Abstract
Objective
To determine baseline predictors of visual acuity (VA) outcomes at 1 year after treatment with ranibizumab or bevacizumab for neovascular age-related macular degeneration (AMD).
Design
Cohort study within the Comparison of AMD Treatments Trials.
Participants
1105 participants with neovascular AMD, baseline VA 20/25 to 20/320, and VA measured at 1 year.
Methods
Participants were randomly assigned to ranibizumab or bevacizumab on either a monthly or as-needed schedule. Masked readers evaluated fundus morphology, and features on optical coherence tomography (OCT). VA was measured using electronic visual acuity testing. Independent predictors were identified using regression techniques.
Main Outcome Measures
VA score, VA score change from baseline, and ≥3-line gain at 1 year.
Results
At one year, the mean VA score was 68 letters, mean improvement from baseline was 7 letters, and 28% of participants gained ≥3-line. Older age, larger area of choroidal neovascularization (CNV), and elevation of retinal pigment epithelium (RPE) were associated with worse VA (all p<0.005), less gain in VA (all p<0.02) and a lower proportion gaining ≥3-lines (all p<0.04). Better baseline VA was associated with better VA at 1 year, less gain in VA, and a lower proportion gaining ≥3-lines (all p<0.0001). Predominantly or minimally classic lesions were associated with worse VA than occult lesions (66 vs. 69 letters, p=0.0003). Retinal Angiomatous Proliferans (RAP) lesions were associated with more gain in VA (10 vs. 7 letters, p=0.03) and a higher proportion gaining ≥3-lines (odds ratio=1.9, 95% confidence interval: 1.2 – 3.1). Geographic atrophy (GA) was associated with worse VA (64 vs. 68 letters, p=0.02). Eyes with total foveal thickness in the 2nd quartile (325 – 425 microns) had the best visual acuity (p=0.01) and were most likely to gain ≥3 lines (p=0.004). Predictors did not vary by treatment group.
Conclusion
For all treatment groups, older age, better baseline VA, larger CNV area, predominantly or minimally classic lesion, absence of RAP lesion, presence of GA, greater total fovea thickness and RPE elevation on OCT were independently associated with less improvement in VA at 1 year.
INTRODUCTION
The visual acuity prognosis among patients who develop choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD) has changed dramatically over the last 7 years since the introduction of treatment with highly effective anti-vascular endothelial growth factor (anti-VEGF) treatments.1–4 The Comparison of Age-related Macular Degeneration Treatments Trials (CATT) recently showed that bevacizumab (Avastin) was equivalent to ranibizumab (Lucentis) in improving visual acuity (VA) of patients with CNV when treatment was administered either monthly or pro re nata (PRN).5 At one year, participants treated monthly with bevacizumab or ranibizumab gained 8.0 and 8.5 letters, respectively, and those treated as needed gained 5.9 and 6.8 letters, respectively. The majority of CATT participants had the same or improved visual acuity relative to their baseline VA. However, response to treatment varied substantially among patients. While VA improved 3 lines or more in 25–34% of CATT participants in the four treatment arms, it worsened by 3 lines or more in 5–8% of participants.5
This report provides a comprehensive evaluation of baseline predictors for VA outcomes at 1 year, including demographic characteristics and medical history, ocular factors and CNV lesion features determined from fundus photographs, fluorescein angiograms, and OCT scans. Identification of these baseline predictors associated with VA outcomes may provide a more accurate assessment of the potential benefit from treatment with ranibizumab or bevacizumab and provide further insight into the mechanisms of action of these anti-VEGF drugs. In addition, identifying these predictors may allow refinement of inclusion criteria for clinical trials evaluating novel therapies for neovascular AMD.
METHODS
Details on the study design and methods have been reported previously5 and on ClinicalTrials.gov (NCT00593450). Only the major features related to the evaluation of predictors for visual outcomes are described here.
Study Participants
The institutional review board associated with each center approved the study protocol and a written consent form was obtained from each participant. Participants were enrolled from 43 clinical centers in the United States between 2008 through 2009, and randomized to one of the four treatment groups: (1) ranibizumab monthly; (2) bevacizumab monthly; (3) ranibizumab as needed (pro re nata, PRN); and (4) bevacizumab PRN. The study enrollment criteria included age of 50 or older, the study eye (one eye per patient) had untreated active CNV due to AMD, and VA between 20/25 and 20/320 on electronic VA testing.6 The presence of active CNV, as seen on fluorescein angiography, and fluid, as seen on time-domain OCT, located either within or below the retina or below the retinal pigment epithelium were required to establish the presence of active CNV. Either neovascularization or its sequela, i.e., pigment epithelium detachment, subretinal or sub- retinal pigment epithelium (RPE) hemorrhage, blocked fluorescence, macular edema, or intraretinal, subretinal, or sub-RPE fluid, needed to be under the fovea.
Study Procedures
During the initial visit, participants provided information on demographic characteristics and medical history. Certified photographers followed a standard protocol for field definition and image sequencing to obtain stereoscopic, color fundus photographs and fluorescein angiograms. Photographs from all clinical centers were digital except photographs from one center (film-based). Optical coherence tomography (OCT) was obtained with a Stratus (version 4.0 or higher) time domain OCT machine (Carl Zeiss Meditec, Dublin, California). Certified OCT imagers followed a standard protocol that included fast macular thickness map (FMTM) and macular thickness map (MTM) protocols. Each protocol included 6 radial lines of 6 mm length placed across the fovea center at 30-degree rotational increments with 128 A-scans per line for FMTM and 512 A-scans per line for MTM.
Two masked trained readers in the CATT Fundus Photographic Reading Center independently evaluated baseline fundus photographs and fluorescein angiograms. Discrepancies between two trained readers were adjudicated between the readers and Director of the Photograph Reading Center. Qualitative evaluations of lesion characteristics included identification of the lesion location, lesion type, lesion composition, Retinal Angiomatous Proliferans (RAP) features, hemorrhage contiguous with the lesion, serous retinal pigment epithelial detachment (SPED), atrophic or fibrotic scars, any hemorrhage associated with lesion (not necessarily contiguous) and geographic atrophy anywhere in the macula. To be considered GA, there should be an area of hypopigmentation and/or hyperfluorescence of at least 250μ in its minimum linear dimension, and have 2 of the 3 following characteristics: (1)circular shape; (2) sharp borders; or (3) visibility of choroidal vessels within the area of GA. Quantitative measurements of the CNV area and of the total area of CNV lesion were made using Image J, a public domain Java image processing program developed by the National Institute of Health and available as a free software from http://rsbweb.nih.gov/ij/ (accessed on March 22, 2012). Total area of CNV lesion include CNV and/or one or many of the following in or adjacent to the location of CNV: SPED, hemorrhage, blocked fluorescence, scar, geographic atrophy, non-geographic atrophy or RPE tear. Fluid was not considered to be part of the total CNV lesion. Two trained readers independently measured the lesions; discrepancies were adjudicated if the difference was >50% of the lesion area obtained by averaging the two measurements or >3.0 mm2. In addition, adjudications were performed if one reader could not grade an image and the other reader made a measurement. Quantitative measurements could not be determined because of poor quality photographs, leakage from the edge of a GA and in relatively flat occult lesions whose borders were indistinct.
Independent trained readers at the OCT Reading Center, masked to the treatment assignment, evaluated the time domain-OCT images, with respect to the presence of fluid, location (intra-retinal, sub-retinal, sub-RPE) of fluid (Figure 1A), foveal fluid and RPE elevation. In addition, trained readers measured the total thickness at the foveal center point which was subdivided into three measurements: thickness of retina, subretinal fluid, and subretinal tissue complex (material between Bruch’s membrane and outer retina or subretinal fluid which includes PED, CNV, blood and fibrosis) from 6 radial fast macular thickness scans (Figure 1B). When the two readers disagreed on a morphological parameter, or when the vertical measurement differed by more than 65 micrometers, or the horizontal measurement differed by more than 220 micrometers, a third independent senior reader resolved the discrepancy, and this arbitrated value was used as the final grade.
Figure 1.
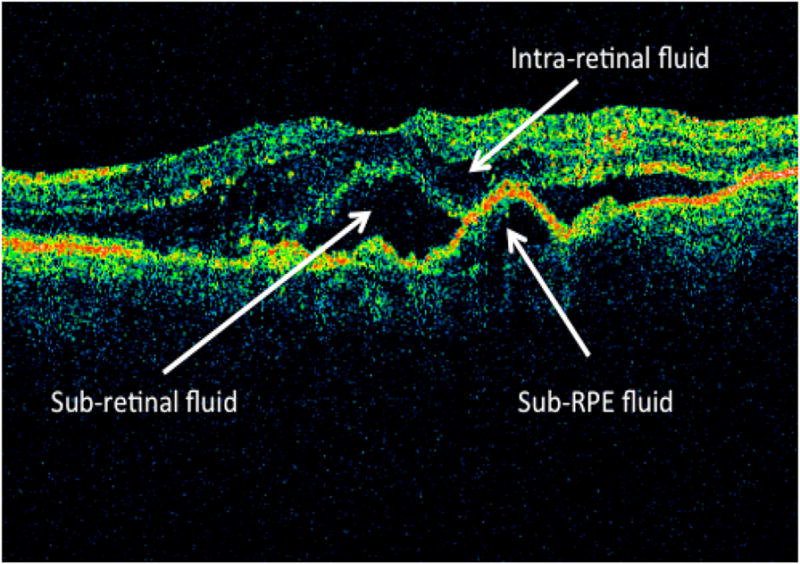
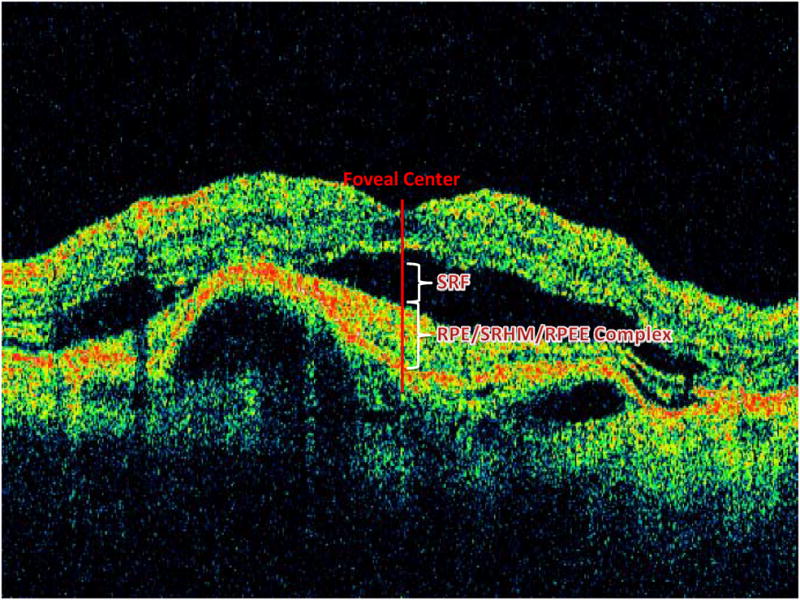
Figure 1A: Location of OCT fluid (intra-retinal fluid, sub-retinal fluid, sub-RPE fluid) at baseline.
Figure 1B: OCT retinal layers and total retinal thickness at fovea center.
OCT= optical coherence tomography, SRF=Sub-retinal fluid, RPE= retinal pigment epithelium; SHRM= subretinal hyper-reflective material; RPEE= RPE elevation.
At baseline and at follow-up weeks 4, 12, 24, 36 and 52, certified visual acuity examiners, masked to the treatment assignment, measured visual acuity after refraction in both eyes using the Electronic Visual Acuity Tester (EVA) following the protocol used in the Diabetic Retinopathy Clinical Research Network.6 The VA scores (the number of letters read correctly on the ETDRS chart, measured with best-corrected visual acuity) from EVA can range from 0 to 100, corresponding to Snellen equivalents of worse than 20/800 to 20/10.
Data Analysis
Hypertension was defined as systolic blood pressure (BP) of 160 mmHg or more, diastolic BP of 95 mmHg or more, or current use of antihypertensive medications. The thickness of retina, subretinal fluid and subretinal tissue complex was calculated as the average of measurements from 6 macular thickness map scans, and the total thickness at the fovea center was calculated as the sum of the 3 averages.
Most of the baseline predictors were measured with respect to the presence or absence of a particular feature (such as lesion features, fluid). For predictors measured on a continuous scale; e.g., VA and retinal thickness, we assessed their association with visual outcomes by modeling them as continuous measures. In addition, we classified continuous measurements into categories for easier clinical interpretation. Categorizations of continuous variables were based on either the normal range (retinal thickness), quartiles of the distribution (total fovea thickness), or clinically relevant cut-points (baseline visual acuity).
We analyzed predictors for three VA outcomes at 1 year including: VA score, change in VA score from baseline, and ≥3-lines (i.e., 15 letters) gain from baseline. The predictors for ≥3-lines loss were not analyzed because only 68 (6%) of patients had ≥3-lines loss from baseline, it didn’t provide enough statistical power to assess the predictors for ≥3-lines loss. Each predictor was first evaluated by univariate analysis (without adjustment for other covariates) using generalized linear models for VA and change in VA, and using logistic regression models for ≥3-lines gain from baseline (yes/no). The predictors with a p-value <0.20 in the univariate analysis were included in a multivariate analysis so that the independent effect of each predictor could be assessed. Interaction between treatment group and each candidate predictor was first evaluated in models containing only the treatment group, the predictor, and treatment group by predictor interaction term. The interaction term that had a p-values ≤ 0.05 was retained for the multivariate analysis. The final multivariate model was created by applying a backward selection procedure that retained only those predictors and interaction terms with a p-value ≤0.05, with the exception of treatment group, which was included in all multivariate models. Adjusted means of VA and VA changed were calculated based on the final multivariate linear models, and adjusted odds ratios (OR) of ≥3-lines gain and their 95% confidence intervals (95% CI) were calculated based on the final multivariate logistic regression model. All data analyses were performed using SAS (v9.2, SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics of Study Participants
The CATT enrolled 1185 participants. Among 1161 participants who survived 1 year after enrollment, visual acuity was measured at 1 year in 1105 (95.2%) participants and these data were included in the analyses. The demographic characteristics of these 1105 participants are shown in the two left columns of Table 1 (available at http://aaojournal.org). The mean age was 79 years (standard deviation (SD) =8 years), 62% were female, 9% were current cigarette smokers, 69% had hypertension, and 17% had diabetes.
Table 1.
Univariate analysis for association of baseline participant characteristics with visual outcomes at 1 year
| # of Subjects at 1 Year (N=1105) | VA score at 1 year | Change in VA score at 1 year | ≥3-lines gain from baseline at 1 year | ||||
|---|---|---|---|---|---|---|---|
| Baseline Participant Characteristics | n(%) | Mean (SE) | P-value* | Mean (SE) | P-value* | n(%) | P-Value† |
| Age (years) | <0.001 | 0.02 | 0.03 | ||||
| 50–69 | 131 (11.9%) | 74.6 (1.5) | 10.9 (1.3) | 51 (38.9%) | |||
| 70–79 | 387 (35.0%) | 70.3 (0.9) | 7.3 (0.7) | 107 (27.6%) | |||
| 80–89 | 512 (46.3%) | 65.2 (0.8) | 6.4 (0.7) | 141 (27.5%) | |||
| ≥90 | 75 (6.8%) | 64.0 (2.0) | 7.6 (1.7) | 28 (37.3%) | |||
| As continuous (1 year increase) | −0.50 (0.07) | <0.001 | −0.18 (0.06) | 0.002 | 0.11 | ||
| Gender | 0.31 | 0.30 | 0.25 | ||||
| Female | 684 (61.9%) | 68.4 (0.7) | 7.7 (0.6) | 211 (30.8%) | |||
| Male | 421 (38.1%) | 67.3 (0.9) | 6.7 (0.7) | 116 (27.6%) | |||
| Cigarette Smoking | 0.75 | 0.93 | 0.74 | ||||
| Never | 475 (43.0%) | 68.2 (0.8) | 7.4 (0.7) | 140 (29.5%) | |||
| Quit | 536 (48.5%) | 68.1 (0.8) | 7.3 (0.6) | 156 (29.1%) | |||
| Current | 94 (8.5%) | 66.7 (1.8) | 6.8 (1.5) | 31 (33.0%) | |||
| Hypertension | 0.03 | 0.41 | 0.32 | ||||
| No | 346 (31.3%) | 69.7 (1.0) | 7.9 (0.8) | 95 (27.5%) | |||
| Yes | 759 (68.7%) | 67.3 (0.7) | 7.1 (0.5) | 232 (30.6%) | |||
| Diabetes | 0.67 | 0.73 | 0.39 | ||||
| No | 915 (82.8%) | 68.1 (0.6) | 7.4 (0.5) | 276 (30.2%) | |||
| Yes | 190 (17.2%) | 67.5 (1.3) | 7.0 (1.1) | 51 (26.8%) | |||
| Treatment Group | 0.45 | 0.16 | 0.09 | ||||
| Ranibizumab Monthly | 284 (25.7%) | 68.8 (1.05) | 8.5 (0.8) | 97 (34.2%) | |||
| Bevacizumab Monthly | 265 (24.0%) | 68.4 (1.09) | 8.0 (1.0) | 83 (31.3%) | |||
| Ranibizumab as needed | 285 (25.8%) | 68.4 (1.05) | 6.8 (0.8) | 71 (24.9%) | |||
| Bevacizumab as needed | 271 (24.5%) | 66.5 (1.08) | 5.9 (1.0) | 76 (28.0%) | |||
VA=Visual Acuity; SE = Standard Error;
P-value was from one-way analysis of variance (ANOVA).
P value was from Monte-Carlo fisher exact test.
P-value in the parenthesis was calculated with category of unknown into comparison, while P-value not in the parenthesis was calculated excluding category of unknown into comparison.
The mean baseline VA score in the study eye was 61 letters (Snellen equivalent =20/63) (SD=13), and in the fellow eye was 66 letters (Snellen equivalent =20/50) (SD=27) (Table 2, available at http://aaojournal.org). The median CNV area at baseline was 3.0 mm2 (range: 0.03 to 28.7 mm2), and the median total area of CNV lesion was 4.3 mm2 (range: 0.05 to 56.9 mm2). Based on the evaluation of fundus photographs and fluorescein angiograms, 71% had a subfoveal lesion, 59% of lesions were occult only, and half of the lesions had CNV only without other lesion components (SPED, fibrosis scar, atrophy scar, hemorrhage, or blocked fluorescence).
Table 2.
Univariate analysis for association of baseline ocular and fundus characteristics with visual outcomes at 1 year
| # of Subjects at 1 year (N=1105) | VA score at 1 year | Change in VA score at 1 year | ≥3-lines gain frombaseline at 1 year | ||||
|---|---|---|---|---|---|---|---|
| Baseline Ocular Characteristics | n (%) | Mean (SE) | P-value* | Mean (SE) | P-value* | n (%) | P-Value† |
| Baseline VA in study eye | <0.001 | <0.001 | <0.001 | ||||
| 68–82 letters, 20/25 - 20/40 | 397 (35.9%) | 77.7 (0.7) | 3.7 (0.7) | 28 (7.1%) | |||
| 53–67 letters, 20/50 - 20/80 | 414 (37.5%) | 69.2 (0.7) | 8.5 (0.7) | 150 (36.2%) | |||
| 38–52 letters, 20/100-20/160 | 223 (20.2%) | 57.8 (1.0) | 11.4 (1.0) | 119 (53.4%) | |||
| 23–37 letters, 20/200-20/320 | 71 (6.4%) | 39.3 (1.7) | 7.8 (1.7) | 30 (42.3%) | |||
| As continuous (1 letter increase) | 0.79 (0.03) | <0.001 | −0.22 (0.03) | <0.001 | <0.001 | ||
| Baseline VA in fellow eye | 0.003 | 0.03 | 0.02 | ||||
| 83–100 letters, 20/20 or better | 331 (30.0%) | 70.7 (1.0) | 8.9 (0.8) | 110 (33.2%) | |||
| 68–82 letters, 20/25-20/40 | 433 (39.2%) | 67.5 (0.9) | 7.2 (0.7) | 135 (31.2%) | |||
| 0–67 letters, 20/50 or worse | 341 (30.9%) | 66.1 (1.0) | 5.9 (0.8) | 82 (24.0%) | |||
| As continuous (1 letter increase) | 0.04 (0.02) | 0.051 | 0.04 (0.02) | 0.04 | 0.005 | ||
| Baseline CNV area (mm2) | <0.001 | 0.007 | 0.27 | ||||
| ≤2.54 | 443 (40.1%) | 70.9(0.8) | (<0.001) | 8.8 (0.7) | (0.007) | 145 (32.7%) | (0.11) |
| >2.54 to ≤5.08 | 219 (19.8%) | 68.4(1.2) | 7.7 (1.0) | 63 (28.8%) | |||
| >5.08 to ≤10.2 | 207 (18.7%) | 66.9(1.2) | 6.6 (1.0) | 65 (31.4%) | |||
| >10.2 | 103 (9.3%) | 63.4(1.8) | 3.4 (1.4) | 24 (23.3%) | |||
| Unknown | 133 (12.0%) | 63.2(1.5) | 5.8 (1.3) | 30 (22.6%) | |||
| As continuous (every 2.54 mm2 increase) | −1.25 (0.33) | <0.001 | −0.60 (0.24) | 0.01 | 0.02 | ||
| Baseline total area of CNV lesion (mm2) | <0.001 | 0.04 | 0.13 | ||||
| ≤2.54 | 355 (32.1%) | 71.3 (0.9) | (<0.001) | 8.1(0.8) | (0.04) | 107(30.1%) | (0.045) |
| >2.54 to ≤5.08 | 240 (21.7%) | 69.6 (1.2) | 8.7(1.0) | 76(31.7%) | |||
| >5.08 to ≤10.2 | 265 (24.0%) | 66.3 (1.1) | 7.3(0.9) | 89(33.6%) | |||
| >10.2 | 202 (18.3%) | 62.7 (1.3) | 5.0(1.0) | 48(23.8%) | |||
| Unknown | 43 (3.9%) | 68.0 (2.7) | 4.3(2.2) | 7 (16.3%) | |||
| As continuous (every 2.54 mm2 increase) | −1.39 (0.21) | <0.001 | −0.58 (0.18) | 0.001 | 0.02 | ||
| Location of lesion | <0.001 | 0.90 | 0.08 | ||||
| Not Subfoveal | 299 (27.1%) | 71.7 (1.0) | (<0.001) | 7.3(0.9) | (0.48) | 77(25.8%) | (0.04) |
| Subfoveal | 788 (71.3%) | 66.5 (0.6) | 7.4(0.5) | 248(31.5%) | |||
| Unknown | 18 (1.6%) | 72.1 (4.2) | 3.2(3.5) | 2 (11.1%) | |||
| Lesion type | <0.001 | 0.94 | <0.001 | ||||
| Predominantly or Minimally classic | 431 (39.0%) | 64.2 (0.9) | (<0.001) | 7.4(0.7) | (0.83) | 156(36.2%) | (<0.001) |
| Occult only | 650 (58.8%) | 70.4 (0.7) | 7.3(0.6) | 167(25.7%) | |||
| Unknown | 24 (2.2%) | 71.6 (3.6) | 5.5(3.0) | 4(16.7%) | |||
| Lesion Composition | 0.19 | 0.09 | 0.003 | ||||
| CNV only | 560 (50.7%) | 69.0 (0.8) | (0.25) | 6.9(0.6) | (0.08) | 152(27.1%) | (0.001) |
| CNV and hemorrhage | 286 (25.9%) | 66.9 (1.1) | 7.4(0.9) | 92(32.2%) | |||
| CNV and blocked fluorescence | 76 (6.9%) | 67.1 (2.1) | 6.5(1.7) | 22(28.9%) | |||
| CNV and SPED | 47 (4.3%) | 70.2 (2.6) | 5.9(2.2) | 8(17.0%) | |||
| CNV and others | 121 (11.0%) | 65.4 (1.6) | 10.8(1.3) | 52(43.0%) | |||
| Unknown | 15 (1.4%) | 71.2 (4.6) | 2.4(3.8) | 1(6.7%) | |||
| RAP lesion | 0.13 | 0.02 | 0.008 | ||||
| No | 966 (87.4%) | 67.6 (0.6) | (0.15) | 7.0(0.5) | (0.06) | 276(28.6%) | (0.009) |
| Yes | 118 (10.7%) | 70.3 (1.7) | 10.3(1.4) | 48(40.7%) | |||
| Unknown | 21 (1.9%) | 72.7 (3.9) | 5.7(3.2) | 3(14.3%) | |||
| Hemorrhage contiguous with lesion | 0.07 | 0.11 | 0.007 | ||||
| No | 699 (63.3%) | 68.7 (0.7) | (0.18) | 6.9(0.6) | (0.09) | 190(27.2%) | (0.002) |
| Yes | 388 (35.1%) | 66.7 (0.9) | 8.4(0.8) | 136(35.1%) | |||
| Unknown | 18 (1.6%) | 70.1 (4.2) | 2.3(3.5) | 1 (5.6%) | |||
| Blocked fluorescence lesion | 0.38 | 0.08 | 0.003 | ||||
| No | 935 (84.6%) | 68.2 (0.6) | (0.47) | 7.1(0.5) | (0.13) | 263 (28.1%) | (0.002) |
| Yes | 157 (14.2%) | 66.8 (1.4) | 9.2(1.2) | 63 (40.1%) | |||
| Unknown | 13 (1.2%) | 72.2 (4.9) | 3.1(4.1) | 1 (7.7%) | |||
| SPED (proportion of total lesion) | 0.81 | 0.77 | 0.33 | ||||
| None | 1027 (92.9%) | 67.9 (0.6) | (0.84) | 7.5 (0.5) | (0.47) | 313 (30.5%) | (0.07) |
| <50% | 21 (1.9%) | 68.2 (3.9) | 7.2 (3.2) | 5 (23.8%) | |||
| >=50% | 40 (3.6%) | 69.8 (2.8) | 5.8 (2.3) | 8 (20.0%) | |||
| Unknown | 17 (1.5%) | 70.7 (4.3) | 2.3 (3.6) | 1 (5.9%) | |||
| Fibrotic or atrophic scar | <0.001 | 0.29 | 0.17 | ||||
| No | 1049 (94.9%) | 68.4 (0.6) | (0.001) | 7.3 (0.5) | (0.33) | 309 (29.5%) | (0.08) |
| Yes | 43 (3.9%) | 58.6 (2.7) | 9.7 (2.3) | 17 (39.5%) | |||
| Unknown | 13 (1.2%) | 72.2 (4.9) | 3.1 (4.1) | 1 (7.7%) | |||
| Hemorrhage (associated with the lesion) | 0.001 | 0.009 | 0.001 | ||||
| None | 420 (38.0%) | 69.3 (0.9) | (0.002) | 5.7 (0.7) | (0.01) | 99 (23.6%) | (<0.001) |
| ≤1 DA | 561 (50.8%) | 68.1 (0.8) | 8.8 (0.6) | 198 (35.3%) | |||
| >1 to ≤2 DA | 55 (5.0%) | 64.1 (2.4) | 7.9 (2.0) | 17 (30.9%) | |||
| >2 DA | 50 (4.5%) | 59.4 (2.5) | 4.9 (2.1) | 11 (22.0%) | |||
| Unknown | 19 (1.7%) | 70.4 (4.1) | 3.8 (3.4) | 2 (10.5%) | |||
| Geographic atrophy | 0.008 | 0.049 | 0.44 | ||||
| None/questionable | 1027 (92.9%) | 68.4 (0.6) | (0.02) | 7.6 (0.5) | (0.14) | 308 (30.0%) | |
| Present | 76 (6.9%) | 62.8 (2.0) | 4.1 (1.7) | 19 (25.0%) | |||
| Unknown | 2 (0.2%) | 53.0 (13) | 6.0 (10) | 0 (0%) | |||
| Glaucoma | 0.15 | 0.97 | 0.53 | ||||
| No | 981 (88.8%) | 68.3 (0.6) | 7.3 (0.5) | 287 (29.3%) | |||
| Yes | 124 (11.2%) | 65.8 (1.6) | 7.3 (1.3) | 40 (32.3%) | |||
| Unknown | 19 (1.7%) | 70.4 (4.1) | 3.8 (3.4) | 2 (10.5%) | |||
| CNV in fellow eye | 0.30 | 0.67 | 0.04 | ||||
| None/questionable | 746 (67.5%) | 67.7(0.7) | (0.56) | 7.4 (0.5) | (0.50) | 235 (31.5%) | (0.09) |
| Present | 334 (30.2%) | 68.9(1.0) | 7.0 (0.8) | 84 (25.1%) | |||
| Unknown | 25 (2.3%) | 67.0 (3.6) | 10.5 (2.9) | 8 (32.0%) | |||
VA = Visual Acuity; SE = Standard Error; VA= Visual Acuity; CNV= choroidal neovascularization; DA= Disc Area; SPED= serous retinal pigment epithelial detachment; RAP=Retinal Angiomatous Proliferans; RPE=Retinal Pigment Epithelium.
P-value was from one-way analysis of variance (ANOVA).
P value was from Monte-Carlo fisher exact test.
P-value in the parenthesis was calculated including the unknown category, while P-value not in parenthesis was calculated excluding the unknown category.
The baseline OCT features are shown in Table 3 (available at http://aaojournal.org). The mean thickness of retina, subretinal fluid, and subretinal tissue complex was 218 microns (SD = 107 microns), 32 microns (SD=70 microns), and 210 microns (SD=176 microns) respectively. The mean total fovea thickness was 460 microns (SD=190 microns). The morphological fluid location was also determined. Subretinal fluid was most common (82%), followed by intraretinal fluid (75%), and sub-RPE fluid (49%). At baseline, all three types of fluid were observed in 30% of study eyes, and 82% of study eyes had fluid involving the foveal center point at baseline. RPE elevation (which could be from drusen, or PED with fluid or reflective material beneath the elevation) was present in 85% of study eyes.
Table 3.
Univariate analysis for association of baseline OCT features with visual outcomes at 1 year
| # of Subjects at 1 year (N=1105) | VA score at 1 year | Change in VA score at 1 year | ≥3-lines gain from baseline at 1 year | ||||
|---|---|---|---|---|---|---|---|
| Baseline OCT features | n (%) | Mean (SE) | P-value* | Mean (SE) | P-value* | n (%) | P-Value† |
| Retinal thickness (microns) | <0.001 | 0.47 | 0.002 | ||||
| <120 | 112 (10.1%) | 68.8 (1.7) | (<0.001) | 6.4(1.4) | (0.66) | 30(26.8%) | (0.005) |
| 120 to 212 | 581 (52.3%) | 70.0 (0.7) | 7.0(0.6) | 150(25.8%) | |||
| >212 | 407 (36.8%) | 64.9 (0.9) | 8.0(0.7) | 146(35.9%) | |||
| Unknown | 5 (0.5%) | 73.6 (7.9) | 9.0(6.6) | 1 (20.0%) | |||
| As continuous (every 10 microns increase) | −0.31 (0.05) | <0.001 | 0.03(0.04) | 0.49 | 0.009 | ||
| Sub-retinal fluid thickness (microns) | 0.13 | 0.13 | 0.002 | ||||
| 0 | 735 (66.5%) | 67.3 (0.7) | (0.21) | 7.9(0.5) | (0.24) | 242(32.9%) | (0.005) |
| >0 to ≤25 | 92 (8.3%) | 70.1 (1.9) | 7.4(1.5) | 24(26.1%) | |||
| >25 | 273 (24.7%) | 69.3 (1.1) | 5.8(0.9) | 60(22.0%) | |||
| Unknown | 5 (0.5%) | 73.6 (8.0) | 9.0(6.6) | 1 (20.0%) | |||
| As continuous (every 10 microns increase) | −0.06 (0.08) | 0.44 | −0.17 (0.06) | 0.006 | 0.02 | ||
| Subretinal tissue complex thickness (microns) by quartile | <0.001 | 0.04 | <0.001 | ||||
| 1st (>0 to ≤75) | 272 (24.6%) | 72.2 (1.1) | (<0.001) | 5.5(0.9) | (0.07) | 53(19.5%) | (<0.001) |
| 2nd (>75 to ≤160) | 263 (23.8%) | 67.9 (1.1) | 7.3(0.9) | 85(32.3%) | |||
| 3rd (>160 to ≤275) | 282 (25.5%) | 67.3 (1.1) | 9.2(0.9) | 101(35.8%) | |||
| 4th (>275) | 283 (25.6%) | 64.8 (1.1) | 7.2(0.9) | 87(30.7%) | |||
| As continuous (every 10 microns increase) | −0.13 (0.03) | <0.001 | −0.00(0.03) | 0.96 | 0.55 | ||
| Retinal + sub-retinal fluid thickness (microns) by quartiles | <0.001 | 0.49 | 0.17 | ||||
| 1st (≤160) | 274 (24.8%) | 69.8 (1.1) | (<0.001) | 6.6(0.9) | (0.64) | 72(26.3%) | (0.26) |
| 2nd (>160 to ≤225) | 273 (24.7%) | 69.8 (1.1) | 8.4(0.9) | 91(33.3%) | |||
| 3rd (>225 to ≤320) | 279 (25.3%) | 68.6 (1.1) | 6.9(0.9) | 75(26.9%) | |||
| 4th (>320) | 274 (24.8%) | 63.7 (1.1) | 7.4(0.9) | 88(32.1%) | |||
| As continuous (every 10 microns increase) | −0.26 (0.04) | <0.001 | −0.04 (0.04) | 0.31 | 0.30 | ||
| Total foveal thickness (microns) by quartile | <0.001 | 0.08 | 0.004 | ||||
| 1st (≤325) | 277 (25.1%) | 71.6 (1.0) | (<0.001) | 5.8 (0.9) | (0.14) | 59 (21.3%) | (0.008) |
| 2nd (>325 to ≤425) | 285 (25.8%) | 72.2 (1.0) | 8.5 (0.9) | 95 (33.3%) | |||
| 3rd (>425 to ≤550) | 253 (22.9%) | 67.9 (1.1) | 8.4 (0.9) | 83 (32.8%) | |||
| 4th (>550) | 285 (25.8%) | 60.4 (1.0) | 6.6 (0.9) | 89 (31.2%) | |||
| Unknown | 5 (0.5%) | 73.6 (7.7) | 9.0 (6.6) | 1 (20.0%) | |||
| As continuous (every 10 microns increase) | −0.22 (0.03) | <0.001 | −0.02 (0.02) | 0.49 | 0.23 | ||
| Intra-retinal fluid | <0.001 | 0.40 | 0.007 | ||||
| No Fluid | 264 (23.9%) | 72.8 (1.1) | (<0.001) | 6.9 (0.9) | (0.58) | 61 (23.1%) | (0.02) |
| Fluid but not in foveal center point | 298 (27.0%) | 68.7 (1.0) | 6.6 (0.9) | 84 (28.2%) | |||
| Fluid in foveal center point | 525 (47.5%) | 65.4 (0.8) | 8.0 (0.6) | 177 (33.7%) | |||
| Unknown | 18 (1.6%) | 64.0 (4.1) | 6.1 (3.5) | 5 (27.8%) | |||
| Sub-retinal fluid | 0.045 | 0.19 | 0.01 | ||||
| No Fluid | 189 (17.1%) | 65.7 (1.3) | (0.10) | 6.4 (1.1) | (0.20) | 62 (32.8%) | (0.02) |
| Fluid but not in foveal center point | 523 (47.3%) | 67.7 (0.8) | 8.1 (0.6) | 169 (32.3%) | |||
| Fluid in foveal center point | 384 (34.8%) | 69.6 (0.9) | 6.5 (0.8) | 92 (24.0%) | |||
| Unknown | 9 (0.8%) | 65.9 (5.9) | 12.9 (4.9) | 4 (44.4%) | |||
| Sub-RPE fluid | 0.77 | 0.76 | 0.01 | ||||
| No Fluid | 483 (43.7%) | 68.7 (0.8) | (0.41) | 7.5 (0.7) | (0.37) | 163 (33.7%) | (0.02) |
| Fluid but not in foveal center point | 194 (17.6%) | 68.1 (1.3) | 6.9 (1.1) | 50 (25.8%) | |||
| Fluid in foveal center point | 344 (31.1%) | 67.8 (1.0) | 6.7 (0.8) | 86 (25.0%) | |||
| Unknown | 84 (7.6%) | 65.2 (1.9) | 9.8 (1.6) | 28 (33.3%) | |||
| Any fluid in foveal center | 0.03 | 0.26 | 0.050 | ||||
| No Fluid in foveal center point | 201 (18.2%) | 70.5 (1.3) | (0.09) | 8.1 (1.0) | (0.59) | 71 (35.3%) | (0.10) |
| Fluid in foveal center point | 901 (81.5%) | 67.5 (0.6) | 7.1 (0.5) | 255 (28.3%) | |||
| Unknown | 3 (0.3%) | 70.7 (10) | 12.7 (8.5) | 1(33.3%) | |||
| RPE elevation | 0.009 | <0.001 | <0.001 | ||||
| No | 145 (13.1%) | 71.6 (1.5) | (0.03) | 11.2 (1.2) | (<0.001) | 61 (42.1%) | (<0.001) |
| Yes | 944 (85.4%) | 67.5 (0.6) | 6.6 (0.5) | 258 (27.3%) | |||
| Unknown | 16 (1.5%) | 68.3 (4.4) | 14.1 (3.7) | 8 (50.0%) | |||
| RPEE maximum height (mm) by quartiles | |||||||
| 1st (≤3.0) | 317 (28.7%) | 68.6 (1.00) | 0.937 | 7.6 (0.83) | 0.631 | 112 (35.3%) | 0.046 |
| 2nd (>3.0 to ≤5.0) | 212 (19.2%) | 68.9 (1.22) | (0.027) | 8.3 (1.01) | (0.684) | 63 (29.7%) | (0.086) |
| 3rd (>5.0 to ≤11.5) | 267 (24.2%) | 68.1 (1.09) | 7.1 (0.90) | 74 (27.7%) | |||
| 4th (>11.5) | 268 (24.3%) | 68.0 (1.08) | 6.7 (0.90) | 67 (25.0%) | |||
| Unknown | 41 (3.7%) | 59.2 (2.77) | 5.6 (2.30) | 11 (26.8%) | |||
| As continuous (1 mm increase) | −0.05 (0.06) | 0.45 | −0.08 (0.05) | 0.12 | 0.002 | ||
| RPEE maximum width (mm) by quartiles | |||||||
| 1st (≤12.0) | 260 (23.5%) | 69.9 (1.10) | 0.094 | 8.3 (0.91) | 0.069 | 92 (35.4%) | 0.02 |
| 2nd (>12 to ≤31.5) | 250 (22.6%) | 68.5 (1.12) | (0.013) | 6.8 (0.93) | (0.100) | 71 (28.4%) | (0.023) |
| 3rd (>31.5 to ≤55.0) | 258 (23.4%) | 69.0 (1.10) | 8.9 (0.91) | 84 (32.6%) | |||
| 4th (>55.0) | 257 (23.3%) | 66.2 (1.11) | 5.8 (0.91) | 60 (23.3%) | |||
| Unknown | 80 (7.2%) | 63.2 (1.98) | 6.0 (1.64) | 20 (25.0%) | |||
| As continuous (1 mm increase) | −0.03 (0.02) | 0.06 | −0.02 (0.02) | 0.12 | 0.02 | ||
OCT= Optical coherence tomography; VA = Visual Acuity; SE = Standard Error; RPE=Retinal Pigment Epithelium; RPEE=RPE elevation.
P-value was from one-way analysis of variance (ANOVA).
P-value was from Monte-Carlo fisher exact test.
P-value in the parenthesis was calculated including the unknown category, while P-value not in parenthesis was calculated excluding the unknown category.
The VA score at 1 year was not available for 80 (6.8%) participants. Among these participants, 24 (2.0%) died before week 052 and the remainder (4.8%) missed the 1-year measurement. The participants with missing VA at 1 year were generally comparable to participants with available VA at one year (data not shown) with respect to the baseline demographic, ocular and OCT characteristics, except that participants without VA score at 1 year were significantly older at baseline (mean 82 vs. 79 years, p=0.002).
Predictors for VA score at 1 year
The mean VA score at one year after treatment was 68 letters (Snellen equivalent =20/40), and did not differ among treatment groups (p=0.45). The unvairate results for the predictors of the VA score at one year were presented in Table 1 (available at http://aaojournal.org) for baseline participant factors, in Table 2 (available at http://aaojournal.org) for the baseline ocular and fundus characteristics of the study eye, and in Table 3 (available at http://aaojournal.org) for the baseline OCT features.
When the baseline factors associated with p<0.20 in the above univariate analyses were considered simultaneously in the multivariate analysis (Table 4), the significant predictors of worse VA score at 1 year were: older age (p=0.0006), worse baseline VA score (p<0.0001), larger CNV area (p=0.001), predominantly or minimally classic lesion (p<0.001), geographic atrophy (p=0.02), thicker total thickness at the fovea (p=0.01) and presence of RPE elevation on OCT (p=0.005). There were no statistically significant interactions between treatment group and any of these predictors.
Table 4.
Multivariate Analysis for Visual Acuity and its Change from Baseline at 1 Year
| VA score (letters) at 1 Year*
|
VA score change (letters) from baseline at 1 Year †
|
||||
|---|---|---|---|---|---|
| Baseline Characteristics | N | Adjusted Mean (SE) | P-value | Adjusted Mean (SE) | P-value |
| Age (years) | |||||
| 50–69 | 131 | 72.1 (1.3) | 0.0006 | 10.8 (1.3) | 0.003 |
| 70–79 | 387 | 68.8 (0.7) | 8.2 (0.8) | ||
| 80–89 | 512 | 66.4 (0.6) | 5.8 (0.6) | ||
| ≥90 | 75 | 67.2 (1.7) | 6.2 (1.7) | ||
| Baseline VA in study eye | |||||
| 68–82 letters, 20/25 - 20/40 | 397 | 76.4 (0.8) | <0.0001 | 3.3 (0.7) | <0.0001 |
| 53–67 letters, 20/50 - 20/80 | 414 | 69.1 (0.7) | 8.4 (0.7) | ||
| 38–52 letters, 20/100 - 20/160 | 223 | 59.0 (1.0) | 11.9 (1.0) | ||
| 23–37 letters, 20/200 - 20/320 | 71 | 41.7 (1.8) | 7.9 (1.7) | ||
| Baseline area of CNV (mm2) | |||||
| ≤2.54 | 443 | 69.9 (0.7) | 0.001 | 8.7 (0.7) | 0.02 |
| >2.54 to ≤5.08 | 219 | 68.0 (1.0) | 7.5 (1.0) | ||
| >5.08 to ≤10.2 | 207 | 67.0 (1.0) | 6.7 (1.0) | ||
| >10.2 | 103 | 64.5 (1.4) | 4.2 (1.4) | ||
| Can’t measure | 133 | 64.9 (1.4) | 4.8 (1.4) | ||
| Lesion type | |||||
| Predominantly or Minimally classic | 431 | 65.8 (0.7) | 0.0003 | -- | |
| Occult only | 650 | 69.3 (0.6) | -- | ||
| RAP lesion | |||||
| No | 966 | -- | 6.9 (0.5) | 0.03 | |
| Yes | 118 | -- | 10.1 (1.3) | ||
| Geographic atrophy | |||||
| None/questionable | 1027 | 68.2 (0.5) | 0.02 | -- | |
| Present | 76 | 63.9 (1.7) | -- | ||
| Total foveal thickness (μ) | |||||
| 1st quartile (≤325) | 277 | 68.0 (0.9) | 0.01 | -- | |
| 2nd quartile (>325 to ≤425) | 285 | 69.7 (0.9) | -- | ||
| 3rd quartile (>425 to ≤550) | 253 | 68.7 (0.9) | -- | ||
| 4th quartile (>550) | 285 | 65.5 (0.9) | -- | ||
| RPE elevation | |||||
| No | 145 | 71.2 (1.2) | 0.005 | 10.5 (1.2) | 0.004 |
| Yes | 944 | 67.5 (0.5) | 6.8 (0.5) | ||
| Treatment Group | |||||
| Ranibizumab Monthly | 284 | 69.4 (0.9) | 0.045 | 8.6 (0.9) | 0.07 |
| Bevacizumab Monthly | 265 | 68.6 (0.9) | 7.9 (0.9) | ||
| Ranibizumab as needed | 285 | 67.5 (0.9) | 6.9 (0.9) | ||
| Bevacizumab as needed | 271 | 66.2 (0.9) | 5.5 (0.9) | ||
SE = Standard Error; VA= Visual Acuity; CNV= choroidal neovascularization; RAP=Retinal Angiomatous Proliferans; RPE=Retinal Pigment Epithelium.
A total of 1061 participants were included in the final multivariate model, and 44 patients were excluded due to missing value in one or more predictors.
A total of 1068 participants were included in the final multivariate model, and 37 patients were excluded due to missing value in one or more predictors.
--: predictor was not included in the final multivariate model, because it was not statistically significant.
Predictors for VA score change from baseline at 1 year
One year after treatment, the mean VA score improved 7 letters from baseline and did not differ among the four treatment groups (p=0.16). The unvariate results for the predictors of the VA score change from baseline at one year were presented in Table 1 (available at http://aaojournal.org) for baseline participant factors, in Table 2 (available at http://aaojournal.org) for the baseline ocular and fundus characteristics of the study eye, and in Table 3 (available at http://aaojournal.org) for the baseline OCT features.
When baseline factors were considered simultaneously in the multivariate analysis (Table 4), the predictors of less gain in VA score at 1 year were: older age (p=0.003), baseline VA 20/40 or better in study eye (p<0.0001), larger CNV area (p=0.02), absence of a RAP lesion (p=0.03), and presence of RPE elevation (p=0.004). There were no statistically significant interactions between treatment group and any of these predictors.
Predictors for ≥3-line (i.e., 15 letters) gain from baseline at 1 year
At one year, 327 (29.6%) participants gained 3 lines or more in visual acuity from baseline. The unvairate results for the predictors of the proportion with ≥3 lines gain at one year were presented in Table 1 (available at http://aaojournal.org) for baseline participant factor, in Table 2 (available at http://aaojournal.org) for the baseline ocular and fundus characteristics of the study eye, and in Table 3 (available at http://aaojournal.org) for the baseline OCT features.
When baseline factors were considered simultaneously in the multivariate logistic regression (Table 5), older age was associated with a lower likelihood of ≥3 lines gained (p=0.008) with an odds ratio (OR) of 0.4 (95% CI: 0.3 – 0.7) for age 80–89 years as compared with 50–69 years old. In the study eye, better VA at baseline was associated with a decreased likelihood of gaining ≥3 lines (p<0.001). Compared to the study eyes with VA 20/50 to 20/80, the OR (95% CI) for gaining ≥ 3 lines was 0.11 (0.07 – 0.18) for eyes with VA 20/40 or better, 2.60 (1.80 – 3.77) for eyes with baseline VA of 20/100 to 20/160, and 1.7 (0.97 – 2.07) for 20/200–320. However, worse VA in the fellow eye was associated with a decreased likelihood of gaining ≥3 lines in the study eye (p=0.005): the OR was 0.5 (95% CI: 0.4–0.8) for fellow eyes with 20/50 or worse when compared to fellow eyes with VA 20/20 or better. Larger area of CNV was associated a lower likelihood of 3-lines gained (p=0.04), and the eyes in which the area of CNV could not be measured were least likely to gain ≥3 lines (OR=0.5, 95% CI: 0.3–0.8). Presence of RAP lesion was associated with increased likelihood of 3-lines gained (OR=1.9, 95% CI: 1.2 – 3.1). Total thickness at the fovea was associated with ≥3 lines gained (p=0.004). However, the relationship was not monotonic. The highest likelihood of ≥3 lines gained occurred when total foveal thickness was in the 2nd quartile (325–425 microns) with OR of 1.7 (95% CI: 1.1–2.7), and lowest likelihood of ≥3 lines gained occurred in the 4th quartile (>550 microns) (OR=0.8, 95% CI: 0.5–1.3). The presence of RPE elevation was associated with decreased likelihood of 3-lines gained (p=0.002). The proportion with 3-lines gained among the 4 treatment groups was statistically significant in the multivariate analysis (p=0.04). The two groups with as needed treatment had a decreased likelihood of ≥3-lines gained with an OR of 0.6 (95% CI: 0.4 – 0.9) when compared to ranibizumab monthly. There were no statistically significant interactions between treatment group and any of the above factors for gaining ≥3 lines of VA.
Table 5.
Analysis for 3-line gain from baseline at 1 Year
| Baseline Characteristics | N | ≥3-line gain (%) | Adjusted OR (95% CI) § | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| 50–69 | 131 | 51(38.9%) | 1.00 | 0.008 |
| 70–79 | 387 | 107(27.6%) | 0.62 (0.37, 1.02) | |
| 80–89 | 512 | 141(27.5%) | 0.44 (0.27, 0.73) | |
| ≥90 | 75 | 28(37.3%) | 0.67 (0.32, 1.41) | |
| Baseline VA in study eye | ||||
| 68–82 letters, 20/25 - 20/40 | 397 | 28(7.1%) | 0.11 (0.07, 0.18) | <0.0001 |
| 53–67 letters, 20/50 - 20/80 | 414 | 150(36.2%) | 1.00 | |
| 38–52 letters, 20/100–20/160 | 223 | 119(53.4%) | 2.60 (1.80, 3.77) | |
| 23–37 letters, 20/200-20/320 | 71 | 30(42.3%) | 1.73 (0.97, 2.07) | |
| Baseline VA in fellow eye | ||||
| 83–100 letters, 20/20 or better | 331 | 110(33.2%) | 1.00 | 0.005 |
| 68–82 letters, 20/25 - 20/40 | 433 | 135(31.2%) | 0.90 (0.63, 1.30) | |
| 0–67 letters, 20/50 or worse | 341 | 82(24.0%) | 0.53 (0.35, 0.80) | |
| Baseline area of CNV (mm2) | ||||
| ≤2.54 | 443 | 145(32.7%) | 1.00 | 0.04 |
| >2.54 to ≤5.08 | 219 | 63(28.8%) | 0.71 (0.47, 1.07) | |
| >5.08 to ≤10.2 | 207 | 65(31.4%) | 0.91 (0.60, 1.37) | |
| >10.2 | 103 | 24(23.3%) | 0.67 (0.38, 1.18) | |
| Can’t measure | 133 | 30 (22.6%) | 0.44 (0.25, 0.76) | |
| RAP lesion | ||||
| No | 966 | 276(28.6%) | 1.00 | 0.006 |
| Yes | 118 | 48(40.7%) | 1.94 (1.21, 3.10) | |
| Total foveal thickness (μ) | ||||
| 1st quartile (≤325) | 277 | 59(21.3%) | 1.00 | 0.004 |
| 2nd quartile (>325 to ≤425) | 285 | 95(33.3%) | 1.74 (1.11, 2.72) | |
| 3rd quartile (>425 to ≤550) | 253 | 83(32.8%) | 1.15 (0.73, 1.82) | |
| 4th quartile (>550) | 285 | 89(31.2%) | 0.80 (0.50, 1.27) | |
| RPE elevation | ||||
| No | 145 | 61(42.1%) | 1.00 | 0.002 |
| Yes | 944 | 258(27.3%) | 0.52 (0.34, 0.79) | |
| Treatment Group | ||||
| Ranibizumab Monthly | 284 | 97 (34.2%) | 1.00 | 0.04 |
| Bevacizumab Monthly | 265 | 83 (31.3%) | 0.80 (0.53, 1.22) | |
| Ranibizumab as needed | 285 | 71 (24.9%) | 0.56 (0.37, 0.86) | |
| Bevacizumab as needed | 271 | 76 (28.0%) | 0.63 (0.41, 0.96) | |
OR = Odds Ratio; CI = Confidence Interval; VA= Visual Acuity; CNV= choroidal neovascularization; RAP=Retinal Angiomatous Proliferans; RPE=Retinal Pigment Epithelium.
A total of 1066 participants were included in the final multivariate model, and 39 patients were excluded due to missing value in one or more predictors.
DISCUSSION
Our evaluation of the predictors for visual outcomes in response to treatment with ranbizumab or bevacizumab is based on prospectively collected clinical data and central grading of images performed in a multicenter randomized clinical trial with a large sample size and broad eligibility criteria. Some predictors of VA improvement identified in CATT, such as younger age, better baseline VA, smaller CNV area, are consistent with predictors identified from two pivotal clinical trials of ranbizumab.7,8,9 In addition, we have identified several new predictors of vision outcomes including total foveal thickness and RPE elevation on OCT. These identified predictors were the same across all four treatment groups of CATT.
Baseline VA in treated eyes was associated with all three visual outcomes, but in different ways. Eyes with worse baseline VA had lower mean VA scores at 1 year. However, the mean increase in VA and the proportion with ≥3-line improvement were greatest for the group of eyes with baseline VA 20/100 to 20/160, and were lowest for the eyes with VA 20/40 or better. This may be explained by the fact that eyes of participants with median age of 81 years having baseline VA 20/40 or better, particularly those having VA of 20/25 or 20/32, are unlikely to improve beyond 20/20 (1 or 2 lines of VA improvement) even if all visual loss imposed by CNV is eradicated. Although eyes with worse baseline visual acuity had greater improvement by 1 year, the average improvement was not sufficient to restore VA at 1 year to the same level for all participants. For example, while eyes with baseline VA of 20/100 to 20/160 improved on average 12 letters, their average VA at 1 year was only 59 letters (20/63), whereas eyes with baseline VA of 20/25 to 20/40 improved on average only 3 letters but had average VA at 1 year approximately 3 lines better (76 letters or 20/32). The detection of CNV before there is a large loss in vision remains extremely important even in this era of highly effective treatment.
CATT evaluated the relationship of baseline OCT features with visual outcomes in response to anti-VEGF treatment. Univariate analysis showed several OCT features were associated with visual outcomes including the presence of fluid and thickness of different layers of the retina, subretinal space, and sub-RPE space. However, after adjusting for demographic characteristics, baseline visual acuity, and other ocular features through multivariate analyses, only greater total foveal thickness and RPE elevation were independently associated with a lower proportion gaining 3 or more lines of VA.
RPE elevation anywhere in the macula was one of the OCT factors most strongly associated with vision outcomes. RPE elevation was common (85%) and independently associated with worse VA, lower mean increase in VA score, and a lower proportion with ≥3 lines gained in VA. This finding is unexpected. Perhaps the eyes without RPE elevation at baseline had unimpaired RPE function, and were thus more likely to have VA improvement than the majority of eyes with RPE elevation. Eyes with RPE elevation include those with sub-RPE hemorrhage, neovascularization or fibrosis or drusen alone, features that could signal abnormal RPE functional activity, and could have consequently adversely affected visual acuity. In MARINA and ANCHOR, increased RPE abnormalities observed on color photographs was strongly associated with VA loss.9 Together, these findings suggest that RPE function may play an important role in the visual acuity response to anti-VEGF treatment.
Sub-foveal hemorrhage secondary to CNV in AMD, if untreated, has a poor visual acuity prognosis.10 In CATT, the presence of hemorrhage and larger size of hemorrhage at baseline were associated with worse VA at 1 year and less gain in visual acuity. However, this association was statistically significant only in the univariate analysis, and not after adjustment for other baseline predictors including baseline VA. Eyes with hemorrhage may be expected to improve, consistent with findings from other studies.11
It is important to note that all subgroups of participants examined in this paper, had improvement in mean visual acuity after treatment with either drug or either regimen. Even the oldest patients or those with larger lesions experienced some improvement in vision and it is just as important to treat these patients despite potentially reduced expectations.
In conclusion, the predictors of visual outcomes did not differ between ranibizumab and bevacizumab or between dosing regimens in CATT. The results of this paper confirmed the predictors (age, baseline VA, lesion size) of VA improvement established from the previous clinical trials involving ranibizumab. This comprehensive evaluation of predictors in CATT also identified total foveal thickness, and RPE elevation as independently associated with VA outcomes. Early detection of CNV remains important for maximizing visual acuity after treatment. In addition, these predictors should not be used to justify reduced interest in treatment since all subgroups experienced some benefit in visual acuity from treatment. Instead, the identification of these predictors allow ophthalmologists and their patients to adjust their visual acuity expectations regarding vision based on the patient’s characteristics at the time of initial treatment. Finally, these predictors may help define eligibility criteria most likely to produce favorable or adverse visual acuity outcomes in future clinical trials for neovascular AMD.
Supplementary Material
Acknowledgments
Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
Reprints requests to: CATT Coordinating Center, University of Pennsylvania, 3535 Market Street, Suite 700, Philadelphia, PA 19104-3309.
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 4.Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.CATT Research Group. Ranibizumab and bevacizumab for neovascular agerelated macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 7.Boyer DS, Antoszyk AN, Awh CC, et al. MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–52. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–7. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Shapiro H, Tuomi L, et al. MARINA Study Group, ANCHOR Study Group. . Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523–30. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Avery R, Fekrat S, Hawkins B, Bressler N. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–9. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
- 11.McKibbin M, Papastefanou V, Matthews B, et al. Ranibizumab monotherapy for sub-foveal haemorrhage secondary to choroidal neovascularisation in age-related macular degeneration. Eye (Lond) 2010;24:994–8. doi: 10.1038/eye.2009.271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


