Abstract
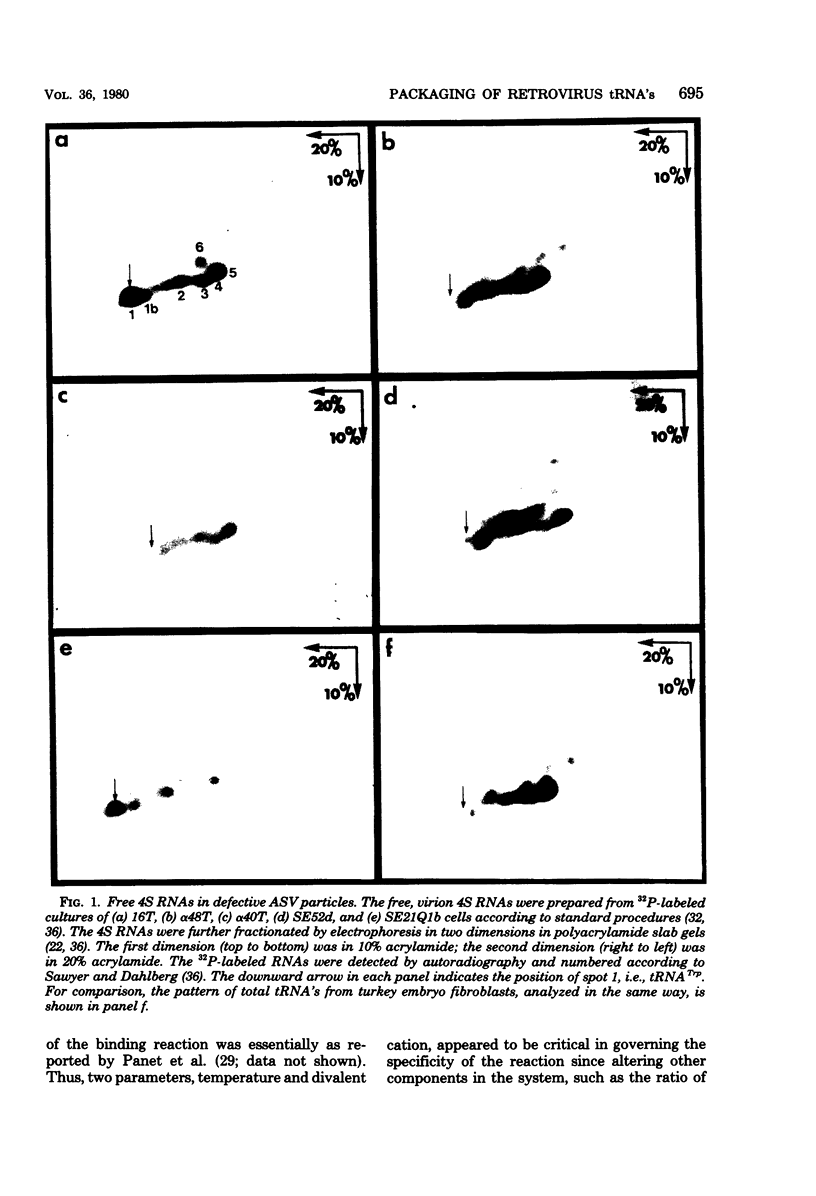
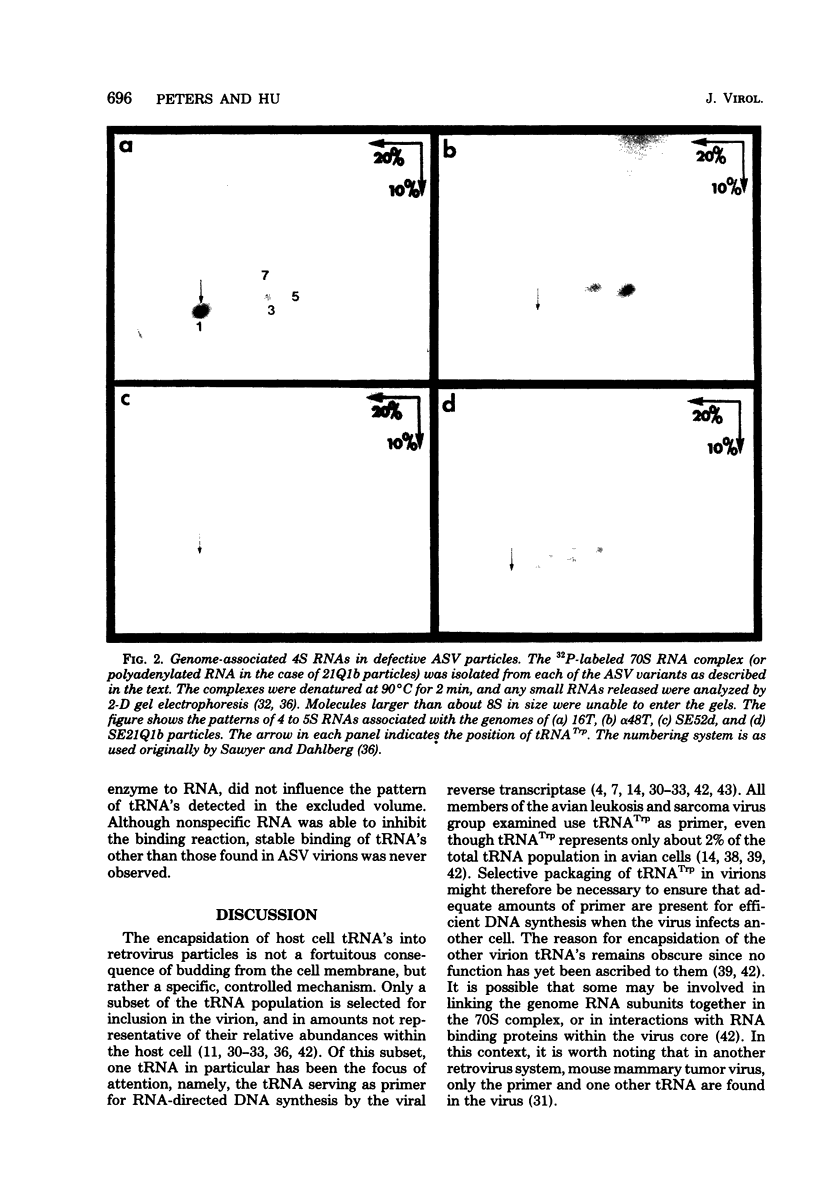
Mutants of avian sarcoma virus which lack a functional DNA polymerase were found to be nonselective in the incorporation of host cell tRNA's into virus particles. In contrast, mutants which possess a functional DNA polymerase but lack the viral genome RNA contained a specific subset of the host cell tRNA population, indistinguishable from that of the wild-type virus. Thus the reverse transcriptase, and not the viral RNA, is probably the major factor determining which tRNA's are incorporated into avian sarcoma virus particles. Supporting evidence was obtained in an in vitro binding assay between purified reverse transcriptase and unfractionated cellular tRNA's. However, the subset of tRNA's which associated with the genome in the 70S complex was determined primarily by the viral RNA. In the absence of DNA polymerase, the 70S RNA complex in mature virus particles contained the normal complement of associated tRNA's with the exception of tRNATrp, the primer for RNA-directed DNA synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Fournier M., Labouesse J., Papas T. S., Chirikjian J. G. tRNATrp (bovine) binding to the reverse transcriptase of avian myeloblastosis virus and function as a heterologous primer. Proc Natl Acad Sci U S A. 1977 May;74(5):1889–1893. doi: 10.1073/pnas.74.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Nichols J. L. Characterization of poly(riboadenylic acid) segments in L-cell messenger ribonucleic acid. Biochemistry. 1973 Sep 25;12(20):3951–3956. doi: 10.1021/bi00744a026. [DOI] [PubMed] [Google Scholar]
- Eiden J. J., Quade K., Nichols J. L. Interaction of tryptophan transfer RNA with Rous sarcoma virus 35S RNA. Nature. 1976 Jan 22;259(5540):245–247. doi: 10.1038/259245a0. [DOI] [PubMed] [Google Scholar]
- Elder K. T., Smith A. E. Methionine transfer RNAs associated with avian oncornavirus 70S RNA. Nature. 1974 Feb 15;247(5441):435–438. doi: 10.1038/247435a0. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Isolation of amino acid acceptor RNA from purified avian myeloblastosis virus. J Mol Biol. 1970 Sep 14;52(2):387–390. doi: 10.1016/0022-2836(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Dibble N. A. RNA-directed DNA synthesis by the DNA polymerase of Rous sarcoma virus: structural and functional identification of 4S primer RNA in uninfected cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):859–863. doi: 10.1073/pnas.72.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Faras A. J. Different states of avian myeloblastosis virus DNA polymerase and their binding capacity to primer rRNATrp. Virology. 1976 Nov;75(1):26–32. doi: 10.1016/0042-6822(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Panet A., Smoler D., Baltimore D., Peters G., Harada F., Dahlberg J. E. Interaction of tryptophan tRNA and avian myeloblastosis virus reverse transcriptase: further characterization of the binding reaction. Biochemistry. 1977 Aug 9;16(16):3625–3632. doi: 10.1021/bi00635a019. [DOI] [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. The RNA-dependent DNA polymerase of avian sarcoma virus B77. Binding of viral and nonviral ribonucleic acids to the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Oct 10;252(19):6878–6884. [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Selective packaging of host tRNA's by murine leukemia virus particles does not require genomic RNA. J Virol. 1979 Jan;29(1):328–335. doi: 10.1128/jvi.29.1.328-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Brown S., Neiman P. A nonconditional mutant of Rous sarcoma virus containing defective polymerase. Virology. 1978 Jun 1;87(1):130–141. doi: 10.1016/0042-6822(78)90165-4. [DOI] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Berliner H. Binding of tRNA to reverse transcriptase of RNA tumor viruses. J Virol. 1978 May;26(2):214–220. doi: 10.1128/jvi.26.2.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Glover C. Low-molecular-weight RNAs and initiation of RNA-directed DNA synthesis in avian reticuloendotheliosis virus. J Virol. 1980 Feb;33(2):708–716. doi: 10.1128/jvi.33.2.708-716.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Rosenthal L. J., Zamecnik P. C. Base composition differences between avian myeloblastosis virus transfer RNA and transfer RNA isolated from host cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3233–3237. doi: 10.1073/pnas.68.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J., Zamecnik P. C. Amino-acid acceptor activity of the "70S-associated" 4S RNA from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1184–1185. doi: 10.1073/pnas.70.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M. An analysis of the role of tRNA species as primers for the transcription into DNA of RNA tumor virus genomes. Biochim Biophys Acta. 1977 Mar 21;473(1):57–71. doi: 10.1016/0304-419x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cordell-Stewart B., Rohde W., Goodman H. M., Bishop J. M. Reassociation of 4 S and 5 S RNA's with the genome of avian sarcoma virus. Virology. 1975 May;65(1):248–259. doi: 10.1016/0042-6822(75)90025-2. [DOI] [PubMed] [Google Scholar]
- Wang S., Kothari R. M., Taylor M., Hung P. Transfer RNA activities of Rous sarcoma and Rous associated viruses. Nat New Biol. 1973 Apr 4;242(118):133–135. doi: 10.1038/newbio242133a0. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Ho T., Yang W. K. Ability of tryptophan tRNA to hybridize with 35S RNA of avian myeloblastosis virus and to prime reverse transcription in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2155–2159. doi: 10.1073/pnas.72.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]