Abstract
It has been proposed that working memory (WM) is updated/manipulated via a fronto-basal-ganglia circuit. One way that this could happen is via the synchronization of neural oscillations. A first step towards testing this hypothesis is to clearly establish a frontal scalp EEG signature of WM manipulation. Although many EEG studies have indeed revealed frontal EEG signatures for WM, especially in the theta frequency band (3–8 Hz), few of them required subjects to manipulate WM, and of those that did, none specifically tied the EEG signature to the manipulation process per se. Here we employed a WM manipulation task that has been shown with imaging to engage the prefrontal cortex and the striatum. We adapted this task to titrate the success of WM manipulation to approximately 50%. Using time-frequency analysis of EEG, we showed that theta power is increased over frontal cortex for successful versus failed WM manipulation, specifically at the time of the manipulation event. This establishes a clear-cut EEG signature of WM manipulation. Future studies could employ this to test the fronto-basal-ganglia hypothesis of WM updating/manipulation.
Keywords: EEG, theta oscillations
Introduction
‘Working memory’ (WM) is a neurocognitive system that includes short-term storage/maintenance and executive operations, such as updating/manipulating mnemonic contents (Baddeley 2003). An influential theory proposes that WM updating/manipulation (hereafter ‘manipulation’ only) is orchestrated by communication between the prefrontal cortex and the basal ganglia. More specifically, it is proposed that the basal ganglia is a gate that allows WM representations in prefrontal cortex to be either maintained or changed (O’Reilly and Frank 2006; Hazy, Frank and O’Reilly 2007; Frank et al. 2001). Consistent with this, recent neuroimaging studies have pointed to frontal and striatal regions underlying WM manipulation (Bäckman et al. 2011; Dahlin et al. 2008; Lewis et al. 2004). However, these imaging studies are limited as tests of the fronto-basal ganglia hypothesis because they do not reveal the temporal dynamics. By contrast, time-frequency analysis of EEG can reveal the neural oscillations underlying WM manipulation with temporal precision. Identifying neural oscillations is important because they can reflect long-distance neural communication (Fries 2006) if they are in synchrony.
Going back more than a decade, frontal-midline theta oscillations (~3–8 HZ) have often been observed in EEG studies employing WM tasks, which may have included the manipulation process, but were not designed to isolate it (Gevins et al. 1997; Jensen and Tesche 2002; Mizuhara et al. 2004; Sauseng et al. 2005; Klimesch et al. 2008; Onton et al. 2005; Pesonen et al. 2007). Moreover, tasks other than WM but also involving executive functions of the frontal cortex (e.g., conflict/error monitoring) also elicit frontal-midline theta oscillations (Cavanagh et al., 2009; Cavanagh et al., 2010; Cavanagh et al., 2012; Luu and Tucker, 2001; Luu et al., 2003; Luu et al. 2004). Taking both of these together, it is thus possible that the frontal-midline theta oscillations in WM tasks partly reflect the executive operation of WM manipulation. Consistent with this, three recent studies that required subjects to manipulate WM contents revealed frontal-midline theta (Dieber et al., 2007; Griesmayr et al. 2010; Kawasaki et al. 2010). However, none of these studies [discussed below] specifically related the theta oscillations to the cognitive manipulation process per se.
Dieber et al. (2007) reported sustained increase in frontal-midline theta activity for a 2-back WM task compared to a 1-back. Since the N-back task requires many WM processes, including actively maintaining a sequence of previously presented items, matching the current item to the N-back item, and recreating a new to-be-maintained sequence, it is difficult to know if the increased theta activity represented an increase in mere maintenace load rather than manupulation per se. Kawasaki et al. (2010) used a task in which subjects had to manipulate relevant features of a mental object. They also found frontal-midline theta, but it was not clear if this was related to increased maintenance load over time rather than manipulation. Further, the way the task was designed did not allow a trial-specific index of manipulation success. Griesmayr et al. (2010) used a task requiring manipulation of letter order, also finding a frontal-midline theta increase. Yet, they interpreted this as a delayed maintenance/rehearsal process because its timing was later than expected. Notably, none of these studies specifically related the theta oscillations to the behavioral success of the WM manipulation. Therefore, the current study aimed to overcome these limitations, moreover for a task that already implicates both frontal cortex and basal ganglia–making it suitable for future investigation of fronto-stratial communication underlying WM manipulation.
We adapted a letter-memory manipulation task previously used in human neuroimaging studies (Bäckman et al. 2011; Dahlin et al. 2008). Each trial contained two delay periods—delay period 1 required only maintenance of the letter sequence and delay period 2 required either mere maintenance or manipulation. This allowed us to (1) examine an oscillatory pattern of EEG during maintenance alone and (2) directly compare manipulation to maintenance, which occur at the same time point relative to the trial onset, thus preventing confounds that might be caused by increased mere maintenance demand over time. Importantly, we carefully titrated individual subject performance to a ~50% success rate of WM manipulation (unlike any of the previous studies), thus allowing the specific comparison between successful and unsuccessful manipulation trials. If theta oscillations are a signature for successful WM manipulation, we predicted that increased theta power for WM manipulation would occur in a temporal window that is distinct from that of mere maintenance. Importantly, we also expected to see increased theta power for successful versus unsuccessful manipulation.
Materials and Methods
Subjects
Seventeen neurologically healthy young adults were recruited from the University of California, San Diego (UCSD) community to participate in the study. All participants provided written informed consent in accord with the human subjects Institutional Review Board at UCSD and were compensated at $15/hour. Four subjects were excluded due to excessive eye or head movements, leaving 13 subjects in the analysis (6 female, all right-handed, 18–29 years; age = 22 years +/− 4).
Apparatus, task, and procedure
Each trial started with an encoding cue (a green fixation) for 500 ms (Figure 1). Four letters then sequentially appeared, each for 300 ms, followed by a 200-ms blank screen. The letters were randomly selected from 13 Latin consonants: B, D, F, H, J, L, N, P, R, T, V, X, and Z (not alphabetically adjacent to prevent the formation of words or orderly alphabetic patterns). A white fixation then appeared for 1500 ms (delay period 1). The task instructions emphasized maintenance of the sequence during this delay. Then, a fifth item was displayed for 300 ms plus a 200-ms blank screen. If it was a symbol (#, %, *, or &), subjects maintained the sequence (no manipulation); if it was a new letter item, they generated a new sequence containing the last three letters from the encoding phase plus the new letter item (manipulation). There followed delay period 2 for 1500 ms. A response cue (a blue fixation) then appeared for 300 ms, followed by a prompt (response mapping) that contained four letters in a row (in random order relative to the actual sequence to obviate a motor plan). Subjects indicated the actual sequence, in the correct order, with the right hand. For this response-sequence method, the probability of getting the entire letter sequence correct by chance is a mere.0039 (1/4*1/4*1/4*1/4). The inter-trial interval was 400 ms. All letters and symbols spanned ~3.0°×4.0 °. The space in-between four letters in the response mapping was ~0.5°.
Figure 1.
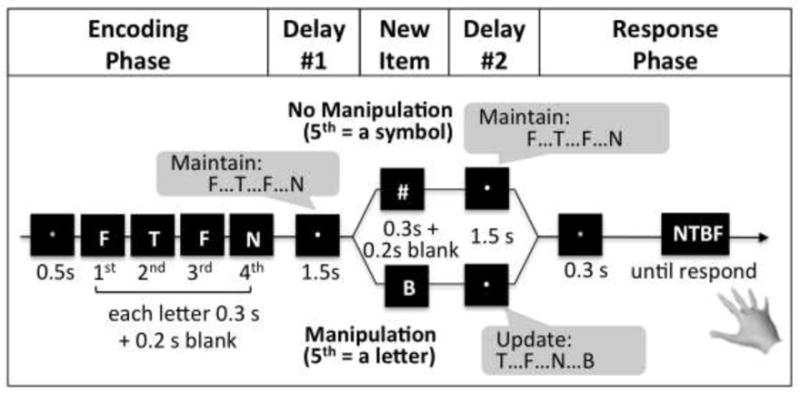
The letter-memory manipulation task. The encoding cue (a green fixation) appeared at the beginning of each WM trial, followed by a sequence of four letters. Subjects encoded the four letters one by one and maintained them in their WM during delay period 1, during a white fixation. In the no manipulation condition a non-letter symbol appeared, and subjects continued maintaining the same letter sequence throughout delay period 2. In the manipulation condition, a new letter item appeared right after delay period 1; subjects then incorporated the new item to recreate a new sequence of the last four letters they saw. After delay period 2, the response cue (blue fixation) appeared followed by a prompt. Subjects indicated the actual sequence, in the correct order, with the right hand.
Before EEG, a training phase varied the number of letter ‘types’ within a 4-letter sequence between 1 and 4 letter types (e.g., during the encoding phase subjects might see ‘BXBX’ and ‘BXNJ’ for 2 and 4 letter types, respectively). This procedure identified the item type number that yielded a 50% manipulation error rate. There were 8 trials per 1, 2, 3, and 4 item types for each of manipulation and no manipulation. The number of letter types yielding ~50% error rate in the manipulation condition was fixed for the EEG experiment. Two subjects had 2 letter types, 4 subjects had 3 letter types, and 7 subjects had 4 letter types. For the EEG session there were 10 runs of the task, with 20 no manipulation and 20 manipulation trials per run, for 400 trials in total.
EEG recording
We used a 32-channel Bio-semi ActiveTwo system (Biosemi Instrumentation), with a sampling rate of 512 Hz. Additional electrodes were placed on the mastoids, and above, below and lateral to the eye.
EEG preprocessing
We used EEGLAB9.0.0.2b (Delorme and Makeig 2004). We re-referenced the data to the mastoids, applied 0.5 Hz high-pass filtering to attenuate drift, and removed non-stereotypic artifacts (based on data improbability and visual inspection), and eye movements using independent component analysis.
EEG analysis
First, single subject data were wavelet-filtered by Gaussian filter of a 0.35-Hz bandwidth into 100 frequencies (from 1–100 Hz), yielding an analytic amplitude signal for each frequency (Canolty et al., 2007; Grossmann and Morlet, 1985, Roach and Mathalon, 2008). Second, single-subject data were time-locked to the first letter, averaged for each WM condition, and baseline-corrected from 0 ms to 400 ms before the first letter. Third, the epoched data were averaged across subjects separately for each condition: correct no manipulation, correct manipulation, and incorrect manipulation. [For incorrect no manipulation, there were too few trials]. One-sample t-tests examined the significance of each time-frequency point compared to baseline. Fourth, between-condition differences were computed (correct manipulation vs. correct no manipulation and correct manipulation vs. incorrect manipulation) for individual subjects, averaged across subjects, and evaluated with paired t-tests.
For each contrast, multiple comparisons were corrected using the False Discovery Rate method (Benjamini and Hochberg, 1995), ranging across all frequencies and from 400 ms before to 9000 ms after the onset of the first letter. The corrected α value was p < 0.05. [Note, although analysis was performed for all channels, we mainly focus on Fz, which had the strongest theta response in topography maps.]
Results
Behavioral results
All subjects significantly outperformed the chance level (0.39%) of getting the entire letter sequence correct for both no manipulation (percentage of correct trials ranged from 56.0%−89.5%, t(12) = 20.86, p<0.001) and manipulation conditions (percentage of correct trials ranged from 26.5%−74.0%, t(12) = 11.88, p<0.001). Mean accuracy was higher for no manipulation (72.67% correct, SD = 12.51%) than manipulation (54.15% correct, SD = 16.30%, t(12) = 5.83, p < 0.001). RTs for correct responses were significantly longer for correct manipulation (Mean = 3303 ms, SD = 661 sec) than correct no manipulation (Mean = 2674 ms, SD = 398 ms), t(12) = 5.30, p<0.001). However, RTs for correct vs. incorrect manipulation (Mean = 3314 ms, SD = 615 ms) were not significantly different (t(12) < 1, n.s.), suggesting that subjects attempted to manipulate a new letter sequence although they failed. Importantly, the number of incorrect manipulation trials (46.85%, SD = 16.30%) was almost equal to the number of correct manipulation trails (54.15%, SD = 16.30%, t(12) < 1, n.s.), allowing an unbiased EEG comparison.
EEG results
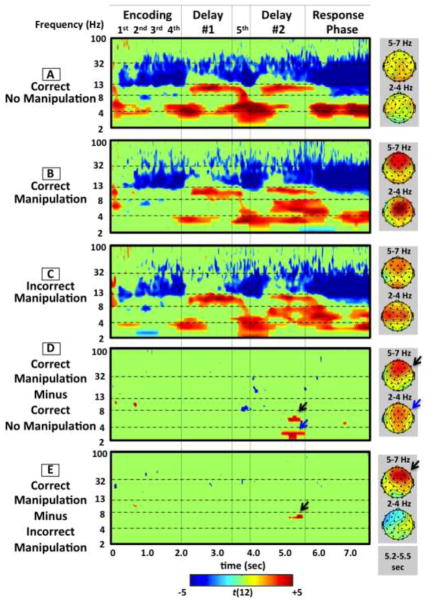
Although our main focus is manipulation during delay period 2, we report results from all phases to compare oscillatory components that possibly differ across encoding, maintenance, manipulation, and response periods (Figure 2A–E). The figure depicts t-scores thresholded to show only significant changes from baseline (t(12) > 2.18, p < 0.05) (Figure 2A–C) or significant between-condition differences (t(12) > 3.78, p < 0.05) (Figure 2D–E). Red and blue show significant increases and decreases in spectral power respectively. Green represents t-values below the FDR-corrected threshold (i.e., n.s.).
Figure 2.
Event-related time frequency plots for correct manipulation (a), correct no manipulation (b), incorrect manipulation (c), correct manipulation versus correct no manipulation contrast (d), and correct versus incorrect manipulation contrast (e). Data in each panel depict t-scores thresholded at p < 0.05 FDR-corrected across all frequencies and time points. Red and blue colors show significant increases and decreases in spectral power respectively. Green depicts t-values below the FDR-corrected threshold (i.e., n.s.). Scalp topographies are shown for high theta and low theta frequencies for the 5.25-to−5.50-second window corresponding to between-condition differences.
Encoding period
For each WM condition (Figures 2A–C), there were significant below-baseline decreases in beta power (~ 13–30 Hz) occurring ~400 ms after each letter, presumably signifying stimulus perception/encoding. Importantly, there were no significant between-condition differences (Figures 2D,E), ensuring that any differences in delay period 2 are not confounded.
Delay period 1
For each WM condition, there was a significant increase in low theta power (~3–5Hz), peaking at the onset of delay period 1 (~500 ms after the fourth letter) and lasting ~500 ms. There followed significantly increased alpha power (~9–12 Hz), starting from ~300 ms and lasting to ~1800 ms after delay onset. Importantly, there were no significant between-condition differences (Figures 2D,E), ensuring that any differences in delay period 2 are not confounded.
Delay period 2
Again, there was a significant decrease in beta power ~400 ms after the fifth item (either symbol or letter) for each WM condition, presumably reflecting stimulus perception/encoding. For the correct no manipulation condition (Figure 2A), there was a similar oscillatory pattern as for delay period 1, i.e. significant increased low theta power (~3–5 Hz) followed by significantly increased alpha power. For the correct manipulation condition, the pattern was similar at first (Figure 2B); however, critically, the increased low theta power (~3–4 Hz) lasted 375ms longer than the correct no manipulation condition (probably reflecting an extended/more difficult WM transfer) (this is statically confirmed by the significant difference between manipulation and no manipulation in Figure 2D, a blue arrow). There was also additional increased theta power at the typical range (~5–8 Hz) from 714–2300ms after the onset of delay period 2. For the between-condition contrast of correct no manipulation and manipulation, these differences were significant from ~1000 to ~1500 ms after delay period 2 onset (Figure 2D, black and blue arrows, respectively). To demonstrate the exact time-course of the power differences for manipulation and no manipulation conditions, we extracted averaged signal change separately for the low theta (2–4Hz) and the high theta bands (5–8Hz) (Figure 3). We found that the percent signal change for the low theta frequency band was significantly increased for the correct manipulation vs. the correct no manipulation condition from 986–1361ms after delay period 2 onset (the delay period 2 onset is at 4000ms in Figure 3). Also, the increased signal change for the high theta frequency band was significant from 1146–1591ms after delay period 2 onset (a similar temporal window).
Figure 3.
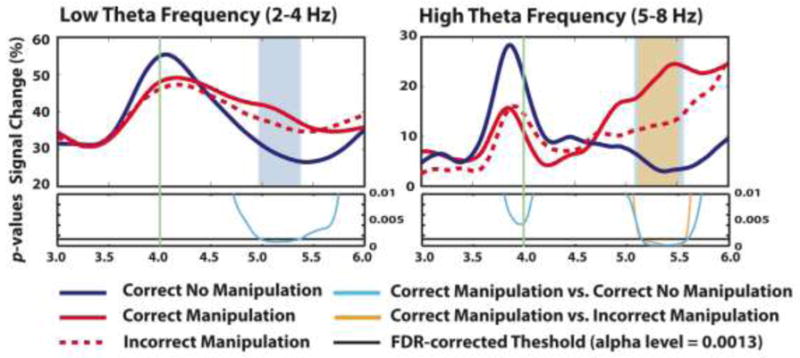
Extracted percent signal change (%) at low theta (2–4Hz, left) and high theta frequency bands (5–8Hz, right) from the frontocentral channel (Fz) during delay period 2 for correct no manipulation (dark blue line), correct manipulation (red line) and incorrect manipulation (red dotted line). The vertical green line at 4000ms indicates the onset of the delay period 2. The bottom of each panel shows p-values from the statistical comparison between ‘correct manipulation and correct no manipulation’ (light blue line) and ‘correct manipulation and incorrect manipulation’ (orange line). The black horizontal line in the bottom panel indicates the FDR-corrected threshold at the alpha level of 0.0013 (p = 0.05 FDR-corrected). The blue and orange areas indicate significant windows for the ‘correct manipulation and correct no manipulation’ comparison and the ‘correct manipulation and incorrect manipulation’ comparison, respectively.
For the key contrast of correct and incorrect manipulation, there was again a significant difference in a similar temporal window, but this time only in a higher (and more typical) theta range (~5–8 Hz) (Figure 2E, a black arrow) (and see Figure 3 for the time course, with the significant difference occurring at 1148–1574ms after delay period 2 onset) but not in the low theta range. Thus, the decisive difference was in the theta band proper (~5–8 Hz), reflecting whether manipulation was successful or not. [Auxiliary analysis affirmed the same results with matched numbers of correct and incorrect manipulation trials for single subjects].
Response period
Increased low theta power occurred for each WM condition. For the correct no manipulation condition, this change was ~800 ms earlier than the correct and incorrect manipulation conditions, consistent with faster RT. There were no notable between-condition differences.
Discussion
To test whether frontal-midline theta power is a neural signature of successful WM manipulation and to determine its precise timing, the present study employed an adapted version of the letter-memory manipulation task, in which human subjects either maintained or manipulated a letter sequence. We carefully titrated behavioral performance to 50% success rate of WM manipulation in order to specifically link theta oscillations with successful WM manipulation. Compared to baseline, for both the maintenance period of delay 1 and delay 2, there was increased low theta power (~3–5Hz) followed by increased alpha power (~9–12Hz) (probably reflecting maintenance operations). Again compared to baseline, for the manipulation period of delay 2, we observed an additional (higher) theta component at the typical range (i.e. between 5–8Hz), which occurred later (714–2300ms after the delay onset). Further analysis showed that this later theta was greater for manipulation compared to no manipulation trials, and also for successful manipulation compared to failed. These results clearly point to frontal theta as a signature of successful WM manipulation.
Behaviorally, there was superior performance for WM maintenance vs. manipulation, which confirms that the experiment was successful at engaging subjects in the effortful manipulation operation. When comparing the EEG spectral power for manipulation and maintenance (i.e. no manipulation), there was a temporally extended increase in low theta power, potentially reflecting sustained WM maintenance, as well as an increase in high theta power at a later point during this period, potentially reflecting the WM manipulation process (Figure 2D). It is possible, however, that the high theta power difference might be confounded by the difference in performance between the two WM conditions. This leads to an alternative account that the late theta increase does not reflect a manipulation process per se, but instead increased maintenance demand or a delay in maintenance during WM manipulation. We consider this unlikely for two reasons. First, it is the other two oscillatory components, an earlier low theta band (~3–5 Hz) and a following alpha band (~9–12 Hz), that are likely related to maintenance (Hsieh et al. 2011; Kawasaki et al. 2010), and not the late theta increase. Consistent with this, these two early components were even observed during delay period 1, where no manipulation was required at all. As it has been demonstrated (by Hsieh et al. 2011) that theta and alpha oscillations during WM maintenance relate to maintenance of temporal order and item information, respectively, we propose that these two signatures reflect the transfer of temporal order information into the more intact WM representation. [Consistent with this, we observed a more sustained low theta power increase for correct manipulation compared correct no manipulation, which probably reflects a more difficult/extended WM maintenance]. A second reason why the late theta increase does not merely reflect difficult/extended maintenance was that this increased power, in a typical theta range (4–8 Hz) (as opposed to the earlier low theta), only occurred during the second half of delay period 2 of the correct manipulation condition. Importantly, this was significant not only for correct manipulation versus correct no manipulation, but also for correct versus incorrect manipulation. Another alternative account could be that the increase in high theta power might reflect other non-WM processes (e.g., a conflict or error processing). However, this would predict higher frontocentral theta power for failed vs. successful manipulation, whereas we found the opposite, which strongly suggests that the increase in higher theta power is indeed a WM process specifically tied to successful manipulation.
A different study, by Griesmayr et al. (2010), also found a theta increase for manipulation versus maintenance at a similar temporal window to ours; however, they argued that due to its late timing, it did not represent manipulation per se but delayed maintenance/rehearsal. This is unlikely in the current experiment because we further demonstrated that this late theta increase was also significant for successful versus unsuccessful manipulation, which better captures the dynamic window of the manipulation process. In addition, considering that there were probably several cognitive processes preceding WM manipulation (e.g., attentional engagement, encoding of a new item, retrieval of old letter sequence, maintenance of relevant items and temporal order information), we believe the late significant window of this theta increase corresponds to the actual manipulation process. Taken together, our observations strongly suggest that the increase frontal theta at a late temporal window is an index of the manipulation process itself and not merely a signature of the increased demand or a delay of the maintenance process.
It is interesting that some aspects of WM maintenance (e.g., maintenance of temporal order information via the early theta component) and WM manipulation (the late theta component) operate in an overlapping frequency band. One simple explanation could be that what is assumed to be two cognitive processes, could actually just be variants of the same cognitive process, that relies on a core neural substrate (though it might be recruited at different times). However, this runs against an influential neurocomputational theory, which proposes that WM maintenance and manipulation are two different cognitive processes (O’Reilly and Frank 2006; Hazy et al. 2007; Frank et al. 2001). Specifically, WM is proposed to be actively maintained via continuous firing of frontal neurons and that manipulation occurs through selective gating by striatal neurons, which thens increases neural synchrony of task-relevant fronto-basal-ganglia circuits (O’Reilly and Frank 2006; Hazy et al. 2007; Frank et al. 2001). This theory is supported by evidence from Parkinson’s Disease studies showing selective impairment of WM manipulation but not simple maintenance (Lewis et al. 2003, 2005), and reduced striatal activity in Parkinson’s disease vs. controls in a WM manipulation task (Lewis et al. 2004).
Thus, we propose that the frontal theta activity for maintenance (early low theta) and for manipulation (late theta) represent two distinct neural substrates. Specifically, the maintenance process might operate via theta synchrony of local frontal neural circuits and/or fronto-parietal communication that supports temporary storage of WM presentation (c.f. Curtis et al. 2005; Cohen et al. 1997; Rowe et al. 2000); on the other hand, the late theta increase during WM manipulation could reflect long-range fronto-basal-ganglia communication (c.f., Cavanagh et al. 2011) and it could underlie the putative role of the basal ganglia in manipulating WM. Further research could specifically test this idea by using the current version of the letter-memory manipulation task along with a manipulation of the basal ganglia, for example using Deep Brain Stimulation (Cavanagh et al. 2011; Swann et al. 2011), specifically to see if this differentially modulates the early and late theta components. More generally, the current results provide an electrophysiological marker with a precise timecourse that bolsters a burgeoning research field that aims to understand how WM contents in the prefrontal cortex are changed when new information comes to light.
Acknowledgments
Thanks to Nicole Swann, Paul Sauseng and Jobi George for discussion. Funding was gratefully received from the Alfred P Sloan Foundation.
References
- Baddeley A. Working memory: looking back and looking forward. Nature Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Neely AS, Virta J, Laine M, Rinne J. Effects of working-memory training on striatal dopamine release. Science. 2011;333(6043):718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Canolty RT, Soltani M, Dalal SS, Edwards E, Dronkers NF, Nagarajan SS, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporaldynamics of word processing in the human brain. Front Neurosci. 2007;1:185–196. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nature Neurosci. 2011;14(11):2860–2870. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua Franca: A common mide-frontal substrate for action monitoring processes. Psychophysiology. 2012;49(2):220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24(16):3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320(5882):1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Missonnier P, Bertrand O, Gold G, Fazio-Costa L, Ibañez V, Giannakopoulos P. Distinction between perceptual and attentional processing in working memory tasks: a study of phase-locked and induced oscillatory brain dynamics. J Cognitive Neurosci. 2007;19:158–172. doi: 10.1162/jocn.2007.19.1.158. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affective Behav Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cong Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu DH. High-resolution EEG mapping of cortical activation related to working memory: effect of task difficulty, type processing, and practice. Cerebral Cortex. 1997;7(4):374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Griesmayr B, Gruber WR, Klimesch W, Sauseng P. Human frontal midline theta and its synchronization to gamma during a verbal delayed match to sample task. Neurobiol of Learn Mem. 2010;93(2):208–215. doi: 10.1016/j.nlm.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Grossmann A, Morlet J. Decomposition of functions into wavelets of constant shape and related transforms. In: Streit L, editor. Mathematics and physics, lectures on recent results. World Scientific; River Edge, NJ: 1985. [Google Scholar]
- Hazy TE, Frank MJ, O’reilly RC. Towards and executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc London [Biol] 2007;362(1485):1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L-T, Ekstrom AD, Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. J Neurosci. 2011;31(30):10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche C. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1400. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Watanabe M. Oscillatory gamma and theta activity during repeated mental manipulations of a visual image. Neurosci lett. 2007;422(2):141–145. doi: 10.1016/j.neulet.2007.04.079. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Kitajo K, Yamaguchi Y. Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. Eur J Neurosci. 2010;31:1683–1689. doi: 10.1111/j.1460-9568.2010.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res. 2008;1235:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J of Neurosci. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43(6):823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Regulating action: Alternating activation of midline frontal and motor cortical networks. Clinical Neurophysiology. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psych Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Mizuhara H, Yamaguchi Y. Neuronal ensemble for visual working memory via interplay of slow and fast oscillations. Eur J Neurosci. 2011;33:1925–1934. doi: 10.1111/j.1460-9568.2011.07681.x. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27(2):341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18(2):283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Pesonen M, Hämäläinen H, Krause CM. Brain Oscillatory 4–30 Hz responses during visual n-back memory task with varying memory load. Brain Res. 2007;23:171–177. doi: 10.1016/j.brainres.2006.12.076. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–60. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Frontoparietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J of Psychophysiol. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease. J Neurosci. 2011;31(15):5721–5729. doi: 10.1523/JNEUROSCI.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]