Abstract
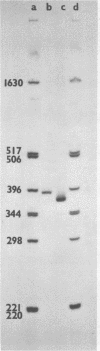
The sequence of about 70 nucleotides at the 5′ end of the RNAs of nine different aphthoviruses (foot-and-mouth disease viruses), including representatives of the seven serotypes of the virus, has been determined by partial enzyme digestion of 32P-end-labeled S fragment—that part of the RNA lying to the 5′ side of the poly(C) tract and including the 5′ end of the molecule. The S fragments were prepared from polyadenylated virus-specific RNA extracted from infected cells by digestion with RNase H in the presence of oligo(dG)12-18. The first 27 nucleotides from the 5′ end were highly conserved in all the RNAs. This region was followed by a more variable region of about 15 nucleotides, showing some length and sequence heterogeneity and including potential but probably nonutilized initiation codons. In agreement with previous homology studies, the sequencing results showed that the European serotypes A, O, and C form a group distinct from the SAT serotypes and that the Asia 1 serotype is closely related to the European group. The lengths of the S fragments of two different RNAs were confirmed as containing 360 to 400 nucleotides by gel electrophoresis with reference to nucleotide markers of known size.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich A., Wimmer E., Toyoda H., Nomoto A. The genome-linked protein of picornaviruses. VI. The 5'-terminal protein of poliovirus type 2RNA is covalently linked to a nonanucleotide identical to that of poliovirus type 1 RNA. Intervirology. 1980;13(3):192–199. doi: 10.1159/000149125. [DOI] [PubMed] [Google Scholar]
- Bachrach H. L., Morgan D. O., Moore D. M. Foot-and-mouth disease virus immunogenic capsid protein VPT: N-terminal sequences and immunogenic peptides obtained by CNBr and tryptic cleavages. Intervirology. 1979;12(2):65–72. doi: 10.1159/000149070. [DOI] [PubMed] [Google Scholar]
- Black D. N., Stephenson P., Rowlands D. J., Brown F. Sequence and location of the poly C tract in aphtho- and cardiovirus RNA. Nucleic Acids Res. 1979 Jun 11;6(7):2381–2390. doi: 10.1093/nar/6.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J. The nucleotide sequence at the 5' end of foot and mouth disease virus RNA. Nucleic Acids Res. 1979 Dec 11;7(7):1765–1785. doi: 10.1093/nar/7.7.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Florkiewicz R. Z. Sequence of picornavirus RNAs containing a radioiodinated 5'-linked peptide reveals a conserved 5' sequence. Proc Natl Acad Sci U S A. 1980 Jan;77(1):303–307. doi: 10.1073/pnas.77.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Lockard R. E., Vamvakopoulos N., Rieser L., RajBhandary U. L., Vournakis J. N. Secondary structure of mouse and rabbit alpha- and beta-globin mRNAs: differential accessibility of alpha and beta initiator AUG codons towards nucleases. Cell. 1980 Jan;19(1):91–102. doi: 10.1016/0092-8674(80)90391-8. [DOI] [PubMed] [Google Scholar]
- Pereira H. G. Subtyping of foot-and-mouth disease virus. Dev Biol Stand. 1976;35:167–174. [PubMed] [Google Scholar]
- Porter A. G., Fellner P., Black D. N., Rowlands D. J., Harris T. J., Brown F. 3'-Terminal nucleotide sequences in the genome RNA of picornaviruses. Nature. 1978 Nov 16;276(5685):298–301. doi: 10.1038/276298a0. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Crowther J. R., King A. M., Brown F. Comparative biochemical and serological analysis of five isolates of a single serotype of foot-and-mouth disease virus. J Gen Virol. 1979 Dec;45(3):579–590. doi: 10.1099/0022-1317-45-3-579. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Doel T. R., Gorman B. M., Brown F. Biochemical aspects of variation in foot-and-mouth disease virus. J Gen Virol. 1980 Jan;46(1):179–193. doi: 10.1099/0022-1317-46-1-179. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Harris T. J., Brown F. An assessment by competition hybridization of the sequence homology between the RNAs of the seven serotypes of FMDV. J Gen Virol. 1977 Nov;37(2):271–276. doi: 10.1099/0022-1317-37-2-271. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Harris T. J., Brown F. More precise location of the polycytidylic acid tract in foot and mouth disease virus RNA. J Virol. 1978 May;26(2):335–343. doi: 10.1128/jvi.26.2.335-343.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangar D. V., Black D. N., Rowlands D. J., Harris T. J., Brown F. Location of the initiation site for protein synthesis on foot-and-mouth disease virus RNA by in vitro translation of defined fragments of the RNA. J Virol. 1980 Jan;33(1):59–68. doi: 10.1128/jvi.33.1.59-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wurst R. M., Vournakis J. N., Maxam A. M. Structure mapping of 5'-32P-labeled RNA with S1 nuclease. Biochemistry. 1978 Oct 17;17(21):4493–4499. doi: 10.1021/bi00614a021. [DOI] [PubMed] [Google Scholar]