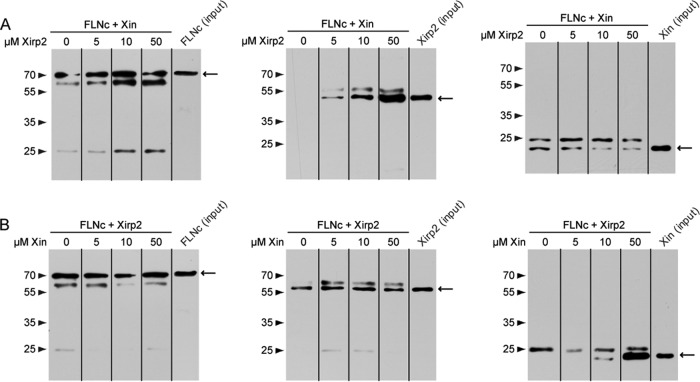
Subsequently, we analyzed whether Xin and Xirp2 compete for binding to FLNc. Competition assays in which 10 μm of a fragment of one of the Xirps was mixed with increasing amounts (0–50 μm) of a fragment of the other protein and both proteins were enabled to bind FLNc. These experiments showed that addition of increased levels of Xirp2 decreases the amount of Xin bound to FLNc, but even at the highest Xirp2 concentration (50 μm) a complete inhibition was not observed. Moreover, the amount of bound Xin seemed not reduced at this Xirp2 concentration compared with the experiment using equimolar amounts of both proteins. In contrast, the addition of increasing amounts of Xin fragment to the FLNc/Xirp2/Xin mixture showed no effect on the quantity of FLNc-associated Xirp2, even though the amount of FLNc-bound Xin protein fragment was highly increased at its highest concentration (Fig. 6).
Fig. 6.
Competition assays. Co-immunoprecipitation of T7-tagged FLNc d18–21 that was mixed with either 10 μm EEF-tagged Xin fragment (1686–1812) (A) or 10 μm myc-tagged Xirp2 fragment (2768–3327) (B) in the presence of increasing amounts (0 μm - 50 μm) of Xirp2 (A) or Xin (B), respectively. FLNc and coprecipitated Xin and Xirp2 fragments were detected using antibodies specific for their immunotags. Precipitated proteins and molecular mass standards are indicated by arrows and arrowheads, respectively. In most blots secondary antibodies also detected heavy and/or light chains of the T7-antibody. Note that increasing amounts of Xirp2 lessen but not completely impede binding of Xin to FLNc, and that Xin does not seem to reduce the efficiency of Xirp2 binding.
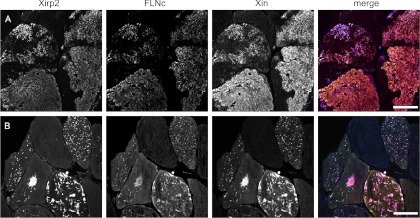
To analyze the localization of FLNc, Xin, and Xirp2, cryosections from a skeletal muscle biopsy from a filaminopathy patient were triple stained with antibodies against these proteins (Fig. 7). Colocalization was evident in all muscle fibers containing massive aggregates. In addition, the precise colocalization in fibers containing only very small aggregates, not only indicates that FLNc and both interacting Xirps colocalize already during early stages of aggregate formation, but also that they might serve as highly sensitive markers for early aggregate detection.
Fig. 7.
FLNc, Xin and Xirp2 colocalize in all protein aggregates observed in filaminopathy muscle fibers. Immunolocalization studies were performed on frozen serial sections of a skeletal muscle biopsy from a filaminopathy patient using antibodies directed against Xirp2, FLNc and Xin as indicated at the top. The proteins not only colocalize in large (A) but also in fibers containing very small aggregates (B). Bar: 50 μm.
DISCUSSION
The histological hallmarks of MFM are focal destruction of myofibrils and massive protein aggregation in skeletal muscle fibers. This is also true for filaminopathy, a subtype of MFM caused by mutations in FLNc. Better knowledge of the precise composition of protein aggregates in filaminopathy might help to unravel disease-specific pathomechanisms and to identify novel MFM candidate genes. In addition, it may help to establish specific biomarkers to differentiate filaminopathy from other MFM subtypes and protein aggregate myopathies.
Usually, accumulation of proteins in aggregates of MFM patients is investigated by immunohistochemistry or immunofluorescence (4, 26, 34). These analyses are mostly restricted to a limited number of proteins suspected to participate in protein aggregation, e.g. binding partners of the mutated proteins. Here, we applied a workflow consisting of laser microdissection and label-free spectral count-based proteomics not restricted to pre-selected proteins (22, 23, 35) with the ultimate goal to get new insights into aggregate composition in filaminopathy muscle samples. To our knowledge, it was the first time that this strategy was performed in immunostained skeletal muscle sections. Aggregates from six filaminopathy patients harboring three different mutations in Ig-like domains 7 and 24 of FLNc were collected and analyzed. Considering the rareness of filaminopathy, this number of patient samples is proportionally high. The comparison with control tissue from the same patients (parts of muscle fibers not harboring aggregates) allowed us to identify proteins not only present but even accumulating in aggregates.
About 400 proteins were identified in the patients' samples. A significantly higher spectral index in aggregates compared with control samples was true for 31 of these proteins. Of these 31 proteins FLNc, desmin, αB-crystallin, Xin, collagen VI, myotilin, and dysferlin have previously been identified by immunofluorescence analyses (4). Our studies confirm, without exception, accumulation of these proteins in aggregates, underlining the power of our proteomic approach. The remaining 24 proteins have not been studied in filaminopathy so far and give new insights in aggregate composition.
The accumulation of proteasome subunits in aggregates is consistent with results of subsequently performed immunohistochemical studies in filaminopathy (Kley et al., Brain, in press) and previous findings in other MFM subtypes (for review see (2)) indicating that protein degradation via the ubiquitin proteasome system plays an important role in pathogenesis of these diseases.
It is noteworthy that most of the proteins accumulated in aggregates are components of, or associated with the Z-disc. The finding that e.g. M-band proteins are decreased in aggregates argues against an involvement of the whole sarcomere in aggregate formation. This is consistent with ultrastructural studies that showed myofibrillar alterations mainly at the Z-disc level (4, 6). But even within the increasing list of Z-disc-associated proteins only a defined subset was consistently revealed: the known FLNc-interacting proteins N-RAP, obscurin and myotilin (19, 31, 36 and unpublished data) were enriched in aggregates, whereas α-actinin and its direct interactors such as myopodin (29, 37), were neither detected in aggregates by our proteomic approach, nor by immunofluorescence studies (unpublished data). This supports our proposal that the Z-disc contains two sets of proteins: one that is more strongly bound to FLNc and is prone to be involved in MFM, and a second group of proteins more firmly associated with α-actinin, probably involved in other myopathies (26).
The second highly abundant group of aggregated proteins includes intermediate filaments and associated proteins: desmin and its binding partners vimentin, nestin (38), syncoilin (39), nexilin (39) and the chaperone αB-crystallin (41). Intermediate filaments laterally interconnect adjacent myofibrils at the Z-disc level and link peripheral myofibrils to the sarcolemma and nuclei (for review see (42)). Taken together, results of both our proteomic and immunofluorescence analyses indicate that aggregates in filaminopathy contain mainly proteins interacting under physiological conditions. This observation suggests that initially aggregates are formed by mutated proteins that subsequently recruit their ligands (43).
As a logical continuation of this argument we further investigated whether we might identify novel FLNc ligands among the aggregate components. Xirp2 brought special interest because it was exclusively identified in aggregate samples with a high spectral index and shares its domain layout with Xin, a known FLNc ligand. Xirp2 did not only colocalize with FLNc in immunofluorescence but was also co-immunoprecipitating with FLNc domains 20–21 revealing Xirp2 as a novel FLNc binding partner. In skeletal muscle, Xirp2 interacts with α-actinin and was suggested to play a role in the regulation of cytoarchitectural integrity (44). Xirp2 shares many characteristics with Xin, and we here add the property that both proteins rely on the presence of the insertion in Ig-like domain 20 that is unique within the family of filamin proteins to interact with FLNc. Competition assays revealed that although increasing amounts of Xirp2 slightly reduce binding efficiency of Xin, increased association of Xin to FLNc does not affect binding of Xirp2 to FLNc. This indicates that although Xirp2 might bind FLNc stronger than Xin, both proteins can simultaneously associate with FLNc. The colocalization of all three proteins in small aggregates in muscle fibers of filaminopathy patients supports this observation. The precise role of FLNc, Xirp2, and Xin in the formation of protein aggregates, or in stabilizing disintegrating myofibrils will have to be investigated in future studies.
A further example of a protein enriched in aggregates, based on mass spectrometry and immunofluorescence microscopy (Kley et al., Brain, in press), was Hsp27. Previous yeast two hybrid screens using specific fragments of FLNc as baits had indicated Hsp27 as potential binding partner, but we first assumed this to be nonspecific (31 and unpublished data). In light of these novel results we re-evaluated this older finding and could indeed confirm binding of Hsp27 to FLNc (our unpublished data). These findings that resemble the identification of an interaction of FLNa with the closely related cardiovascular small heat shock protein cvHSP/HspB7 (45) may even be of therapeutic relevance: The small heat shock protein Hsp27 is a multifunctional protein acting as a powerful molecular chaperone in protein folding and preventing accumulation of aggregated proteins, but also facilitating degradation via the UPS (46, 47). Hsp27 forms oligomeric complexes with αB-crystallin (ABC; (48)), another known MFM gene (49, 50). Interestingly, ABC mutations alter its interaction with Hsp27 (51). Both in cell- and animal models of αB-crystallinopathy, overexpression of Hsp27 or treatment with the heat shock protein-inducing drug geranylgeranylacetone (GGA) reduced aggregate formation and improved cardiac function and survival (52, 53). It is therefore tempting to try to attenuate aggregate formation in filaminopathy by overexpressing Hsp27, thus opening new therapeutic options.
The third protein that we were able to confirm as novel aggregate component by immunofluorescence analysis is Rab35, a small GTPase with a function in actin dynamics [for review see (54)]. This finding may be of high relevance, because FLNa was shown to interact with the small GTPase RalA (55). It still has to be investigated, whether Rab35 is also present in aggregates of other MFM subtypes, or whether it is specific for filaminopathy. Its role in pathogenesis of filaminopathy is still unknown.
In contrast to proteins associated with aggregates, we found that the M-band proteins myomesin-1 and myomesin-2 are almost completely lacking in aggregates. Because both proteins were positive in aggregates in other MFM subtypes (26), they might be suited to serve as biomarkers to differentiate between MFM subtypes.
The fact that immunostaining confirmed both, proteins with a higher and proteins with a lower spectral index in aggregate samples compared with controls, strongly substantiates the reliability of our proteomic approach, and indicates that this method is an applicable tool for validation of proteomic results in MFM.
In reducing body myopathy (RBM), proteomic analysis of laser-microdissected intracytoplasmatic inclusions revealed FHL1 as the protein with the highest Mascot score (20). Subsequent studies demonstrated that mutations in FHL1 are causative for RBM. We therefore arranged our list of proteins accumulating in aggregates in the order of decreasing spectral index and also found that FLNc, the disease causing protein, was at the top. If this trend also holds true for other genetically proven forms of MFM subtypes, proteomic analysis of aggregate proteins might offer a valuable diagnostic tool for MFM patients with unknown mutation (currently about 60–70% of all MFM cases).
Although, the proteomic approach presented here is, in contrast to conventional genetic analyses, not limited to informative families, it clearly has its drawbacks. A grading of proteins solely by height of spectral index is only semiquantitative, because the MS-based analysis depends on the ionization of all the peptides derived from any protein. Moreover, differences in protein mass result in different numbers of peptides that can be identified by MS. Finally, the number of unique peptides, which were used for the calculation of the spectral index within this study, differs from protein to protein. Especially large protein families often result in a small number of unique peptides as a result of sequence homologies. These limitations are, however, less critical in case of interindividual comparisons, because the supposed bias is similar in samples processed in the same way. In our filaminopathy patients, we found a notably homogeneous pattern, independent from individual causative mutations (Supplemental Table S1). The top proteins were identical in all patients and the order of subsequent proteins was very similar, suggesting that different MFM-associated FLNc mutations lead to highly similar pathological protein aggregations. It has to be further verified by comparative analyses if other MFM subtypes also show a specific aggregate composition that differs from the one detected in filaminopathies.
In conclusion, compared with previous studies our approach enables the identification of an unprecedented number of components of highly insoluble pathologic protein aggregates within skeletal muscle fibers. A highly similar aggregate composition with high abundance of disease causing FLNc in patients with different FLNC mutations not only indicates reproducibility of the assay, but also identical pathomechanisms. Our data confirmed several previously identified aggregate components. In addition, many novel components were identified and in part verified as new filaminopathy markers by immunostaining. Some of these should be considered as new MFM candidate genes in so far unspecified MFM patients. Furthermore, novel binding partners of FLNc were identified.
The findings of our proteomic approach provide the basis for further biochemical and functional studies to elucidate the pathogenesis of filaminopathy and to generate strategies for treatment of this disease. There is a need for comparative studies to verify if other MFM subtypes show an aggregate composition different from that detected in our filaminopathy patients. This may simplify and fasten the diagnostic workup of MFM patients in the future. Specific protein signatures may also help to overcome a diagnostic dilemma: Genetic testing of MFM genes frequently reveals sequence changes that have not been observed in other patients before and are of unknown pathogenicity. This leads to uncertainty for counseling and management of these patients. The detection of a defined aggregate composition in a given patient that is typical for an MFM subtype caused by mutations in the gene in which a sequence change has been found may therefore strongly indicate a pathogenic mutation.
Supplementary Material
Acknowledgments
We thank the federal state North Rhine-Westphalia for funding within the project PURE. We thank the patients for participation in this study.
Footnotes
* This research was supported by grants from the Ruhr-University Bochum (FoRUM F680-2009 and F599R-2008), the German Research Foundation (KL 2487/1-1, FOR1228, and FOR1352), the German Ministry of Education and Research (BMBF, MD-NET, and 01GM0887), the Sophia and Fritz Heinemann Foundation and the Heimer Foundation.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- FA
- formic acid
- FHL1
- four and a half LIM domain 1
- LMD
- laser microdissection
- MFM
- myofibrillar myopathy
- RBM
- reducing body myopathy
- SI
- spectral index
- hsp
- heat shock protein
- (s)IBM
- (sporadic) inclusion body myositis
- FLNc
- filamin C
- ABC
- αB-crystallin
- RT
- room temperature
- PET
- polyethylene terephthalate
- FDR
- false discovery rate
- PSM
- peptide spectrum match
- BSA
- bovine serum albumin
- GGA
- geranylgeranylacetat.
REFERENCES
- 1. Selcen D. (2011) Myofibrillar myopathies. Neuromuscul. Disord. 21, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olivé M. (2009) Extralysosomal protein degradation in myofibrillar myopathies. Brain Pathol. 19, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrer I., Olivé M. (2008) Molecular pathology of myofibrillar myopathies. Expert. Revol. Med. 10, e25. [DOI] [PubMed] [Google Scholar]
- 4. Kley R. A., Hellenbroich Y., van der Ven P. F. M., Fürst D. O., Huebner A., Bruchertseifer V., et al. (2007) Clinical and morphological phenotype of the filamin myopathy: a study of 31 German patients. Brain 130, 3250–3264 [DOI] [PubMed] [Google Scholar]
- 5. Luan X., Hong D., Zhang W., Wang Z., Yuan Y. (2010) A novel heterozygous deletion-insertion mutation (2695–2712 del/GTTTGT ins) in exon 18 of the filamin C gene causes filaminopathy in a large Chinese family. Neuromuscul. Disord. 20, 390–396 [DOI] [PubMed] [Google Scholar]
- 6. Shatunov A., Olivé M., Odgerel Z., Stadelmann-Nessler C., Irlbacher K., van L. F., et al. (2009) In-frame deletion in the seventh immunoglobulin-like repeat of filamin C in a family with myofibrillar myopathy. Eur. J. Hum. Genet. 17, 656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vorgerd M., van der Ven P. F. M., Bruchertseifer V., Löwe T., Kley R. A., Schröder R., et al. (2005) A mutation in the dimerization domain of filamin c causes a novel type of autosomal dominant myofibrillar myopathy. Am. J. Hum. Genet. 77, 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fischer D., Kley R. A., Strach K., Meyer C., Sommer T., Eger K., et al. (2008) Distinct muscle imaging patterns in myofibrillar myopathies. Neurology 71, 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wattjes M. P., Kley R. A., Fischer D. (2010) Neuromuscular imaging in inherited muscle diseases. Eur. Radiol. 20, 2447–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capitanio D., Vasso M., Fania C., Moriggi M., Vigano A., Procacci P., et al. (2009) Comparative proteomic profile of rat sciatic nerve and gastrocnemius muscle tissues in ageing by 2-D DIGE. Proteomics 9, 2004–2020 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira R., Vitorino R., Alves R. M. P., Appell H. J., Powers S. K., Duarte J. A., et al. (2010) Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10, 3142–3154 [DOI] [PubMed] [Google Scholar]
- 12. Ohlendieck K. (2011) Skeletal muscle proteomics: current approaches, technical challenges and emerging techniques. Skelet. Muscle 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis C., Carberry S., Ohlendieck K. (2009) Proteomic profiling of x-linked muscular dystrophy. J. Muscle Res. Cell Motil. 30, 267–269 [DOI] [PubMed] [Google Scholar]
- 14. Thongboonkerd V., Kanlaya R., Sinchaikul S., Parichatikanond P., Chen S. T., Malasit P. (2006) Proteomic identification of altered proteins in skeletal muscle during chronic potassium depletion: Implications for hypokalemic myopathy. J. Proteome. Res. 5, 3326–3335 [DOI] [PubMed] [Google Scholar]
- 15. de Palma S., Morandi L., Mariani E., Begum S., Cerretelli P., Wait R., et al. (2006) Proteomic investigation of the molecular pathophysiology of dysferlinopathy. Proteomics 6, 379–385 [DOI] [PubMed] [Google Scholar]
- 16. Li J., Yin C., Okamoto H., Jaffe H., Oldfield E. H., Zhuang Z., et al. (2006) Proteomic analysis of inclusion body myositis. J. Neuropathol. Exp. Neurol. 65, 826–833 [DOI] [PubMed] [Google Scholar]
- 17. Sitek B., Luttges J., Marcus K., Kloppel G., Schmiegel W., Meyer H. E., et al. (2005) Application of fluorescence difference gel electrophoresis saturation labelling for the analysis of microdissected precursor lesions of pancreatic ductal adenocarcinoma. Proteomics 5, 2665–2679 [DOI] [PubMed] [Google Scholar]
- 18. Poschmann G., Sitek B., Sipos B., Ulrich A., Wiese S., Stephan C., et al. (2009) Identification of proteomic differences between squamous cell carcinoma of the lung and bronchial epithelium. Mol. Cell. Proteomics 8, 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu B. J. (2010). Combining laser capture microdissection and proteomics: methodologies and clinical applications. Proteomics. Clin. Appl 4, 116–γÇô123 [DOI] [PubMed] [Google Scholar]
- 20. Schessl J., Zou Y., McGrath M. J., Cowling B. S., Maiti B., Chin S. S., et al. (2008) Proteomic identification of FHL1 as the protein mutated in human reducing body myopathy. J. Clin. Invest 118, 904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reidegeld K. A., Eisenacher M., Kohl M., Chamrad D., Korting G., Bluggel M., et al. (2008) An easy-to-use Decoy Database Builder software tool, implementing different decoy strategies for false discovery rate calculation in automated MS/MS protein identifications. Proteomics 8, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 22. Spitzer P., Klafki H. W., Blennow K., Buee L., Esselmann H., Herruka S. K., et al. (2010) cNEUPRO: Novel Biomarkers for Neurodegenerative Diseases. Int. J. Alzheimers Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Müller T., Schrötter A., Loosse C., Helling S., Stephan C., Ahrens M., et al. (2011) Sense and nonsense of pathway analysis software in proteomics. J. Proteome Res. 10, 5398–5408 [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y., Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerfull approach to multiple testing. J. Roy. Statist. Soc. Ser. 57, 289–300 URL: http://www.jstor.org/stable/2346101 [Google Scholar]
- 25. Vinkemeier U., Obermann W., Weber K., Fürst D. O. (1993) The globular head domain of titin extends into the center of the sarcomeric M band. cDNA cloning, epitope mapping and immunoelectron microscopy of two titin-associated proteins. J. Cell Sci. 106, 319–330 [DOI] [PubMed] [Google Scholar]
- 26. Claeys K. G., van der Ven P. F. M., Behin A., Stojkovic T., Eymard B., Dubourg O., et al. (2009) Differential involvement of sarcomeric proteins in myofibrillar myopathies: a morphological and immunohistochemical study. Acta Neuropathol. 117, 293–307 [DOI] [PubMed] [Google Scholar]
- 27. Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl. K. (1995) Short protocols in molecular biology. New York: Wiley and Sons, Inc. [Google Scholar]
- 28. Obermann W. M. J., Gautel M., Steiner F., van der Ven P. F. M., Weber K., Fürst D. O. (1996) The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J. Cell Biol. 134, 1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linnemann A., van der Ven P. F. M., Vakeel P., Albinus B., Simonis D., Bendas G., et al. (2010) The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur. J. Cell Biol. 89, 681–692 [DOI] [PubMed] [Google Scholar]
- 30. Wehland J., Willingham M. C. (1983) A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. II. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of Golgi elements. J. Cell Biol. 97, 1476–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Ven P. F. M., Ehler E., Vakeel P., Eulitz S., Schenk J. A., Milting H., et al. (2006) Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp. Cell Res 312, 2154–2167 [DOI] [PubMed] [Google Scholar]
- 32. van der Ven P. F. M., Wiesner S., Salmikangas P., Auerbach D., Himmel M., Kempa S., et al. (2000) Indications for a novel muscular dystrophy pathway. gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin. J. Cell Biol 151, 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu S., Carroll S. L., Herrera A. H., Ozanne B., Horowits R. (2003). New N-RAP-binding partners alpha-actinin, filamin and Krp1 detected by yeast two-hybrid screening: implications for myofibril assembly. J. Cell Sci 116, 2169–2178 [DOI] [PubMed] [Google Scholar]
- 34. Selcen D., Engel A. G. (2004). Mutations in myotilin cause myofibrillar myopathy. Neurology 62, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 35. Müller T., Loosse C., Schrötter A., Schnabel A., Helling S., Egensperger R., Marcus K. (2011) The AICD interacting protein DAB1 is up-regulated in Alzheimer frontal cortex brain samples and causes deregulation of proteins involved in gene expression changes. Curr Alzheimer Res 8, 573–582 [DOI] [PubMed] [Google Scholar]
- 36. Dalkilic I., Thompson T. G., Brossius M. A., Puca A. A., Feener C., Muntoni F., et al. (2002) Obscurin interacts with skeletal muscle specific filamin. Journal of the Neurological Sciences 199, 82 [Google Scholar]
- 37. Faul C., Dhume A., Schecter A. D., Mundel P. (2007) Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol. Cell Biol 27, 8215–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinert P. M., Chou Y. H., Prahlad V., Parry D. A., Marekov L. N., Wu K. C., et al. (1999) A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J. Biol. Chem. 274, 9881–9890 [DOI] [PubMed] [Google Scholar]
- 39. Poon E., Howman E. V., Newey S. E., Davies K. E. (2002) Association of syncoilin and desmin: linking intermediate filament proteins to the dystrophin-associated protein complex. J. Biol. Chem. 277, 3433–3439 [DOI] [PubMed] [Google Scholar]
- 40. Hassel D., Dahme T., Erdmann J., Meder B., Huge A., Stoll M., et al. (2009) Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat. Med. 15, 1281–1288 [DOI] [PubMed] [Google Scholar]
- 41. Bennardini F., Wrzosek A., Chiesi M. (1992) Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ. Res. 71, 288–294 [DOI] [PubMed] [Google Scholar]
- 42. Capetanaki Y., Milner D. J., Weitzer G. (1997) Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct. Funct. 22, 103–116 [DOI] [PubMed] [Google Scholar]
- 43. Löwe T., Kley R. A., van der Ven P. F. M., Himmel M., Huebner A., Vorgerd M., et al. (2007) The pathomechanism of filaminopathy: altered biochemical properties explain the cellular phenotype of a protein aggregation myopathy. Hum. Mol. Genet. 16, 1351–1358 [DOI] [PubMed] [Google Scholar]
- 44. Huang H. T., Brand O. M., Mathew M., Ignatiou C., Ewen E. P., McCalmon S. A., et al. (2006) Myomaxin is a novel transcriptional target of MEF2A that encodes a Xin-related alpha-actinin-interacting protein. J. Biol. Chem. 281, 39370–39379 [DOI] [PubMed] [Google Scholar]
- 45. Krief S., Faivre J. F., Robert P., Le D. B., Brument-Larignon N., Lefrere I., et al. (1999) Identification and characterization of cvHsp. A novel human small stress protein selectively expressed in cardiovascular and insulin-sensitive tissues. J. Biol. Chem. 274, 36592–36600 [DOI] [PubMed] [Google Scholar]
- 46. Lanneau D., Wettstein G., Bonniaud P., Garrido C. (2010) Heat shock proteins: cell protection through protein triage. Sci. World J. 10, 1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kostenko S., Moens U. (2009) Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol. Life Sci. 66, 3289–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kato K., Goto S., Inaguma Y., Hasegawa K., Morishita R., Asano T. (1994) Purification and characterization of a 20-kDa protein that is highly homologous to alpha B crystallin. J. Biol. Chem. 269, 15302–15309 [PubMed] [Google Scholar]
- 49. Vicart P., Caron A., Guicheney P., Li Z., Prevost M. C., Faure A., et al. (1998) A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20, 92–95 [DOI] [PubMed] [Google Scholar]
- 50. Selcen D., Engel A. G. (2003) Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann. Neurol. 54, 804–810 [DOI] [PubMed] [Google Scholar]
- 51. Simon S., Fontaine J. M., Martin J. L., Sun X., Hoppe A. D., Welsh M. J., et al. (2007) Myopathy-associated alphaB-crystallin mutants: abnormal phosphorylation, intracellular location, and interactions with other small heat shock proteins. J. Biol. Chem. 282, 34276–34287 [DOI] [PubMed] [Google Scholar]
- 52. Chavez Zobel A. T., Loranger A., Marceau N., Theriault J. R., Lambert H., Landry J. (2003) Distinct chaperone mechanisms can delay the formation of aggresomes by the myopathy-causing R120G alphaB-crystallin mutant. Hum. Mol. Genet. 12, 1609–1620 [DOI] [PubMed] [Google Scholar]
- 53. Sanbe A., Daicho T., Mizutani R., Endo T., Miyauchi N., Yamauchi J., et al. (2009) Protective effect of geranylgeranylacetone via enhancement of HSPB8 induction in desmin-related cardiomyopathy. PLoS One 4, e5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chua C. E., Lim Y. S., Tang B. L. (2010) Rab35–a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett. 584, 1–6 [DOI] [PubMed] [Google Scholar]
- 55. Ohta Y., Suzuki N., Nakamura S., Hartwig J. H., Stossel T. P. (1999) The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. U. S. A. 96, 2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.