Abstract
Cancer vaccines based on human tumor associated antigens (TAA) have been tested in patients with advanced or recurrent cancer, in combination with or following standard therapy. Their immunogenicity and therapeutic efficacy has been difficult to properly evaluate in that setting characterized by multiple highly suppressive effects of the tumor and the standard therapy on the patient’s immune system. In animal models of human cancer, vaccines administered in the prophylactic setting are most immunogenic and effectively prevent cancer development and progression. We report results of a clinical study that show that in patients without cancer but with a history of premalignant lesions (advanced colonic adenomas, precursors to colon cancer), a vaccine based on the TAA MUC1 was highly immunogenic in 17/39 (43.6%) of vaccinated individuals, eliciting high levels of anti-MUC1 IgG and long-lasting immune memory. Lack of response in 22/39 individuals was correlated with high levels of circulating myeloid derived suppressor cells pre-vaccination. Vaccine-elicited MUC1-specific immune response and immune memory were not associated with any toxicity. Our study shows that vaccines based on human tumor associated antigens are immunogenic and safe and capable of eliciting long term memory that is important for cancer prevention. We also show that in the premalignant setting, immunosuppressive environment (e.g. high levels of MDSC) might already exist in some individuals, suggesting an even earlier premalignant stage or preselection of non-immunosuppressed patients for prophylactic vaccination.
Keywords: immunopevention, advanced adenoma, antibody, myeloid derived suppressor cells (MDSC), regulatory T cells (Treg)
Introduction
Colorectal cancer is under strong immune surveillance. The presence of tumor specific antibodies (1, 2) or infiltrating T cells in primary tumors can prolong time to disease recurrence and extend survival (3, 4). Immunosurveillance begins early in the neoplastic process as tumor-specific antibodies and T cells are found in subjects with premalignant adenomas (5, 6). A successful prophylactic colon cancer vaccine would boost or improve natural immune surveillance leading to elimination of premalignant lesions before their progression to malignant disease (7, 8).
Many candidate tumor-associated antigens have been identified for vaccines against cancer (9–11), including several for colon cancer (5, 12, 13). MUC1 glycoprotein is one such antigen (14, 15). In contrast to low level luminal or apical expression of the heavily glycosylated MUC1 on normal colonic epithelial cells, neoplastic cells express high levels of the hypoglycosylated form of MUC1 that lacks luminal polarity. This abnormal expression induces humoral and cellular immune responses (16–20). Abnormal expression of MUC1 is also found on premalignant colorectal adenomas where it promotes malignant transformation by interacting with β-catenin, ras and other tumor-promoting signaling pathways (21–24).
Ever since the first characterization of MUC1 as a tumor antigen (16) and successful cloning of the muc1 gene (25), MUC1 has been a promising candidate for vaccine-based interventions against human adenocarcinomas. Many different MUC1 vaccines such as MUC1 peptides with adjuvants, MUC1 loaded dendritic cells, or MUC1 DNA expressed in viral vectors have been tested in Phase I/II trials in patients with cancer who had failed standard therapy (26–33). These therapeutic vaccines were well tolerated, but only mildly immunogenic. In contrast, many of these same vaccines tested in the prophylactic setting in animal models (34–37) were highly immunogenic and resulted in immune protection against either transplantable or spontaneous MUC1+ tumors. To date, with the exception of the study we are reporting here, no cancer vaccine based on a tumor associated antigen has been tested in the prophylactic setting in humans.
In patients with cancer, it has been difficult to determine if the low vaccine immunogenicity is due to the wrong antigen choice (e.g. some TAA may be mostly self molecules and thus subject to self-tolerance), bad vaccine design (e.g. weak or ineffective adjuvant), the immunosuppressive tumor microenvironment, the immunosuppressive effect of previous therapy, patient circumstances such as advanced age, or a combination of some or all of the above.
We evaluated the immunogenicity of a MUC1 peptide vaccine in the absence of cancer by assessing the elicited immune response in the premalignant setting in individuals with a history of an advanced adenoma of the colon. Patients with advanced adenomas are at higher risk for subsequent colorectal cancer (38) and are recommended to undergo more frequent surveillance colonoscopy (39, 40). Because these patients do not have invasive cancer nor have they undergone immunosuppressive chemotherapy, the response to a vaccine could be assessed in the absence of these and other confounding factors that are present in patients with cancer.
The vaccine was immunogenic in 43.6% of subjects and capable of inducing long-term memory responses. A large number of responders provided the opportunity for comparison with non-responders (56.4%) for host-specific factors that control vaccine response. Non-responders had a significantly higher percentage of circulating myeloid derived suppressor cells (MDSC) prior to vaccination.
Materials and Methods
Subjects
All subjects provided informed consent and the study was monitored by Data Safety Monitoring Board of the Clinical Translational Science Institute of the University of Pittsburgh. The primary eligibility criteria included: 1) Age 40 – 70; 2) a history of an advanced colorectal adenoma(s) defined as: a) ≥ 1cm in size, or b) with villous or tubulovillous histology, or c) with high-grade dysplasia; 3) normal (within specified parameters) hemoglobin, liver and renal testing; and 4) ANA ≤1:160. Subjects were excluded if they had a history of a heritable cancer syndrome, autoimmune disease, or a malignancy within 5 years prior to the enrollment, excluding non-melanoma skin cancer. Subjects with use of corticosteroids within 12 weeks prior to enrollment or current or planned use of immunomodulators were excluded.
Peripheral blood mononuclear cells (PBMC) from healthy, age-matched, non-smoking donors were collected under a separate protocol via recruitment at community organizations and events.
Vaccine preparation and administration
A certified clinical grade 100-amino acid synthetic MUC1 peptide with the molecular structure of H2N-(GVTSAPDTRPAPGSTAPPAH)5-CONH2, was synthesized at the University of Pittsburgh Peptide Synthesis Facility. The adjuvant, toll like receptor (TLR) 3 agonist, Poly-ICLC (Hiltonol®), was supplied by Oncovir Inc. (Washington, DC) in single-dose vials of 1mL solution containing 2mg poly-IC, 1.5mg poly-L-lysine, and 5mg sodium carboxymethylcellulose in 0.9% sodium chloride, adjusted to pH 7.6–7.8 with sodium hydroxide. The vaccine consisted of 100 micrograms of the MUC1 100mer peptide dissolved in 50µL of sterile saline, admixed with 500µg of Hiltonol® in 250µL, for a total injection volume of 300µL. Vaccine was administered subcutaneously in the same upper thigh on each occasion. The vaccine received an Investigational New Drug (IND) approval from the FDA. The trial was registered at ClinicalTrial.gov with NCT-007773097.
Vaccine protocol
This was a phase I/II open label study to evaluate the immunogenicity (anti MUC1 IgG) of the 100mer MUC1 peptide with the adjuvant polyinosinic-polycytidylic acid stabilized with poly-L-lysine and carboxymethylcellulose (Poly-ICLC) (Hiltonol®), a toll-like receptor (TLR) 3 agonist (41). Vaccine was administered at week 0, 2 and 10. To assess memory response a booster dose was given at week 52. Subjects underwent blood draws immediately prior to each vaccination at week 2, 10, and 52, and post vaccination at week 12, 28 and 54.
Anti MUC1 IgG response was the main measure of vaccine immunogenicity because elicitation of IgG antibody requires activation not only of MUC1-specific B cells, but also of MUC1-specific helper T cells that promote anti-MUC1 antibody isotype switching from IgM to IgG. The preset criterion for considering subjects as responders to the vaccine was a ratio of anti-MUC1 IgG levels at week 12 to pre-vaccination levels at week 0 ≥2. This criterion was based on results previously obtained with the same or a similar vaccine in cancer subjects (27). Lacking examples from trials in cancer patients where vaccine-elicited memory responses could not be evaluated, the criterion for a positive memory response was arbitrarily set at a ratio of IgG levels at week 54 (two weeks post booster administration) to pre booster levels at week 52 of ≥2.
Monitoring for Adverse Events
The NCI common terminology criteria for adverse events (CTCAE3.0) were used to monitor toxicity. Laboratory monitoring including CBC, BUN, creatinine and liver function tests was performed at baseline, prior to each vaccine dose, and at week 28 and 54. A repeat ANA test was performed at week 52 prior to booster vaccination. Physical examination was performed at baseline and at week 52. Phone calls to subjects were made at week 6, 16 and 40.
Immunological assays
Immediately after collection, heparinized blood was centrifuged over a density gradient (Ficoll) to separate the plasma and PBMC. Plasma was collected, aliquoted and stored at −20°C. PBMC were washed several times, aliquoted, slowly frozen to −80°C in fetal bovine serum with 20% DMSO and stored in the vapor phase of liquid nitrogen.
Anti-MUC1 IgG was measured by Enzyme-Linked Immunosorbent Assay (ELISA) as previously published (32). Immulon 4 (Thermo-Fisher Scientific, MA) microtiter plates were coated overnight at 4°C with 1µg of synthetic MUC1 100mer peptide (vaccine antigen) dissolved in 0.9% Dulbecco phosphate buffered saline (PBS). Corresponding control plates received PBS but no antigen. The plates were washed three times with and incubated with 2.5% bovine serum albumin (BSA) in PBS (PBS-BSA) to fully coat the microtiter plate wells with protein and block non-specific binding. PBS-BSA was removed and plasma diluted in PBS-BSA was added to the wells. After one-hour incubation at room temperature the plates were washed five times with PBS with 0.1% tween-20 (Sigma-Aldrich, MO), and alkaline phosphatase-conjugated anti-human IgG secondary antibody (Sigma-Aldrich) in PBS-BSA was added. Following a one-hour incubation the plates were washed five times and the substrate, p-nitrophenyl phosphate (Sigma-Aldrich), was added to each well. The reaction was terminated after one hour by adding 0.5M NaOH. The results were read at OD 405nm on a spectrophotometer. The OD values from the control wells containing no antigen were subtracted from the OD values in test wells coated with peptide. Every sample was assayed multiple times at multiple dilutions, in at least triplicate wells.
For detecting myeloid derived suppressor cells (MDSC), PBMC were thawed and stained with APC-labeled mouse anti-human CD11b antibody (clone: ICRF44, BD Biosciences), PE-Cyanine 7 (PE-Cy7) labeled mouse anti human CD14 antibody (clone: M5E2, BD Biosciences), PE -labeled mouse anti human CD33 antibody (clone: WM53, BD Biosciences) and FITC-labeled mouse anti human HLA-DR antibody (clone: G46-6, BD Biosciences). MDSC were defined as CD11b+ CD33+/low HLA-DR−/low cells.
For the MDSC functional assay, MDSCs were depleted from PBMC with anti human CD15 antibody-conjugated MicroBeads and MACS MD separation column according to manufacturer’s instruction (Miltenyi Biotech, CA). PBMC (MDSC-depleted or not) were cultured in 96 well round bottom plates overnight in a CO2 incubator at 37°C and then the T cells were stimulated for 48 hours with anti-CD3 and anti-CD28 antibodies conjugated on beads (Dynabeads, Invitrogen Dynal, Oslo, Norway). Interferon gamma (INFγ) concentration in the cultures was measured using human IFNγ ELISA kit (BD Biosciences). Regulatory T cells were analyzed by flow cytometry for surface expression of CD4 and CD25 and intracellular expression of Foxp3. Previously frozen PBMC were first stained with fluoroisothyocyanate (FITC)-labeled mouse anti human CD4 antibody (clone: RPA-T4, BD Biosciences CA) and allophycocyann (APC)-labeled mouse anti-human CD25 antibody (clone: 2A3, BD Biosciences) and then stained for intracellular Foxp3 using the human Foxp3 buffer set (BD Biosciences) and phycoerythrin (PE)-labeled mouse anti-human Foxp3 antibody (clone: 236A/E7, BD Biosciences)
Statistical analysis
The association of two variables was assessed as follows: Fisher’s exact test for two categorical variables; Wilcoxon rank-sum test for one continuous and one dichotomous variable; Spearman rank-correlation test for two continuous variables. A two-sided p-value less than 0.05 was considered indicative of a true association, but no corrections were applied for multiple comparisons.
Results
MUC1 vaccine is immunogenic
Of the 46 subjects who consented to participate, 6 did not receive vaccine: four had abnormal screening laboratory tests, one did not meet criteria for an advanced adenoma, and one declined to participate. One patient dropped out after receiving the 1st injection because of travel distance, leaving a total of 39 evaluable subjects. The characteristics of the study subjects are presented in Table 1. The mean age was 58 and 55% were men. Most subjects met the criteria of having an advanced adenoma by having an adenoma that measured ≥1 cm. The median time between the most recent diagnosis of an advanced adenoma and receipt of the first dose of vaccine was 572 days (range 168 – 3499).
Table 1.
Characteristics of study subjects1
| Age: mean (range) | 58.0 (43.5–70.8) |
| Gender: N (%) | |
| Male | 22 (55) |
| Female | 18 (45) |
| Race: N (%) | |
| White | 36 (90) |
| Black | 3 (7.5) |
| Other | 1 (2.5) |
| BMI: mean (range) | 27.4 (18.1– 43.5) |
| Family History of CRC2 | 8 (20.0) |
| Advanced Adenoma3: N (%) | |
| Size ≥ 1 cm | 37 (77.5) |
| Tubulovillous/Villous | 18 (45) |
| High Grade Dysplasia | 6 (15) |
| Time from most recent advanced adenoma to receipt of vaccine, Days, median (mean, range) | 572 (824,168–3499) |
N=40 (includes one patient treated only on week 0)
In a first-degree relative
May meet more than one criterion
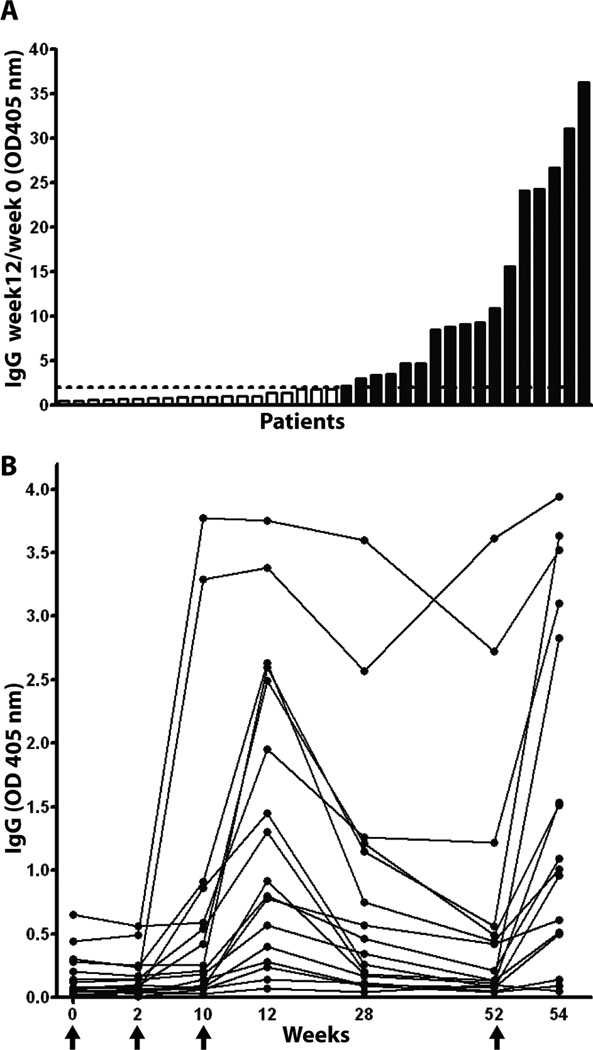
Figure 1A shows that week 12 IgG/prevaccination IgG ratio ≥ 2.0 was observed in 17/39 subjects (43.6%; range in ratio among responders: 2.2 to 36.3). Antibody generally began to appear after the second injection (measured at week 10), measured still higher at week 12, declined at week 28, and declined further at week 52 (Figure 1B). The antibody end-point titers at week 12 ranged from 1:320 to 1:2560 (Fig. S1.).
Fig. 1.
Vaccine elicited anti-MUC1 IgG responses. A. Ratio of week 12/week 0 anti MUC1 IgG in ascending order. Subjects with ratio >2 were considered responders (black bars) and those with ratio <2 were non-responders (white bar). Data are presented as OD 405 values for 1:40 dilution of plasma. Dashed line represents ratio of 2. B. Time and kinetics of anti MUC1 IgG development in responders. Vaccine was administered at Week 0, 2, 10 and 52 (arrows).
MUC1 vaccine-elicited immune response is safe
There were no adverse events above Grade 1 and vaccine administration was on schedule for all. Adverse events related to vaccination consisted of erythema experienced by 35/40 patients (87.5%), discomfort at the injection site in 32/40 (80%), and flu-like symptoms in 15/40 (37.5%), shown for the first injection. There was no association between these adverse events and response to the vaccine.
Of the 39 subjects who completed the protocol to week 52, two did not receive booster vaccine. One was found to have elevated anti-nuclear antibody (ANA) at week 52. Retesting of pre-vaccination serum at week 12 showed the ANA to have been elevated prior to vaccination and at all other time points along with SSA and Ro antigens, leading us to conclude that the initial immunofluorescence-based ANA test at enrollment was falsely negative. One patient at 11 months post vaccination developed clinical hypothyroidism with an elevated thyroid-stimulating hormone (TSH) of 27.2 (nl <5). Testing of serum pre-vaccination showed a TSH level of 3.5, however with significantly elevated thryroglobulin and thyroid peroxidase antibody levels, consistent with Hashimoto’s thyroiditis, leading us to conclude that the condition predated vaccination.
The vaccine elicited a memory response
A booster injection at 52 weeks to evaluate the long-term memory response was administered to 37 subjects. Of those who responded (ratio ≥2) at week 12 and received a booster injection at week 52, 12/16 (75.0%) had a response to the booster. Of the 4 subjects who did not respond to the booster, 3 had persistently high levels of antibody at week 52 (OD of 0.42, 2.72, and 3.61). One patient, although classified as a responder at week 12, had a low titer antibody response (.02 at baseline and .07 at week 12) and did not respond to the booster. Of the 21 subjects who were non-responders at week 12 and received a booster injection, two (9.5%) responded to the booster by increasing antibody levels at week 54 by two fold, however the antibody levels achieved were relatively low, and did not exceed OD of 0.21.
Response to the vaccine correlated with pre-vaccination levels of circulating myeloid derived suppressor cells (MDSC) but not T regulatory cells (Treg)
Comparing vaccine responders to non-responders (Table 1) we found no association of response with age (p=0.75), family history of CRC (p=1.0), BMI (p=0.37), the criterion for advanced adenoma (p=0.71 for size ≥ 1 cm, 0.75 for villous, and 0.68 for high-grade dysplasia) or with the length of time from adenoma removal to vaccination (p=0.94). Women were more likely to respond to the vaccine (11/18, 61%) than men (6/21, 29%), but this was of borderline significance (p=0.06). There was no association between response and HLA-DR (Table S.1) or DQ (Table S2) types, which were similar in frequency to general population.
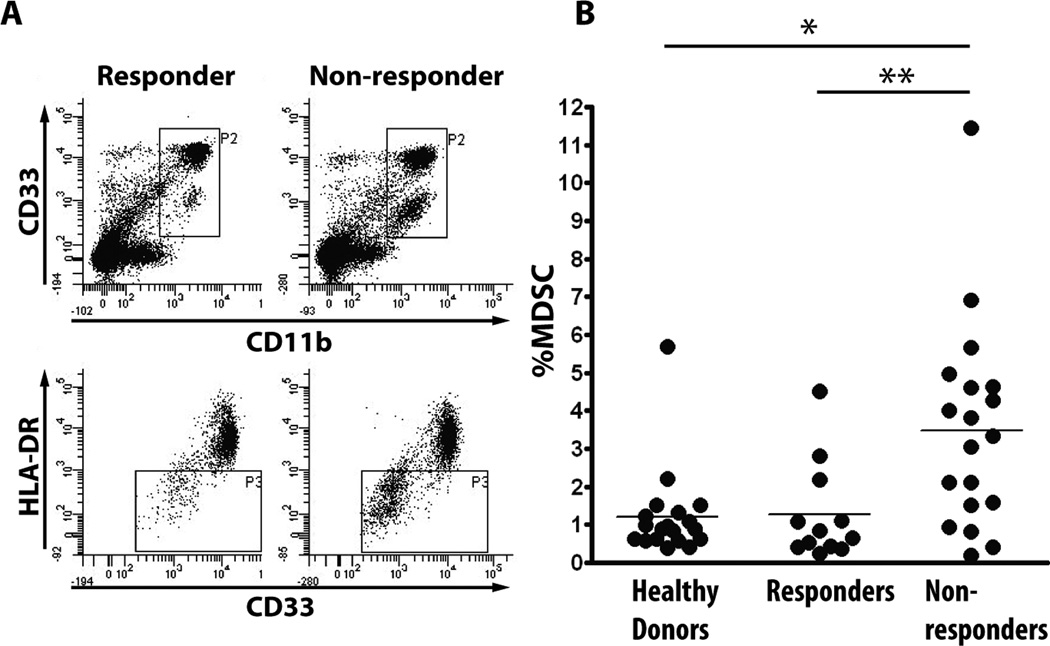
When analyzing subjects’ PBMC by flow cytometry (Fig. 2A) we observed a presence of a non-lymphoid cell population in non-responders that was very low or absent in responders. Phenotypic analysis identified these cells as CD11b+, CD33+/low and HLA-DR−/low myeloid derived suppressor cells (42). Non-responders (N=19) had a significantly higher percentage of these cells pre-vaccination compared to responders (N=12; p>0.05) whose MDSC levels were similar to healthy, age-matched controls (N=19) (Figure 2B).
Fig. 2.
PBMC of non-responders contain increased levels of myeloid derived suppressor cells (MDSC). A. Representative PBMC flow cytometry profile of a responder (left) and a non-responder (right) showing a difference in the CD33 +/low, CD11b+ and HLA-DR- cell populations (MDSC). B. MDSC percentage in PBMC of healthy donors (N=19) compared to pre-vaccination PBMC of vaccine responders (N=12) and vaccine non-responders (N=19). 9 patients were not evaluated due to insufficient number of PBMC. * <0.01, **<0.05
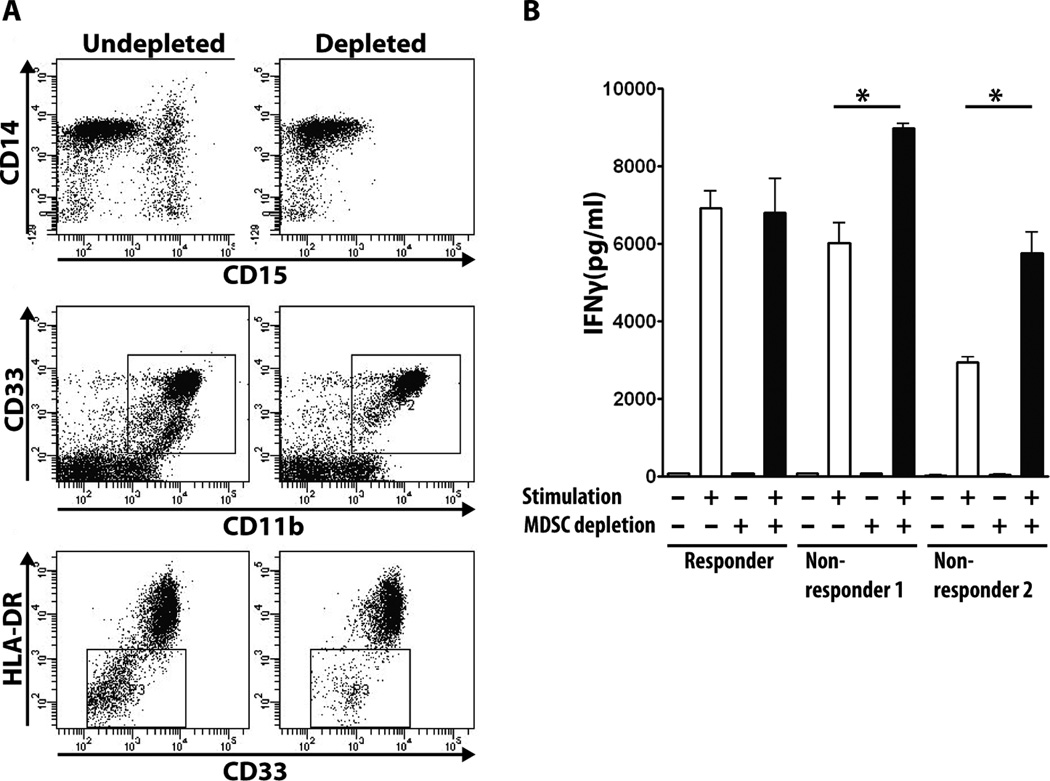
Abnormally high percentages of these cells had been described in the blood and at the tumor site in many different malignancies and correlated with high-level suppression of both innate and immune anti-tumor effector mechanisms (43). Their presence in the setting of premalignant disease, and especially in individuals with only a history of premalignant disease, had not been explored. We evaluated the functional consequence of increased MDSCs on the T cell effector function in 3 vaccinated subjects, one responder with low percent of MDSC and two non-responders with higher percent of MDSC, by measuring T cell responses to stimulation with anti-CD3 and anti-CD28 antibody before and after MDSC depletion from the PBMC. Fig. 3A shows successful depletion of MDSCs with magnetic beads conjugated to antibody against CD15, a cell surface fucosyl transferase expressed on MDSC (44, 45). Fig. 3B shows that T cells from the two non-responders produce significantly higher amounts of INF-γ after CD15+ MDSC depletion, whereas the same depletion procedure performed on the PBMC of a responder had no effect on the T cell response.
Fig. 3.
Depletion of MDSC improves T cell response. A. Representative flow cytometry result showing that depletion of CD15+ cells from PBMC removes the CD33+/low, CD11b+ HLA-DRlow MDSC population. B. IFNγ production by T cells stimulated with anti-CD3/anti-CD28 antibody before and after MDSC depletion from PBMC of one responder and two non-responders. MDSC depletion does not affect IFNγ production in responder but increases response in non-responders (* <0.01).
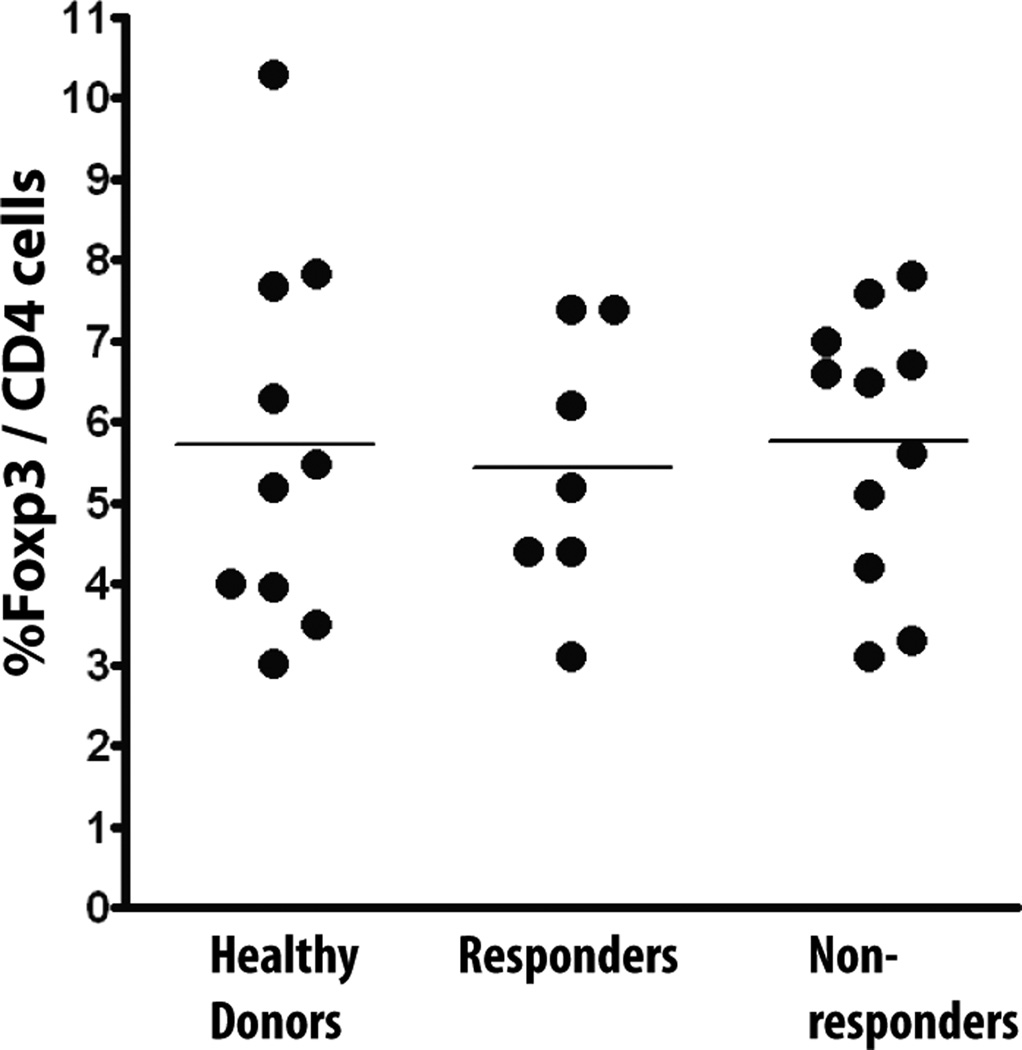
Regulatory T cells (Treg) (46) are another cell population known to suppress anti-tumor adaptive immunity, including in colon cancer (47). There were no differences in Tregs between responders and non-responders, and when the levels in both groups were compared to healthy controls there was no difference (Fig. 4).
Fig. 4.
Regulatory T cells (Treg) are not increased in vaccine non-responders. Percentage of Foxp3+ CD4 T cells analyzed by flow cytometry in healthy donors (N=10), vaccine responders (N=7) and vaccine non-responders (N=11).
Discussion
Immunoprevention of cancer through the use of cancer vaccines has the potential for non-invasive, non-toxic and, due to the specificity of the immune response and its long-term memory, prolonged protection. Vaccines based on viral antigens, such as HBV and HPV, are established approaches for prevention of liver and cervical cancer (48). We report the first experience with a cancer vaccine based not on a viral antigen but on a tumor-associated antigen administered to individuals without cancer. We tested the immunogenicity and safety of a MUC1 vaccine in subjects with a presumably healthy immune system. Various forms of MUC1 vaccine have been given to patients with MUC1+ tumors (49). With rare exceptions, those patients had failed multiple rounds of standard chemotherapy and had advanced recurrent disease. The diminished immune response to these vaccines was attributed to multiple factors, including tumor and therapy-induced suppression (50) and impaired T cell function (51). Lack of a strong response to MUC1 vaccines has also been attributed to self-tolerance to MUC1 antigen, demonstrated in a transgenic mouse model (52, 53). In our trial, nearly 44% of vaccinated subjects developed anti MUC1 IgG antibody. We had set the increase of 2-fold over pre-vaccination IgG levels as the criterion for response based on studying antibody responses in cancer patients receiving MUC1 vaccines (27, 32). In the absence of heterologous help, such as the often-used keyhole limpet hemocyanin (KLH) (30), helper T cell responses resulting in isotype switching by B cells from IgM to IgG were only rarely found. In our previous trial of a MUC1 100mer peptide plus adjuvant vaccine in patients with resected pancreatic tumors, only one of 16 patients (6.25%) developed IgG and only a 2.16-fold increase from prevaccination OD of 0.168 to post-vaccination OD of 0.368 (32). In contrast, in this trial in the premalignant setting, of the nearly 44% that responded, over 76% had greater than a 4-fold increase and over 47% had more than a 9-fold increase in antibody titer. Furthermore, the highest IgG OD value that we measured in the pancreatic cancer trial was 0.561 in one patient’s plasma at 1:20 dilution. In this study in the premalignant setting, the majority of responders had post-vaccination OD values above 10 at a plasma dilution of 1:40, the highest being an OD of 36.3. Of responders, over 76% had greater than a 4 fold increase and over 47% had more than a 9 fold increase in antibody titer.
Anti-MUC1 IgG levels measured at week 12, after the first three injections, in most patients decreased over time, as would be expected of a response to an antigen that is cleared from the system. We tested the ability of the vaccine to elicit a memory response by giving a booster injection at one year and the levels of IgG increased again. The response to the booster injection was another indication, in addition to isotype switching, that the vaccine had elicited a T cell response and T cell memory participating in the response to the booster injection.
Importantly, development of high titer of anti-MUC1 antibodies was not associated with significant adverse events. In particular, we observed no incidence of clinical autoimmune disease developing subsequent to vaccination and there was no increase in ANA titer over 1 year of observation. The inability to induce a significant anti-MUC1 immune response in cancer patients made the safety of a high titer response impossible to assess. In this trial, even the subjects that had over a 30-fold increase in IgG titer had no evidence of clinical adverse effect. We have reported previously that healthy individuals can have an immune response against MUC1 (measured by anti-MUC1 IgG levels) presumably elicited via exposure to abnormal forms of MUC1 induced by acute inflammatory conditions, such as mastitis or mumps. Anti-MUC1 immunity in these individuals correlated with positive outcomes, such as lower risk of cancer, rather than being detrimental (54–57). While the beneficial clinical effects of the MUC1 vaccine will need to be tested in future randomized trials, our results here clearly show that the vaccine-elicited anti-MUC1 immune response is not seemingly detrimental to the patient’s overall health.
Our study was carried out in a population at increased risk for colorectal cancer by virtue of having a history of advanced adenoma. We expected that these subjects would not harbor the same immunosuppressive environment as cancer patients, such as increased numbers of immunosuppressive regulatory T cells (58). Surprisingly, we observed significantly higher levels of MDSCs in pre-vaccination PBMC in subjects who did not respond to the vaccine compared to those who did. Abnormally high percentages of these cells had been described in the blood and at the tumor site in many different malignancies and correlated with high-level suppression of both innate and immune anti-tumor effector mechanisms (43). Higher levels of MDSC in the setting of premalignant disease, and especially in individuals with only a history of premalignant disease, has not been previously explored or described. Increased MDSCs, like T regulatory cells, have been primarily associated with advanced cancer (43) and some chronic infections (59, 60) where they have been shown to suppress adaptive immunity by producing arginase 1, inducible nitric oxide synthase (iNOS), nitric oxide (NO) and reactive oxygen species (ROS)(42, 45). In mice MDSCs increase during the development of spontaneous inflammatory bowel disease (IBD)(37, 61) and pancreatic cancer (62). Increased MDSCs have also been reported in humans with IBD (61). It is not known if development of IBD or premalignant polyps causes an increase in MDSC or is preceded by an increase in MDSC. We also don’t know why some subjects with advanced adenoma have significantly increased levels of MDSC and others do not. What is clear, however, is that a response to the vaccine is compromised by the presence of MDSC. Further research on a much larger sample may establish MDSCs as biomarkers for selection of subjects who are likely to respond to vaccines or other forms of immunotherapy that depend on activating endogenous immunity.
Cancer vaccines given in the therapeutic setting are beginning to assume greater role in the overall care of cancer patients and despite compromised immunogenicity in advanced disease, there is evidence from Phase III clinical trials of their positive impact on disease free and overall survival in prostate cancer (63), melanoma (64) and follicular lymphoma (65). Ours is the first study to administer a cancer vaccine against a tumor-associated antigen in the prophylactic setting, in subjects at high risk for subsequent malignancy. The vaccine proved to be highly immunogenic, much more so than has been previously observed when similar type vaccines were administered to cancer patients, raising hope that this increased immunogenicity will translate to a highly effective anti-tumor response. The vaccine was well tolerated without evidence of autoimmunity in these immunocompetent hosts. Unlike the majority of peptide vaccines that are restricted to one or only a limited number of HLA types, MUC1 100mer peptide was immunogenic in individuals of most HLA-DR and HLA-DQ types precluding the need for HLA-typing prior to vaccination. Immunogenicity of the vaccine can be monitored by a simple and inexpensive ELISA for MUC1 specific IgG. Subsequent studies will include evaluation of whether the vaccine can impact a clinical endpoint, such as reducing adenoma recurrence leading to colon cancer prevention.
Supplementary Material
Acknowledgments
Financial support: This study was funded by the NCI grant P01 CA73743 to OJF and RES, NIH grants UL1RR024153 and UL1TR000005 and by The Nathan Arenson Fund.
Footnotes
Conflicts of interest: none
References
- 1.Nakamura H, Hinoda Y, Nakagawa N, Makiguchi Y, Itoh F, Endo T, et al. Detection of circulating anti-MUC1 mucin core protein antibodies in patients with colorectal cancer. J Gastroenterol. 1998;33:354–361. doi: 10.1007/s005350050096. [DOI] [PubMed] [Google Scholar]
- 2.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 5.Nagorsen D, Thiel E. Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin Cancer Res. 2006;12:3064–3069. doi: 10.1158/1078-0432.CCR-05-2788. [DOI] [PubMed] [Google Scholar]
- 6.Silk AW, Schoen RE, Potter DM, Finn OJ. Humoral immune response to abnormal MUC1 in subjects with colorectal adenoma and cancer. Mol Immunol. 2009;47:52–56. doi: 10.1016/j.molimm.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn OJ, Forni G. Prophylactic cancer vaccines. Curr Opin Immunol. 2002;14:172–177. doi: 10.1016/s0952-7915(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 8.Finn OJ. Premalignant lesions as targets for cancer vaccines. J Exp Med. 2003;198:1623–1626. doi: 10.1084/jem.20031787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 10.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 11.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosolits S, Ullenhag G, Mellstedt H. Therapeutic vaccination in patients with gastrointestinal malignancies. A review of immunological and clinical results. Ann Oncol. 2005;16:847–862. doi: 10.1093/annonc/mdi192. [DOI] [PubMed] [Google Scholar]
- 13.Silk AW, Finn OJ. Cancer vaccines: a promising cancer therapy against all odds. Future Oncol. 2007;3:299–306. doi: 10.2217/14796694.3.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Turner MS, McKolanis JR, Ramanathan RK, Whitcomb DC, Finn OJ. Mucins in gastrointestinal cancers. Cancer Chemother Biol Response Modif. 2003;21:259–274. doi: 10.1016/s0921-4410(03)21012-7. [DOI] [PubMed] [Google Scholar]
- 15.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 16.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993;151:1654–1662. [PubMed] [Google Scholar]
- 18.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–2860. [PubMed] [Google Scholar]
- 19.Graham RA, Burchell JM, Taylor-Papadimitriou J. The polymorphic epithelial mucin: potential as an immunogen for a cancer vaccine. Cancer Immunol Immunother. 1996;42:71–80. doi: 10.1007/s002620050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiltbold EM, Ciborowski P, Finn OJ. Naturally processed class II epitope from the tumor antigen MUC1 primes human CD4+ T cells. Cancer Res. 1998;58:5066–5070. [PubMed] [Google Scholar]
- 21.Ajioka Y, Watanabe H, Jass JR. MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. J Clin Pathol. 1997;50:417–421. doi: 10.1136/jcp.50.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho SB, Ewing SL, Montgomery CK, Kim YS. Altered mucin core peptide immunoreactivity in the colon polyp-carcinoma sequence. Oncol Res. 1996;8:53–61. [PubMed] [Google Scholar]
- 23.Zotter S, Lossnitzer A, Hageman PC, Delemarre JF, Hilkens J, Hilgers J. Immunohistochemical localization of the epithelial marker MAM-6 in invasive malignancies and highly dysplastic adenomas of the large intestine. Lab Invest. 1987;57:193–199. [PubMed] [Google Scholar]
- 24.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Gendler SJ, Burchell JM, Duhig T, Lamport D, White R, Parker M, et al. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987;84:6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 30.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 31.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulieres D, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 32.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- 33.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acres B, Apostolopoulos V, Balloul JM, Wreschner D, Xing PX, Ali-Hadji D, et al. MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–594. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pecher G, Finn OJ. Induction of cellular immunity in chimpanzees to human tumor-associated antigen mucin by vaccination with MUC-1 cDNA-transfected Epstein-Barr virus-immortalized autologous B cells. Proc Natl Acad Sci U S A. 1996;93:1699–1704. doi: 10.1073/pnas.93.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barratt-Boyes SM, Vlad A, Finn OJ. Immunization of chimpanzees with tumor antigen MUC1 mucin tandem repeat peptide elicits both helper and cytotoxic T-cell responses. Clin Cancer Res. 1999;5:1918–1924. [PubMed] [Google Scholar]
- 37.Beatty PL, Narayanan S, Gariepy J, Ranganathan S, Finn OJ. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev Res (Phila) 2010;3:438–446. doi: 10.1158/1940-6207.CAPR-09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc. 71:111–117. doi: 10.1016/j.gie.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-Cancer Incidence and Mortality with Screening Flexible Sigmoidoscopy. N Engl J Med. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med. 208:2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 35:107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 44.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 45.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Boussiotis VA. Molecular and functional heterogeneity of T regulatory cells. Clin Immunol. 141:244–252. doi: 10.1016/j.clim.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, et al. Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. doi: 10.1136/gutjnl-2011-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanwar RK, Singh N, Gurudevan S, Kanwar JR. Targeting hepatitis B virus and human papillomavirus induced carcinogenesis: novel patented therapeutics. Recent Pat Antiinfect Drug Discov. 6:158–174. doi: 10.2174/157489111796064560. [DOI] [PubMed] [Google Scholar]
- 49.Tang CK, Katsara M, Apostolopoulos V. Strategies used for MUC1 immunotherapy: human clinical studies. Expert Rev Vaccines. 2008;7:963–975. doi: 10.1586/14760584.7.7.963. [DOI] [PubMed] [Google Scholar]
- 50.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 53.Ryan SO, Turner MS, Gariepy J, Finn OJ. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 70:5788–5796. doi: 10.1158/0008-5472.CAN-09-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cramer DW, Titus-Ernstoff L, McKolanis JR, Welch WR, Vitonis AF, Berkowitz RS, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–1131. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 55.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 23:265–271. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cramer DW, Vitonis AF, Pinheiro SP, McKolanis JR, Fichorova RN, Brown KE, et al. Mumps and ovarian cancer: modern interpretation of an historic association. Cancer Causes Control. 21:1193–1201. doi: 10.1007/s10552-010-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terry KL, Titus-Ernstoff L, McKolanis JR, Welch WR, Finn OJ, Cramer DW. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:30–35. doi: 10.1158/1055-9965.EPI-06-0688. [DOI] [PubMed] [Google Scholar]
- 58.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 59.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 55:343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 207:1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. 81, e1–e5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 62.Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, et al. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology. 2009;128:141–149. doi: 10.1111/j.1365-2567.2009.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 64.Schwartzentruber DJ, Lawson MD, Richards JM, Conrey RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination with patient-specifci tumor-derived antigen in first remission imporves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.