Abstract
Eukaryotic translation initiation factor 5A (eIF5A) is the only cellular protein that contains the unusual amino acid hypusine [Nε-(4-amino-2-hydroxybutyl)lysine]. The role of hypusine formation in the eIF5A protein in the regulation of cell proliferation and apoptosis is addressed in the present review. Moreover, vertebrates carry two genes that encode two eIF5A isoforms, eIF5A-1 and eIF5A-2, which, in humans, are 84% identical. However, the biological functions of these two isoforms may be significantly different. In fact, eIF5A-1 is demonstrable in most cells of different histogenesis, whereas eIF5A-2 protein is detectable only in certain human cancer cells or tissues, suggesting its role as a potential oncogene. In this review we focus our attention on the involvement of eIF5A-1 in the triggering of an apoptotic program and in the regulation of cell proliferation. In addition, the potential oncogenic role and prognostic significance of eIF5A-2 in the prediction of the survival of cancer patients is described. eIF5A-1 and/or the eIF5A-2 isoform may serve as a new molecular diagnostic or prognostic marker or as a molecular target for anti-cancer therapy.
Keywords: eIF5A isoform, Hypusine, Tissue transglutaminase, Cancer, Apoptosis, Prognostic markers, Hypusine synthesis inhibitors
Introduction
The eukaryotic initiation factor 5A (eIF5A) is unusual in that its activity is modulated by post-translational modifications that culminate in the formation of the unusual amino acid hypusine. Hypusine [Nε-(4-amino-2-hydroxybutyl)lysine] is formed by the transfer of the butylamine portion from the polyamine spermidine to the ε-amino group of a specific lysine residue of eIF5A precursor (Wolff et al. 1990) catalyzed by deoxyhypusine synthase (DHS) and by the subsequent hydroxylation at carbon 2 of the transferred 4-aminobutyl moiety by deoxyhypusine hydroxylase (DOHH) (Abbruzzese et al. 1986; Park et al. 1993, 2006). eIF5A probably acts in the final stage of the initiation phase of protein synthesis by promoting the formation of the first peptide bond, but recent reports also suggest its role in translation elongation (Zanelli and Valentini 2007; Gregio et al. 2009; Saini et al. 2009). Hypusine plays a key role in the regulation of eIF5A function because its precursors, which do not contain hypusine, do not have activity in the in vitro assay of methionyl-puromycin synthesis (Smit-McBride et al. 1989; Park et al. 1991). These biochemical correlates make eIF-5A unique. In fact, it is the hypusine-containing eIF-5A form that is active and hypusine is contained only in this factor. Consequently, intracellular hypusine content in general reflects cellular eIF-5A activity. The correlation between hypusine, and thus eIF5A activity, and cell proliferation (Abbruzzese et al. 1988; Park et al. 2010) suggests that activated eIF5A plays a role in cell growth and differentiation (Schnier et al. 1991 see review Park et al. 2010).
Two human isoforms of eIF5A exist, eIF5A-1 and eIF5A-2 (Jenkins et al. 2001; Guan et al. 2001). Both isoforms harbor the hypusine modification (Clement et al. 2003). The first isoform, eIF5A-1, is constitutively expressed in all mammalian cells and is abundant in proliferating cells (Hanauske-Abel et al. 2002; Cracchiolo et al. 2004; Clement et al. 2006). In fact, eIF5A-1 mRNA is under control of the myc oncogene (Coller et al. 2000; Boon et al. 2001) and eIF5A-1 is overexpressed in human cancer tissues (Cracchiolo et al. 2004). In contrast, the second isoform, eIF5A-2, protein is normally too low to be detected in mammalian cells and tissues (Clement et al. 2003). Its mRNA is expressed only in specific tissues like testis and parts of the brain or in certain cancer cell lines (Jenkins et al. 2001; Clement et al. 2006) suggesting a differentiated function of the second isoform, relevant to chemotherapeutic intervention of eIF-5A function in cancers (Caraglia et al. 2000, 2003; Clement et al. 2002).
Amplification and overexpression of the EIF5A2 gene have frequently been detected in various human malignancies such as ovarian, and colorectal cancer tissues and cell lines (Guan et al. 2001; Clement et al. 2006) and can cause cellular transformation (Guan et al. 2001, 2004; Tang et al. 2010) indicating its potential role in cancer development. In fact, overexpression of eIF5A-2 has been reported to be associated with metastasis development in multiple cancer types, including colon (Xie et al. 2008), ovarian (Yang et al. 2009), bladder (Chen et al. 2009) and liver cancer (Tang et al. 2010).
Gene disruption and mutation studies in yeast and higher eukaryotes have provided valuable information on the essential nature of eIF5A and the deoxyhypusine/hypusine modification in cell growth and in protein synthesis. In fact, disruption of both eIF5A genes or inactivation of the single deoxyhypusine synthase gene, in the yeast Saccharomyces cerevisiae, or substitution with a mutated eIF5A gene (Lys50Arg) causes growth arrest (Schnier et al. 1991; Sasaki et al. 1996; Park et al. 1998). Knock-down of eIF5A-1 or the DHS gene is embryonic lethal in mice (Nishimura et al. 2011). Furthermore, inhibitors of deoxyhypusine synthase, such as N1-guanyl-1,7-diaminoheptane, and of deoxyhypusine hydroxylase, such as ciclopirox, exert strong anti-proliferative effects in mammalian cells, including various human cancer cell lines, and cause arrest of cell cycle progression (Park et al. 1994; Hanauske-Abel et al. 1994; Chen et al. 1996; Clement et al. 2002; Nishimura et al. 2005).
Role of hypusine synthesis and eIF5A-1 in apoptosis
It is well established that eIF5A is essential for proliferation and survival of eukaryotic cells and this aspect has been extensively discussed in previous reviews (Park et al. 1998, 2010; Chen and Liu 1997). Therefore, we will focus on another aspect of eIF5A-1 activity: its potential involvement in the regulation of apoptosis in mammalian cells.
Endogenous eIF5A exists predominantly as the hypusinated form. The unhypusinated eIF5A precursor protein accumulates only when deoxyhypusine synthesis is blocked by inhibitors of DHS or by deprivation of the polyamine spermidine. The potential involvement of this polyamine-dependent modification of eIF5A in the triggering of apoptosis in tumour cells was initially suggested by Tome and Gerner (1997; Tome et al. 1997). In the hepatoma cell line DH23A/b, expressing high levels of stable ornithine decarboxylase, excess accumulation of putrescine led to induction of apoptosis and suppression of the formation of hypusine-containing eIF5A-1. Furthermore, we have obtained evidence for a role for eIF5A-1 in the cellular apoptosis triggered by tissue transglutaminase (tTG). Tissue transglutaminase catalyzes an in vitro modification of eIF5A-1 by forming a γ-glutamyl hypusine bond between eIF5A-1 and dimethylcasein (Beninati et al. 1995). Interestingly, stable transfection of tTG in Balb-C 3T3 cells strongly reduced cellular hypusine levels and increased γ-glutamyl hypusine linkage in cellular proteins (Beninati et al. 1998). These effects occurred together with a significant reduction of cell proliferation, suggesting a role for eIF5A-1 in the regulation of apoptosis. In an independent study, we have reported that interferon-α (IFNα) induced growth inhibition and concomitant reduction of the hypusinated eIF5A-1 level and eIF5A-1 activity in human epidermoid cancer KB cells and that these effects were antagonized by EGF (Caraglia et al. 1997). IFNα induced a strong inhibition of hypusine synthesis and thereby of eIF5A-1 activity, while the total level of eIF5A-1 protein (hypusine modified plus unmodified) increased. This finding suggests a further reduction of the active fraction of eIF5A-1 (hypusine-containing eIF5A:total eIF5A ratio) and an increase in unmodified eIF5A precursor upon treatment with IFNα. On the other hand, EGF antagonized the apoptosis induced by IFNα and caused a restoration of hypusine synthesis and an increase of extracellular signal regulated kinase (ERK) activity. On the basis of these results, we have investigated whether eIF5A could be really critical for the biological effects induced by IFNα. We have used a potent inhibitor of deoxyhypusine synthase, N1-guanyl-1,7-diaminoheptane (GC7), that inhibits hypusine formation thereby leading to accumulation of unmodified eIF5A precursor (Nishimura et al. 2005). We have found that this agent synergized with IFNα in inducing cell growth inhibition and apoptosis suggesting a critical role for eIF5A-1 in the modulation of cell proliferation in human epidermoid cancer cells (Caraglia et al. 2003). All these data support the hypothesis of an involvement of eIF5A-1 in the apoptosis induced by IFNα in human epithelial cells.
More recently, the potential role of eIF5A-1 in apoptosis was directly addressed by suppression of eIF5A-1 expression by use of siRNAs (Taylor et al. 2004, 2007) or by overexpression of eIF5A-1 using a plasmid vector (Li et al. 2004) or adenoviral vector (Taylor et al. 2007; Sun et al. 2010). Parenthetically, it is important to emphasize that exogenous overexpression of eIF5A results in accumulation of unhypusinated eIF5A precursor, but not of the hypusinated eIF5A (Park et al. 2006), because exogenously expressed eIF5A precursor is not effectively modified by endogenous DHS and DOHH. In primary lamina cribrosa cells of the human optic nerve head, siRNA-mediated suppression of eIF5A-1 expression protected cells from TNF-α-induced apoptosis (Taylor et al. 2004) and reduced the cytotoxic effects of actinomycin D (Taylor et al. 2007). The role of eIF5A-1 in apoptosis induction was also investigated in colon cancer cells using adenoviral vectors encoding eIF5A-1 wild type or mutant protein (K50A) that cannot be hypusinated. Infection with either wild type or the mutant adenoviral vector led to a high accumulation of eIF5A-1 mainly as the unhypusinated form with concomitant decrease in hypusinated eIF5A-1 and caused a drastic increase in apoptosis, again suggesting a role for the un-hypusinated eIF5A-1 in apoptotic cell death (Taylor et al. 2004, 2007; Sun et al. 2010). Recent studies have further indicated that apoptosis induction by eIF5A-1 involves activation of the intrinsic mitochondrial pathway (Sun et al. 2010). In HCT116 cells, eIF5A-1 level was increased by mitomycin C treatment in a p53-dependent manner, suggesting that eIF5A-1 is a target of p53-mediated transcriptional control (Rahman-Roblick et al. 2007). In lung cancer cells, exogenous overexpression of eIF5A-1 enhanced apoptosis in a p53-dependent manner (Li et al. 2004).
Thus, the connection between eIF5A and apoptosis induction is rather complex. Apoptosis was induced in various mammalian cells under conditions where hypusinated eIF5A was reduced or where unhypusinated eIF5A precursor was accumulated or overproduced. However, it is not clear whether the growth inhibition and apoptosis induction by IFNα, TNFα or GC7 is mediated by the reduced level of hypusinated eIF5A-1 or by increased accumulation of unhypusinated eIF5A-1 precursor. It is also not clear how unhypusinated eIF5A-1 exerts apoptotic effects, either by interfering with the activity of hypusinated eIF5A-1 or by a new activity of its own.
In a mouse diabetes model system, hypusinated eIF5A-1 has been implicated in apoptosis induction through its role in the expression of inflammatory cytokines (Maier et al. 2010). In this system, both eIF5A-1 siRNA and the deoxyhypusine synthesis inhibitor GC7 were able to prevent apoptotic death of pancreatic cells by inhibition of inflammatory cytokine expression. Thus, reduction of eIF5A-1 expression or inhibition of hypusine modification may cause induction of apoptosis or suppression of apoptosis, depending on the biological system.
In addressing the biological functions of hypusinated and unhypusinated eIF5A, their subcellular localization may hold significance to their cellular function. A few previous reports suggested that eIF5A was distributed throughout the cytoplasm and nucleus (Ruhl et al. 1993; Rosorius et al. 1999; Jao and Chen 2002), whereas other studies indicated that eIF5A was largely localized in the cytoplasm and absent from the nucleus (Shi et al. 1996, 1997; Valentini et al. 2002; Jin et al. 2003; Cracchiolo et al. 2004). These initial studies were carried out without distinction between endogenous, hypusinated eIF5A from those epitope-tagged exogenously expressed eIF5A that stays as unmodified precursor. A more recent study analyzed the subcellular distribution of unhypusinated eIF5A generated from an eIF5A vector versus its hypusinated counterpart formed by co-expression of eIF5A and its two modification enzymes, DHS and DOHH. Unhypusinated GFP-eIF5A was distributed in whole cell (cytoplasm as well as nuclei), whereas hypusinated GFP-eIF5A was mainly localized in the cytoplasm, suggesting a role for hypusine modification in eIF5A subcellular localization (Lee et al. 2009), probably through its hypusine-dependent binding to exportin 4 (Lipowsky et al. 2000). A rapid translocation of eIF5A-1 from the cytoplasm to the nucleus was observed by Taylor et al. (2007) in colon cancer cells sensitized with IFN-γ and treated with TNF-α. Probably, eIF5A is retained in the cytoplasm until an apoptotic stimulus triggers its translocation to the nucleus where it may have pro-apoptotic functions (Taylor et al. 2007). The exact role of nuclear eIF5A in apoptosis induction warrants further investigation.
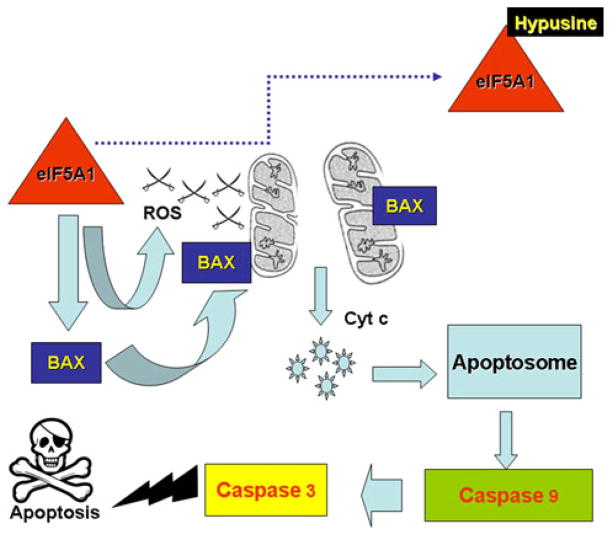
The mechanism by which eIF5A-1 contributes to apoptosis induction has been investigated in conjunction with p53. In various mammalian cells treated with cytokines or cytotoxic drugs such as IFNγ, TNFα, mitomycin C or actinomycin D, induction of apoptosis was often accompanied by a notable increase in the levels of eIF5A-1 and p53 (Rahman-Roblick et al. 2007; Taylor et al. 2007) (Fig. 1).
Fig. 1.
Mechanism of apoptosis induction by eIF5A-1/eIF5A-1 precursor. Over-expression of eIF5A-1 (unhypusinated precursor form) induces loss of mitochondrial transmembrane potential, translocation of Bax to the mitochondria, release of cytochrome c, caspase 9 and 3 activation. These events lead to apoptosis. On the other hand, over-expression of eIF5A-1 can also increase reactive oxygen species (ROS) generation that, creating mitochondrial dysfunction, releases cytochrome c and activates caspases leading to apoptosis. Hypusine-containing eIF5A-1 loses the ability to induce Bax translocation to mitochondria and/or ROS elevation
Oncogenic role of eIF5A-2
Unlike eIF5A-1, which is ubiquitously expressed, eIF5A-2 protein is normally not detected and its mRNA is expressed in a tissue-dependent manner. Enhanced expression of eIF5A-1 was observed in human neoplastic or cancer tissues and (Cracchiolo et al. 2004; Lam et al. 2010; Bala-banov et al. 2007), EIF5A2 overexpression and gene amplification have been specifically implicated in various human cancers (Guan et al. 2001; He et al. 2011; Xie et al. 2008; Chen et al. 2003, 2009; Tang et al. 2010; Marchet et al. 2007; Lee et al. 2010 Yang et al. 2009) (Fig. 2).
Fig. 2.
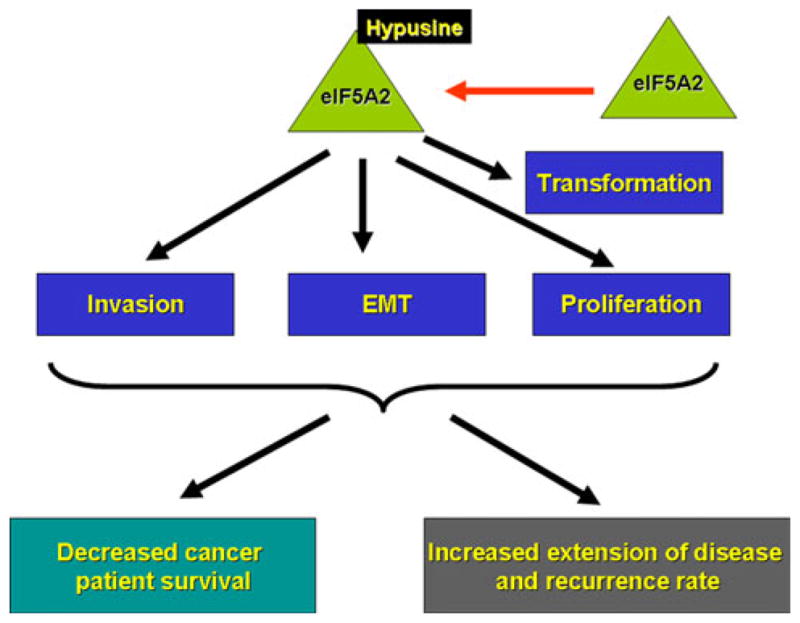
Role of eIF5A-2 in human tumors. Over-expression of eIF5A2 (preferentially hypusinated) is able to transform NIH-3T3 fibroblasts and is correlated with tumor cell invasion, proliferation and epithelial-mesenchymal transition (EMT). Its over-expression in human cancer tissues is also associated with decreased cancer patient survival and increased extension of disease and recurrence rate
Overexpression of eIF5A-2 mRNA has also been observed in various cancer cell lines (Clement et al. 2006; Guan et al. 2001), and eIF5A-2 has been suggested as a candidate oncogene associated with different cancers (Guan et al. 2004). The EIF5A2 gene is commonly amplified and/or over-expressed in several types of human cancers, including ovarian (Guan et al. 2004; Yang et al. 2009), colorectal (Xie et al. 2008) and bladder cancer (Chen et al. 2009) with a higher risk of lymph node metastasis, in human gastric adenocarcinoma (Marchet et al. 2007), hepatocellular carcinoma (HCC) (Tang et al. 2010; Lee et al. 2010), lung adenocarcinoma (Chen et al. 2003) and other non-small cell lung cancers (He et al. 2011). EIF5A2 gene is localized at a frequently amplified region at chromosome 3q26 of a primary ovarian cancer cell line (Jenkins et al. 2001; Guan et al. 2001). Amplification of chromosome 3q has been frequently detected in several human malignancies (Sham et al. 2002), including lung cancer (Kettunen et al. 2000), suggesting that an oncogene(s) localized in this region might play important roles in carcinogenesis.
The potential oncogenic function of eIF5A-2 was investigated by use of anti-sense DNA, siRNA or by overexpression of eIF5A-2 in mammalian cells. In the ovarian cancer cell line UACC-1598, which overexpresses eIF5A-2 owing to EIF5A2 gene amplification, cell growth could be inhibited by treatment with eIF5A-2 antisense DNA (Guan et al. 2004). Furthermore, overexpression of eIF5A-2 in NIH3T3 cells and human liver cell line LO2 caused cell transformation/anchorage-independent growth, and tumorigenicity in nude mice, respectively (Guan et al. 2004). Regarding the specific mechanism of eIF5A-2 in carcinogenesis, some insights were provided from studies in HCC, in which eIF5A-2 expression was associated with the metastatic stage and cancer progression (Tang et al. 2010). Interestingly, the invasive border between tumor and non-tumor tissues showed a higher level of eIF5A-2 expression, indicating that this protein may contribute to a more malignant and invasive phenotype of the cancer cells. Over-expression of eIF5A-2 significantly enhanced cell motility and invasiveness of tumor cells, whereas silencing of eIF5A-2 expression by siRNA against EIF5A2 resulted in significant inhibition of cell motility (Tang et al. 2010) or cell proliferation in HCC cell lines (Lee et al. 2010). Hepatocellular carcinoma cells over-expressing eIF5A-2 probably undergo epithelial mesenchymal transition (EMT) to achieve higher motility and invasiveness through activation of RhoA and Rac1 (Tang et al. 2010). These data provide strong evidence that increased expression of eIF5A-2 may be involved in the invasive and/or metastatic processes of several types of human cancer.
An oncogenic function of eIF5A-2 in liver cancer was also revealed from an independent approach using an oncogenomics-based in vivo RNAi screen designed to search for tumor suppressors in liver cancer (Zender et al. 2008). In this study, exportin 4, the proposed nuclear transporter of eIF5A (Lipowsky et al. 2000) was identified as a tumor suppressor. Retroviral transduction of eIF5A-2 cDNA into p53−/−, Myc hepatocytes promoted hepato-cellular carcinoma, whereas cells transduced with eIF5A-1 retroviral vector did not develop tumors (Zender et al. 2008). Apparently, nuclear accumulation of eIF5A-1 or eIF5A-2 was observed in exportin 4 deficient cells, suggesting a possibility that nuclear accumulation of eIF5A-2 is involved in cellular transformation.
Expression level of eIF5A-2, measured by immunohistochemistry, RTPCR or by microarray analysis, was correlated with clinical status in various human cancer tissues including HCC (Lee et al. 2007, 2010), gastric cancer (Marchet et al. 2007), ovarian cancer (Yang et al. 2009), lung adenocarcinoma (Chen et al. 2003), urothelial carcinoma of the bladder (Chen et al. 2009) and small cell lung cancer (He et al. 2011). Overexpression of eIF5A-2 protein was found to correlate with an ascending clinical stage and poor patient prognosis in ovarian cancer patients (Yang et al. 2009). Similar to that observed in ovarian carcinoma, in non-small cell lung cancer (NSCLC) specimens, an over-expression of eIF5A-2 was frequently detected, and the frequency of eIF5A-2 over-expression increased with an ascending pT stage in NSCLC (He et al. 2011). Thus, examination of eIF5A-2 expression by immunohistochemistry was suggested as a useful molecular marker in distinguishing stage I NSCLC patients with unfavorable prognosis from those with better prognosis (He et al. 2011). Also in human tissues from urothelial carcinoma of the bladder (UC); up-regulated expression of eIF5A-2 mRNA was observed in 50% of specimens, although the protein expression level of eIF5A-2 did not always coincide with gene amplification (Chen et al. 2009). More importantly, stratified survival analysis of UC histopathologic grade and/or pTN stage showed correlation between up-regulated expressions of EIF5A2 with a poor prognostic phenotype (Chen et al. 2009). High expression level of both eIF5A-1 and eIF5A-2 in liver tumor tissues correlated with the appearance of many tumor nodules and tumor venous infiltration, respectively, in HCC patients, for which the number of tumor nodules is one of the criteria indicating the degree of disease severity (Lee et al. 2010).
Conclusions
Eukaryotic translation initiation factor 5A is an old factor in the protein synthesis machinery with new and unexpected properties in the regulation of cell proliferation, apoptosis and carcinogenesis of eukaryotic cells. The regulation of eIF5A function is complicated by the presence of two isoforms and of different eIF5A forms with respect to modification status for each isoform. The two isoforms appear to have different functions, and the hypusinated and unhypusinated eIF5A proteins may also exert opposite functions in cells. Under normal conditions (mammalian cells and tissues), hypusinated eIF5A-1 is the predominant form and the eIF5A-2 isoform expression is silenced. However, in certain human malignancies, eIF5A-2 gene on 3q26 is often amplified or activated leading to a high level of eIF5A-2 protein. When addressing the biological effects of exogenous expression of eIF5A in cells by transduction or transfection, attention should be paid to the hypusine modification status, because exogenously expressed eIF5A mainly exists as an unmodified eIF5A precursor and co-expression of two modifying enzymes are usually required for overproduction of hypusinated eIF5A. Another factor that may contribute to the intracellular activity of eIF5A isoforms (modified and unmodified) is subcellular localization of eIF5A. Although hypusinated eIF5A is mainly distributed in cytoplasm and the unhypusinated eIF5A throughout the cytoplasm and nucleus, their subcellular localization and function may be altered under treatment with cytokines or drugs. Hypusine modification appears to be required for the proliferative activity of eIF5A in most cells, including cancer cells. eIF5A-1 precursor protein (not hypusinated) seems to be involved in the triggering of the programmed cell death in different cell models of different histogenesis. Inversely, eIF5A-2 is often over-expressed in cancer cells and its expression has been directly correlated with the biological aggressiveness of the tumors and with the presence of invasion and metastases. From a clinical point of view, both eIF5A-1 and its isoform can be proposed as new diagnostic markers in several cancers, whereas eIF5A-2 seems to be a good predictor of the clinical outcome of patients affected by different neoplasms. Moreover, eIF5A-2 may represent a molecular target for gene therapy approaches based upon the use of siRNAs eventually delivered with appropriate nanocarriers. All these results provide strong evidence that both eIF5A-1 and eIF5A-2 can be explored as a tool in cancer management, one as a modulator of apoptosis and the other to block tumor formation and metastasis. Moreover, the use of inhibitors of hypusine formation has been shown as a promising strategy in an attempt to increase the anti-cancer activity of chemotherapeutic agents such as type I interferons and warrant further investigations in pre-clinical cancer models. For example, inhibition of eIF5A hypusination was demonstrated to have anti-tumorigenic effect on leukemia cells when given either alone or in combination with imatinib (Balabanov et al. 2007).
Acknowledgments
This paper is dedicated to the memory of Dr. J.E. Folk (Oct 29, 1925–Dec 27, 2010). In addition to his pioneering work on the mechanism of transglutaminase, he and his colleagues discovered the hypusine modification pathway. The research was supported in part by the Intramural Research Program of National Institute of Dental and Craniofacial Research (NIDCR).
Contributor Information
M. Caraglia, Email: michele.caraglia@unina2.it, Department of Biochemistry and Biophysics, Second University of Naples, Via Costantinopoli, 16, 80138 Naples, Italy
M. H. Park, The Oral and Pharyngeal Cancer Branch, NIDCR, National Institutes of Health, Bethesda, MD 20892, USA
E. C. Wolff, The Oral and Pharyngeal Cancer Branch, NIDCR, National Institutes of Health, Bethesda, MD 20892, USA
M. Marra, Department of Biochemistry and Biophysics, Second University of Naples, Via Costantinopoli, 16, 80138 Naples, Italy
A. Abbruzzese, Department of Biochemistry and Biophysics, Second University of Naples, Via Costantinopoli, 16, 80138 Naples, Italy
References
- Abbruzzese A, Liguori V, Park MH. Deoxyhypusine hydroxylase. Adv Exp Med Biol. 1988;250:459–466. doi: 10.1007/978-1-4684-5637-0_40. [DOI] [PubMed] [Google Scholar]
- Abbruzzese A, Park MH, Folk JE. Deoxyhypusine hydroxylase from rat testis: partial purification and characterization. J Biol Chem. 1986;261:3085–3089. [PubMed] [Google Scholar]
- Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M, Brassat U, Priemer M, Hauber I, Wilhelm T, Schwarz G, Kanz L, Bokemeyer C, Hauber J, Holyoake TL, Nordheim A, Brümmendorf TH. Hypusination of eukaryotic initiation factor 5A(eIF5A): a novel therapeutic target in BCR-ABL–positive leukemias identified by a proteomics approach. Blood. 2007;109(4):1701–1711. doi: 10.1182/blood-2005-03-037648. [DOI] [PubMed] [Google Scholar]
- Beninati S, Nicolini L, Jakus J, Passeggio A, Abbruzzese A. Identification of a substrate site for transglutaminases on the human protein synthesis initiation factor 5. Biochem J. 1995;305:725–728. doi: 10.1042/bj3050725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninati S, Gentile V, Caraglia M, Lentini A, Tagliaferri P, Abbruzzese A. Tissue transglutaminase expression affects hypusine metabolism in BALB/c 3T3 cells. FEBS Lett. 1998;437:34–38. doi: 10.1016/s0014-5793(98)01191-0. [DOI] [PubMed] [Google Scholar]
- Boon K, Caron HN, van Asperen R, Valentijn L, Hermus MC, van Sluis P, Roobeek IIW, Voute PA, Schwab M, Versteeg R. N-myc enhances the expression of a large set of genes functioning in ribosome biogenesis and protein synthesis. EMBO J. 2001;20:1383–1393. doi: 10.1093/emboj/20.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraglia M, Passeggio A, Beninati S, Leardi A, Nicolini L, Improta S, Pinto A, Bianco AR, Tagliaferri P, Abbruzzese A. Interferon alpha2 recombinant and epidermal growth factor modulate proliferation and hypusine synthesis in human epidermoid cancer KB cells. Biochem J. 1997;324:737–741. doi: 10.1042/bj3240737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraglia M, Budillon A, Vitale G, Lupoli G, Tagliaferri P, Abbruzzese A. Modulation of molecular mechanisms involved in protein synthesis machinery as a new tool for the control of cell proliferation. Eur J Biochem. 2000;267:3919–3936. doi: 10.1046/j.1432-1327.2000.01465.x. [DOI] [PubMed] [Google Scholar]
- Caraglia M, Marra M, Giuberti G, D’Alessandro AM, Baldi A, Tassone P, Venuta S, Tagliaferri P, Abbruzzese A. The eukaryotic initiation factor 5A is involved in the regulation of proliferation and apoptosis induced by interferon-alpha and EGF in human cancer cells. J Biochem (Tokyo) 2003;133:757–765. doi: 10.1093/jb/mvg097. [DOI] [PubMed] [Google Scholar]
- Chen KY, Liu AY. Biochemistry and function of hypusine formation on eukaryotic initiation factor 5A. Biol Signals. 1997;6:1105–1109. doi: 10.1159/000109115. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Yan YP, Ding QJ, Knapp S, Potenza JA, Schugar HJ, Chen KY. Effects of inhibitors of deoxyhypusine synthase on the differentiation of mouse neuroblastoma and erythroleukemia cells. Cancer Lett. 1996;105:233–239. doi: 10.1016/0304-3835(96)04287-5. [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Thomas DG, Huang CC, Misek DE, Kuick RD, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Proteomic analysis of eIF5A in lung adenocarcinomas. Proteomics. 2003;3:496–504. doi: 10.1002/pmic.200390063. [DOI] [PubMed] [Google Scholar]
- Chen W, Luo JH, Hua WF, Zhou FJ, Lin MC, Kung HF, et al. Overexpression of EIF-5A2 is an independent predictor of outcome in patients of urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Epidemiol Biomarkers Prev. 2009;18:400–408. doi: 10.1158/1055-9965.EPI-08-0754. [DOI] [PubMed] [Google Scholar]
- Clement PM, Hanauske-Abel HM, Wolff EC, Kleinman HK, Park MH. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer. 2002;100:491–498. doi: 10.1002/ijc.10515. [DOI] [PubMed] [Google Scholar]
- Clement PMJ, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JWB, Park MH, Johansson HE. Identification and characterization of eukaryotic initiation factor 5A–2. Eur J Biochem. 2003;147:4254–4263. doi: 10.1046/j.1432-1033.2003.03806.x. [DOI] [PubMed] [Google Scholar]
- Clement PMJ, Johansson HE, Wolff EC, Park MH. Differential expression of eIF5A–1 and eIF5A–2 in human cancer cells. FEBS J. 2006;273:1102–1114. doi: 10.1111/j.1742-4658.2006.05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller HA, Grandori C, Tamayo P, Colbert T, Lander ES, Eisenman RN, Golub TR. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc Natl Acad Sci USA. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracchiolo BM, Heller DS, Clement PM, Wolff EC, Park MH, Hanauske-Abel HM. Eukaryotic initiation factor 5A-1 (eIF5A-1) as a diagnostic marker for aberrant proliferation in intraepithelial neoplasia of the vulva. Gynecol Oncol. 2004;94:217–222. doi: 10.1016/j.ygyno.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Gregio AP, Cano VP, Avaca JS, Valentini SR, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001;61:3806–3809. [PubMed] [Google Scholar]
- Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Wu QL, Fang Y, Sham JS. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res. 2004;64:4197–4200. doi: 10.1158/0008-5472.CAN-03-3747. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel HM, Park MH, Hanauske AR, Popowicz AM, Lalande M, Folk JE. Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim Biophys Acta. 1994;1221:115–124. doi: 10.1016/0167-4889(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel HM, Heller D, Wolff EC, Hameed M, Clement PMJ, Park MH, et al. Eukaryotic translation initiation factor 5A, an emerging target for cytostatic compounds, localizes to proliferative regions in human tissue. Cancer Epidemiol Bio-markers Prev. 2002;11:1145s. [Google Scholar]
- He LR, Zhao HY, Li BK, Liu YH, Liu MZ, Guan XY, Bian XW, Zeng YX, Xie D. Overexpression of eIF5A–2 is an adverse prognostic marker of survival in stage I non-small-cell lung cancer patients. Int J Cancer. 2011;129(1):143–150. doi: 10.1002/ijc.25669. [DOI] [PubMed] [Google Scholar]
- Jao DLE, Chen KY. Subcellular localization of the hypusine-containing eukaryotic initiation factor 5A by immunofluorescent staining and green fluorescent protein tagging. J Cell Biochem. 2002;86:590–600. doi: 10.1002/jcb.10235. [DOI] [PubMed] [Google Scholar]
- Jenkins ZA, Haag PG, Johansson HE. Human EIF5A2 on chromosome 3q25–q27, is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics. 2001;71:101–109. doi: 10.1006/geno.2000.6418. [DOI] [PubMed] [Google Scholar]
- Jin BF, He K, Wang HX, Wang J, Zhou T, Lan Y, Hu MR, Wei KH, Yang SC, Shen BF, Zhang XM. Proteomic analysis of ubiquitin–proteasome effects: insight into the function of eukaryotic initiation factor 5A. Oncogene. 2003;22:4819–4830. doi: 10.1038/sj.onc.1206738. [DOI] [PubMed] [Google Scholar]
- Kettunen E, el-Rifai W, Bjorkqvist AM, Wolff H, Karjalainen A, Anttila S, Mattson K, Husgafvel-Pursiainen K, Knuutila S. A broad amplification pattern at 3q in squamous cell lung cancer: a fluorescence in situ hybridization study. Cancer Genet Cytogenet. 2000;117:66–70. doi: 10.1016/s0165-4608(99)00146-6. [DOI] [PubMed] [Google Scholar]
- Lam FF, Jankova L, Dent OF, Molloy MP, Kwun SY, Clarke C, Chapuis P, Robertson G, Beale P, Clarke S, Bokey EL, Chan C. Identification of distinctive protein expression patterns in colorectal adenoma. Proteomics Clin Appl. 2010;4:60–70. doi: 10.1002/prca.200900084. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheung ST, Poon RT, Fan ST, Luk JM. Genomic and proteomic biomarkers for diagnosis and prognosis of hepatocellular carcinoma. Biomarkers Med. 2007;1:273–284. doi: 10.2217/17520363.1.2.273. [DOI] [PubMed] [Google Scholar]
- Lee SB, Park JH, Kaevel JK, Sramkova M, Weigert R, Park MH. The effect of hypusine modification on the intracellular localization of eIF5A. Biochem Biophys Res Commun. 2009;383:497–502. doi: 10.1016/j.bbrc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NP, Tsang FH, Shek FH, Mao M, Dai H, Zhang C, Dong S, Guan X, Poon RTP, Luk JM. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127:968–976. doi: 10.1002/ijc.25100. [DOI] [PubMed] [Google Scholar]
- Li AL, Li HY, Jin BF, Ye QN, Zhou T, Yu XD, Pan X, Man JH, He K, Yu M, Hu MR, Wang J, Yang SC, Shen BF, Zhang XM. A novel eIF5A complex functions as a regulator of p53 and p53-dependent apoptosis. J Biol Chem. 2004;279:49251–49258. doi: 10.1074/jbc.M407165200. [DOI] [PubMed] [Google Scholar]
- Lipowsky G, Bischoff FR, Schwarzmaier P, Kraft R, Kostka S, Hartmann E, Kutay U, Görlich D. Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 2000;19(16):4362–4371. doi: 10.1093/emboj/19.16.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE, Dondero RS, Lewis EC, Dinarello CA, Nadler JL, Mirmira RG. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–2170. doi: 10.1172/JCI38924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchet A, Mocellin S, Belluco C, Ambrosi A, DeMarchi F, Mammano E, Digito M, Leon A, D’Arrigo A, Lise M, Nitti D. Gene expression profile of primary gastric cancer: towards the prediction of lymph node status. Ann Surg Oncol. 2007;14:1058–1064. doi: 10.1245/s10434-006-9090-0. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Murozumi K, Shirahata A, Park MH, Kashiwagi K, Igarashi K. Independent roles of eIF5A and polyamines in cell proliferation. Biochem J. 2005;385:779–785. doi: 10.1042/BJ20041477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Lee SB, Park JH, Park MH. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 2011 doi: 10.1007/s00726-011-0986-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Wolff EC, Folk JE. Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors. 1993;4(2):95–104. [PubMed] [Google Scholar]
- Park J-H, Aravind L, Wolff EC, Kaevel J, Kim YS, Park MH. Molecular cloning, expression and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci USA. 2006;103:51–56. doi: 10.1073/pnas.0509348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Joe YA, Kang KR. Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:1677–1683. doi: 10.1074/jbc.273.3.1677. [DOI] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional Significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Wolff EC, Smit-McBride Z, Hershey JW, Folk JE. Comparison of the activities of variant forms of eIF-4D. The requirement for hypusine or deoxyhypusine. J Biol Chem. 1991;266(13):7988–7994. [PubMed] [Google Scholar]
- Park MH, Wolff EC, Lee YB, Folk JE. Antiproliferative effects of inhibitors of deoxyhypusine synthase: inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem. 1994;269:27827–27832. [PubMed] [Google Scholar]
- Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, Liu T, Becker S, Hirschberg D, Jornvall H, Auer G, Wiman KG. p53 targets identified by protein expression profiling. Proc Natl Acad Sci USA. 2007;104:5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosorius O, Reichart B, Kratzer F, Heger P, Dabauvalle MC, Hauber J. Nuclear pore localization and nucleocytoplasmic transport of eIF-5A: evidence for direct interaction with the export receptor CRM1. J Cell Sci. 1999;112:2369–2380. doi: 10.1242/jcs.112.14.2369. [DOI] [PubMed] [Google Scholar]
- Ruhl M, Himmelspach M, Bahr GM, Hammerschmid F, Jaksche H, Wolff B, Aschauer H, Farrington GK, Probst H, Bevec D, Hauber J. Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J Cell Biol. 1993;123:1309–1320. doi: 10.1083/jcb.123.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Abid MR, Miyazaki M. Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett. 1996;384:151–154. doi: 10.1016/0014-5793(96)00310-9. [DOI] [PubMed] [Google Scholar]
- Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham JS, Tang TC, Fang Y, Sun L, Qin LX, Wu QL, Xie D, Guan XY. Recurrent chromosome alterations in primary ovarian carcinoma in Chinese women. Cancer Genet Cytogenet. 2002;133:39–44. doi: 10.1016/s0165-4608(01)00567-2. [DOI] [PubMed] [Google Scholar]
- Shi XP, Yin KC, Zimolo ZA, Stern AM, Waxman L. The subcellular distribution of eukaryotic translation initiation factor, eIF-5A, in cultured cells. Exp Cell Res. 1996;225:348–356. doi: 10.1006/excr.1996.0185. [DOI] [PubMed] [Google Scholar]
- Shi XP, Yin KC, Waxman L. Effects of inhibitors of RNA and protein synthesis on the subcellular distribution of the eukaryotic translation initiation factor, eIF-5A, and the HIV-1 Rev protein. Biol Signals. 1997;6:143–149. doi: 10.1159/000109120. [DOI] [PubMed] [Google Scholar]
- Smit-McBride Z, Schnier J, Kaufman RJ, Hershey JW. Protein synthesis initiation factor 4D. Functional comparison of native and unhypusinated forms of the protein. J Biol Chem. 1989;264:18527–18530. [PubMed] [Google Scholar]
- Sun Z, Cheng Z, Taylor CA, McConkey BJ, Thompson JE. Apoptosis induction by eIF5A1 involves activation of the intrinsic mitochondrial pathway. J Cell Physiol. 2010;223(3):798–809. doi: 10.1002/jcp.22100. [DOI] [PubMed] [Google Scholar]
- Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51(4):1255–1263. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Senchyna M, Flanagan J, Joyce EM, Cliche DO, Boone AN, Culp-Stewart S, Thompson JE. Role of eIF5A in TNF-alpha-mediated apoptosis of lamina cribrosa cells. Invest Ophthalmol Visual Sci. 2004;45:3568–3576. doi: 10.1167/iovs.03-1367. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Sun Z, Cliché DO, Ming H, Eshaque B, Jin S, Hopkins MT, Thai B, Thompson JE. Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor a signaling. Exp Cell Res. 2007;313:437–449. doi: 10.1016/j.yexcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Tome ME, Gerner EW. Cellular eukaryotic initiation factor 5A content as a mediator of polyamine effects on growth and apoptosis. Biol Signals. 1997;6(3):150–156. doi: 10.1159/000109121. [DOI] [PubMed] [Google Scholar]
- Tome ME, Fiser SM, Payne CM, Gerner EW. Excess putrescine accumulation inhibits the formation of modified eukaryotic initiation factor 5A (eIF-5A) and induces apoptosis. Biochem J. 1997;328:847–854. doi: 10.1042/bj3280847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini SR, Casolari JM, Oliveira CC, Silver PA, McBride AE. Genetic interactions of yeast eukaryotic translation initiation factor 5A (eIF5A) reveal connections to poly(A)-binding protein and protein kinase C signalling. Genetics. 2002;160:393–405. doi: 10.1093/genetics/160.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EC, Park MH, Folk JE. Cleavage of spermidine as the first step in deoxyhypusine synthesis. The role of NAD. J Biol Chem. 1990;265:4793–4799. [PubMed] [Google Scholar]
- Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu GQ, Kung HF, Guan XY. Overexpression of EIF-5A2 is associated with metastasis of human colorectal carcinoma. Hum Pathol. 2008;39:80–86. doi: 10.1016/j.humpath.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua WF, Wu HM, Kung HF, Zeng YX, Guan XY. Expression and amplification of eIF-5A2 in human epithelial ovarian tumors and overexpression of EIF-5A2 is a new independent predictor of outcome in patients with ovarian carcinoma. Gynecol Oncol. 2009;112:314–318. doi: 10.1016/j.ygyno.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Zanelli CF, Valentini SR. Is there a role for eIF5A in translation? Amino Acids. 2007;33:351–358. doi: 10.1007/s00726-007-0533-0. [DOI] [PubMed] [Google Scholar]
- Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, McCombie RW, Wigler M, Hicks J, Hannon GJ, Powers S, Lowe SW. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–864. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]