Abstract
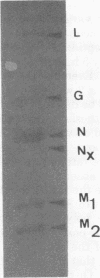
Serial passage of viral hemorrhagic septicemia virus at gradually increasing temperature selected for a variant virus that replicates at 25 degrees C and has a low pathogenicity for rainbow trout. Viral hemorrhagic septicemia virus-specific polypeptide synthesis was examined in epithelioma papulosum cyprini cells infected with either a wild-type strain or a thermoresistant variant. The wild-type N and M1 proteins were synthesized throughout the course of infection, whereas L, G, and M2 were more actively translated later in the replication cycle. The wild-type strain was more cytotoxic at 25 than at 14 degrees C despite the fact that no translation could be evidenced when the temperature was raised. When epithelioma papulosum cyprini cells were infected with the variant virus, the kinetic study was obstructed since protein synthesis was difficult to observe by the pulse method at a low multiplicity of infection and aborted when the multiplicity of infection was raised. The variant was less cytotoxic at 25 degrees C than wild-type virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Emerson S. U., Wagner R. R. RNA- temperature-sensitive mutants of vesicular stomatitis virus: L-protein thermosensitivity accounts for transcriptase restriction of group I mutants. J Virol. 1976 May;18(2):596–603. doi: 10.1128/jvi.18.2.596-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenoir G., de Kinkelin P. Fish rhabdoviruses: comparative study of protein structure. J Virol. 1975 Aug;16(2):259–262. doi: 10.1128/jvi.16.2.259-262.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Rabies virus protein synthesis in infected BHK-21 cells. J Virol. 1977 Apr;22(1):102–112. doi: 10.1128/jvi.22.1.102-112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Virion RNA polymerases of two salmonid rhabdoviruses. J Virol. 1977 Jun;22(3):839–843. doi: 10.1128/jvi.22.3.839-843.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- de Kinkelin P., Le Berre M. Mass virus production in fish cell system. Dev Biol Stand. 1979;42:99–104. [PubMed] [Google Scholar]