Abstract
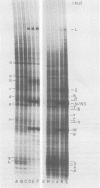
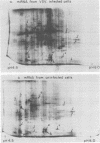
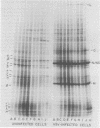
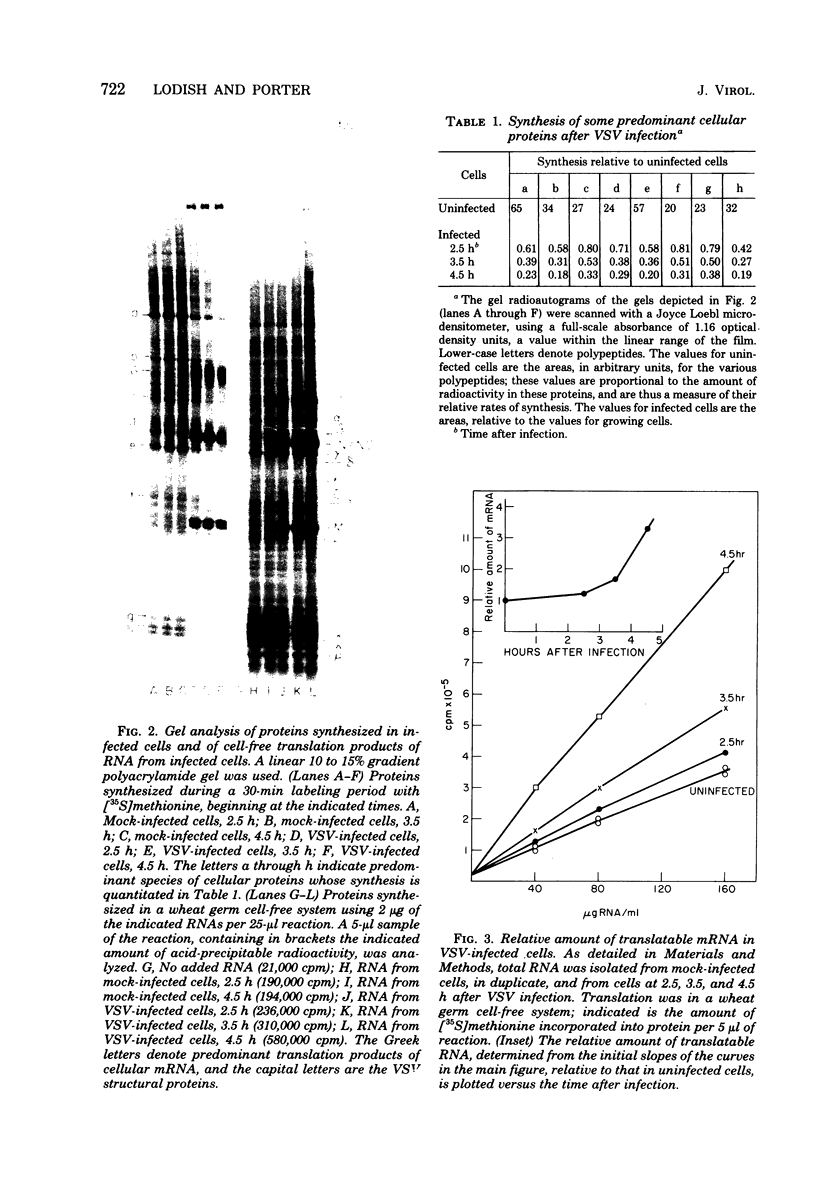
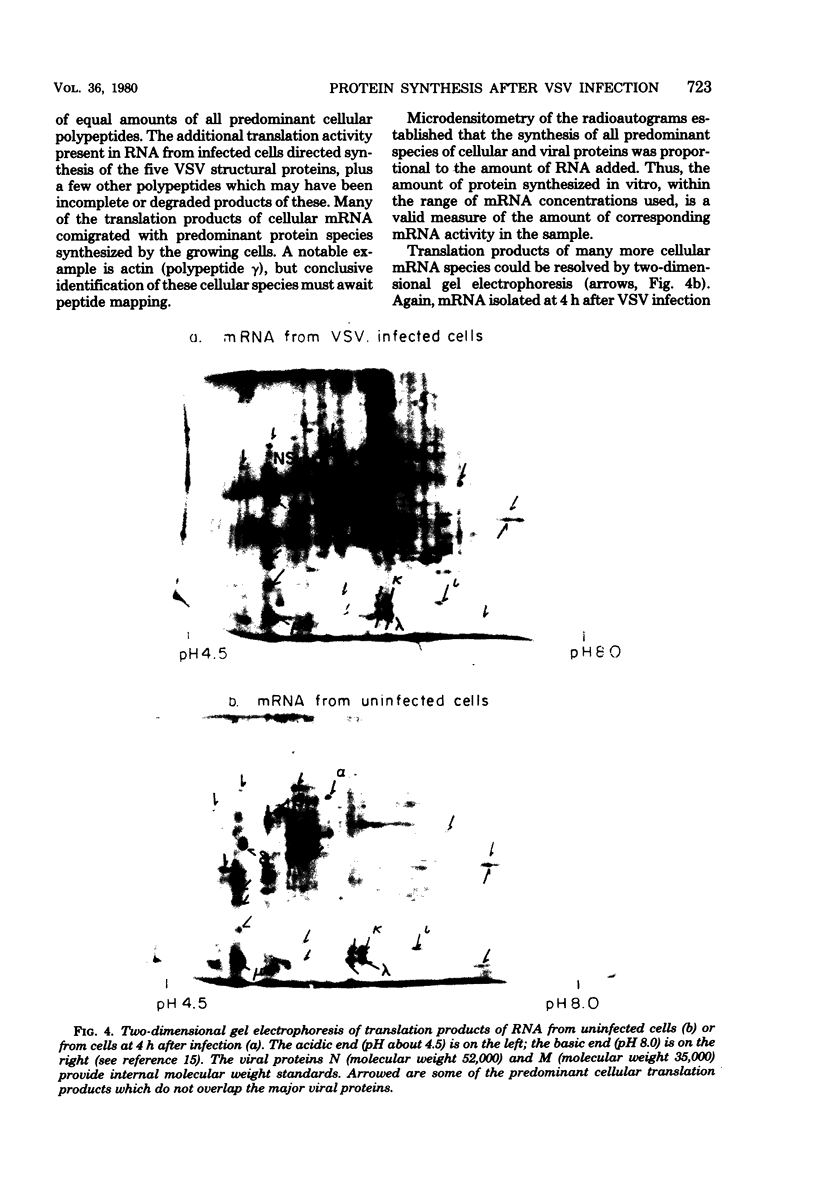
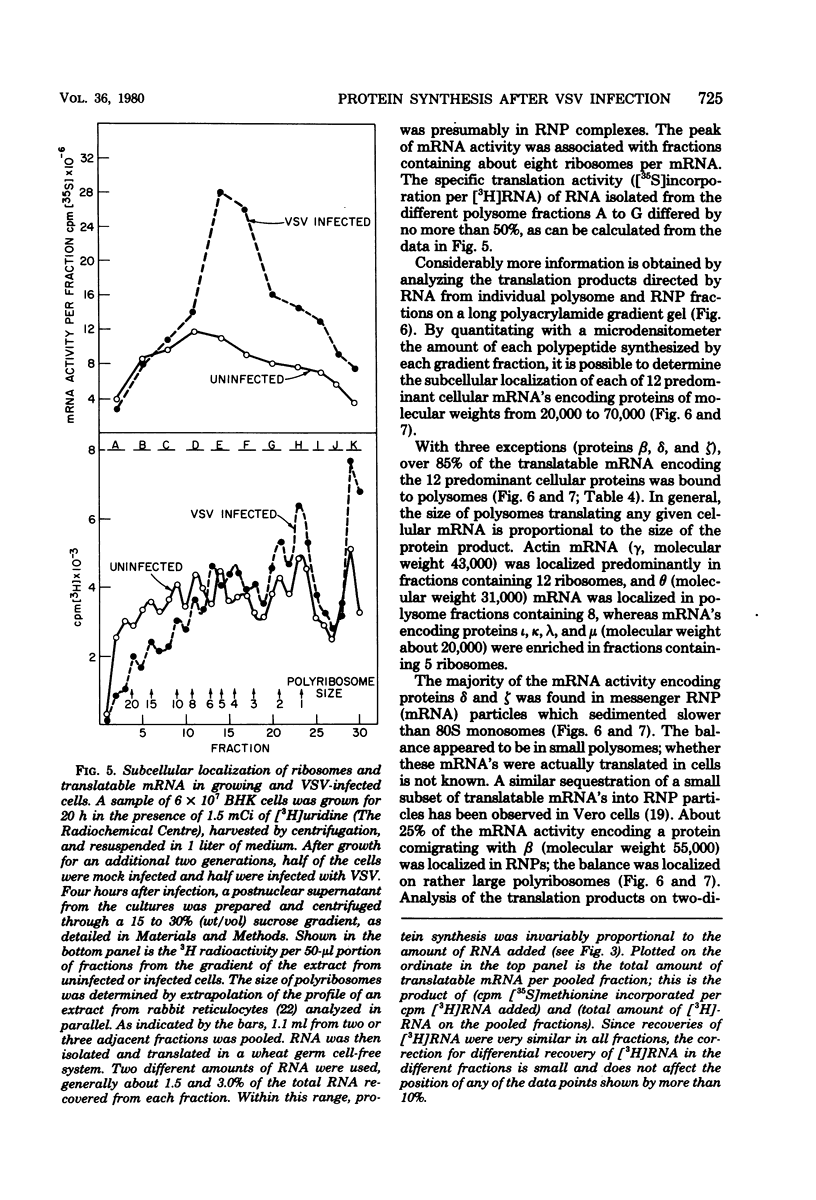
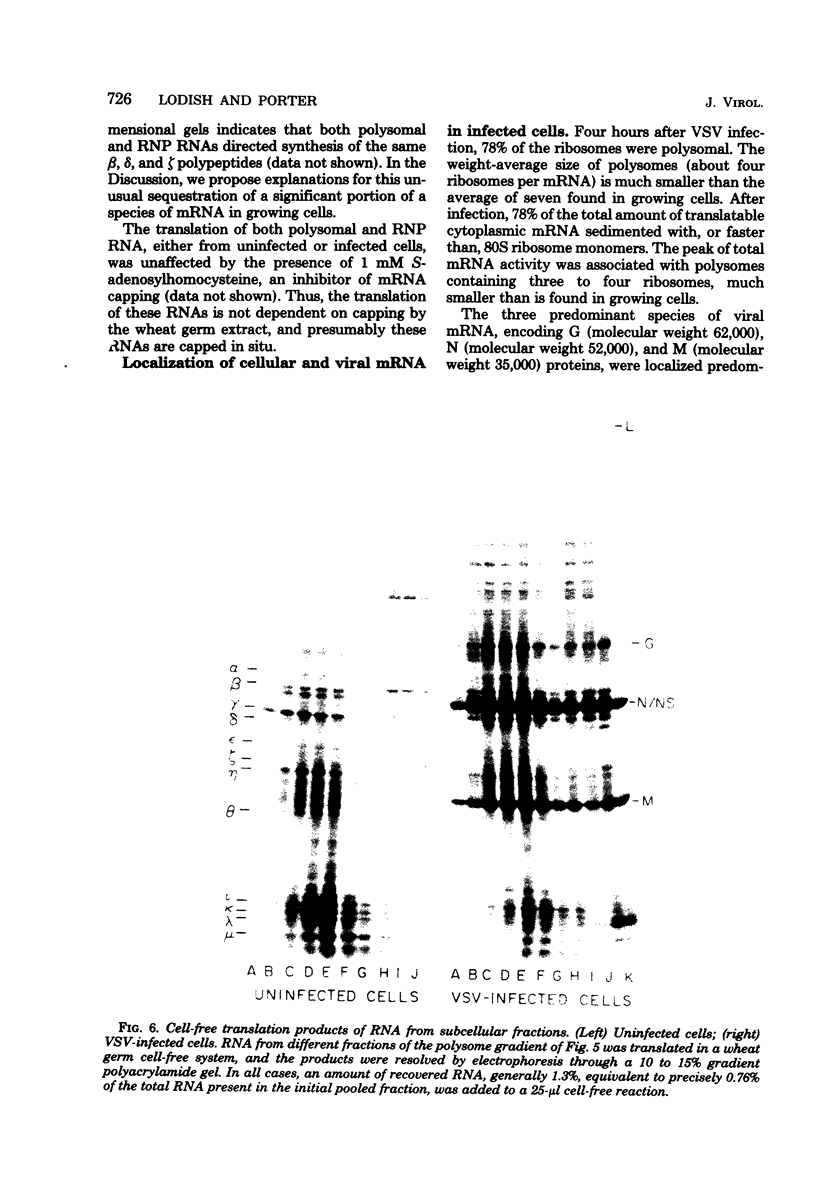
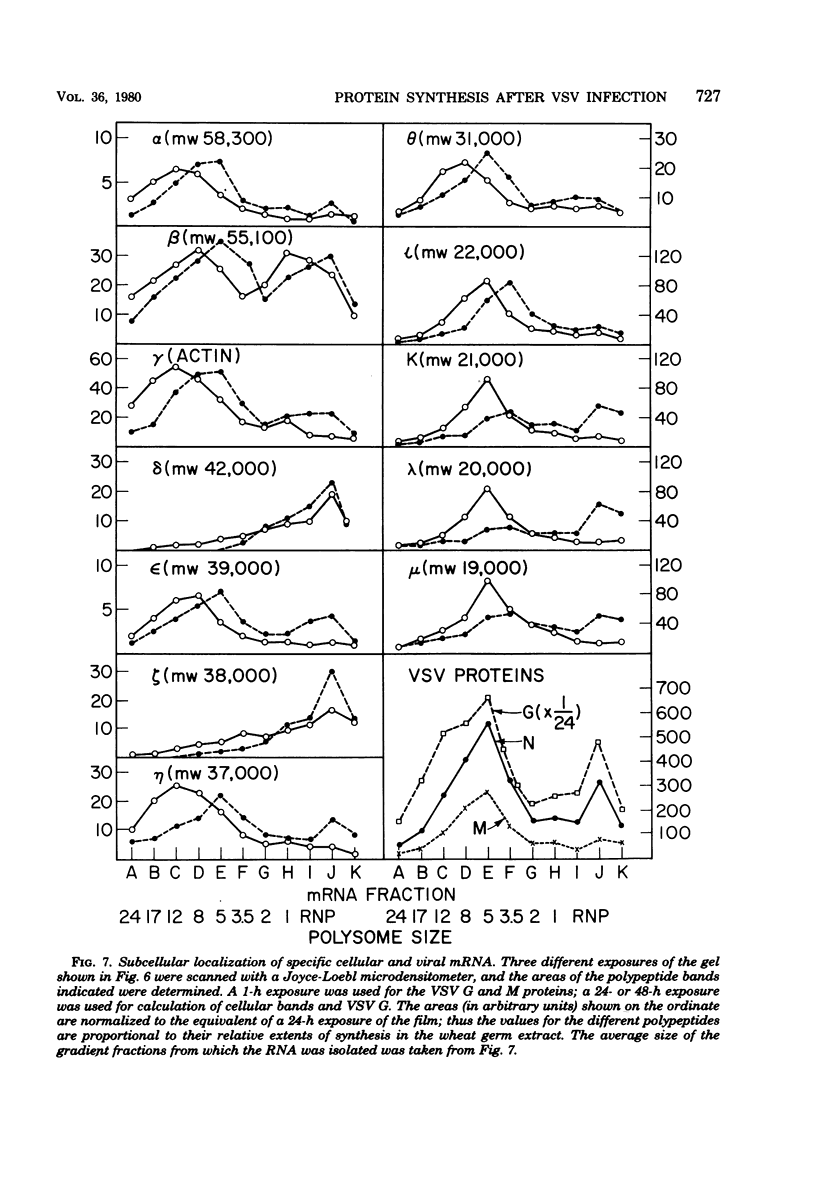
Four hours after infection of BHK cells by vesicular stomatitis virus (VSV), the rate of total protein synthesis was about 65% that of uninfected cells and synthesis of the 12 to 15 predominant cellular polypeptides was reduced to a level about 25% that of control cells. As determined by in vitro translation of isolated RNA and both one- and two-dimensional gel analyses of the products, all predominant cellular mRNA's remained intact and translatable after infection. The total amount of translatable mRNA per cell increased about threefold after infection; this additional mRNA directed synthesis of the five VSV structural proteins. To determine the subcellular localization of cellular and viral mRNA before and after infection, RNA from various sizes of polysomes and nonpolysomal ribonucleoproteins (RNPs) was isolated from infected and noninfected cells and translated in vitro. Over 80% of most predominant species of cellular mRNA was bound to polysomes in control cells, and over 60% was bound in infected cells. Only 2 of the 12 predominant species of translatable cellular mRNA's were localized to the RNP fraction, both in infected and in uninfected cells. The average size of polysomes translating individual cellular mRNA's was reduced about two- to threefold after infection. For example, in uninfected cells, actin (molecular weight 42,000) mRNA was found predominantly on polysomes with 12 ribosomes; after infection it was found on polysomes with five ribosomes, the same size of polysomes that were translating VSV N (molecular weight 52,000) and M (molecular weight 35,000) mRNA. We conclude that the inhibition of cellular protein synthesis after VSV infection is due, in large measure, to competition for ribosomes by a large excess of viral mRNA. The efficiency of initiation of translation on cellular and viral mRNA's is about the same in infected cells; cellular ribosomes are simply distributed among more mRNA's than are present in growing cells. About 20 to 30% of each of the predominant cellular and viral mRNA's were present in RNP particles in infected cells and were presumably inactive in protein synthesis. There was no preferential sequestration of cellular or viral mRNA's in RNPs after infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Lucas-Lenard J. Cellular protein synthesis shutoff by mengovirus: translation of nonviral and viral mRNA's in extracts from uninfected and infected Ehrlich ascites tumor cells. J Virol. 1976 Apr;18(1):182–194. doi: 10.1128/jvi.18.1.182-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Smith K. D., Noyes A. N., Mullen M. A. Immunological characterization of rabbit hemoglobin alpha and beta chain-synthesizing polysomes. J Biol Chem. 1974 Nov 25;249(22):7210–7219. [PubMed] [Google Scholar]
- Colby D. S., Finnerty V., Lucas-Lenard J. Fate of mRNA of L-cells infected with mengovirus. J Virol. 1974 Apr;13(4):858–869. doi: 10.1128/jvi.13.4.858-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. E. Control of vesicular stomatitis virus protein synthesis. Virology. 1976 May;71(1):217–229. doi: 10.1016/0042-6822(76)90107-0. [DOI] [PubMed] [Google Scholar]
- Golini F., Thach S. S., Birge C. H., Safer B., Merrick W. C., Thach R. E. Competition between cellular and viral mRNAs in vitro is regulated by a messenger discriminatory initiation factor. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3040–3044. doi: 10.1073/pnas.73.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Egberts E., Traub P. Translation of ascites and mengovirus RNA in fractionated cell-free systems from uninfected and mengovirus-infected Ehrlich-ascites-tumor cells. Eur J Biochem. 1978 Feb;83(2):341–352. doi: 10.1111/j.1432-1033.1978.tb12100.x. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen G., Detjen B. M., Thach R. E. Shutoff of HeLa cell protein synthesis by encephalomyocarditis virus and poliovirus: a comparative study. J Virol. 1980 Jul;35(1):150–156. doi: 10.1128/jvi.35.1.150-156.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Kaumeyer J. F., Young E. M., Raff R. A. A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchine eggs. Dev Biol. 1978 Apr;63(2):279–298. doi: 10.1016/0012-1606(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., Lodish H. F. A role for cyclic AMP in expression of developmentally regulated genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1044–1048. doi: 10.1073/pnas.77.2.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Gurdon J. B., Partington G. A. Protein synthesis in oocytes of Xenopus laevis is not regulated by the supply of messenger RNA. Cell. 1977 Jun;11(2):345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. T., Engelhardt D. L. Peptide coding capacity of polysomal and non-polysomal messenger RNA during growth of animal cells. J Mol Biol. 1979 Apr 5;129(2):221–233. doi: 10.1016/0022-2836(79)90278-x. [DOI] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel J. B., Woodland H. R. Initiation does not limit the rate of globin synthesis in message-injected Xenopus oocytes. Eur J Biochem. 1974 Aug 15;47(1):47–56. doi: 10.1111/j.1432-1033.1974.tb03666.x. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Binding of viral glycoprotein mRNA to endoplasmic reticulum membranes is disrupted by puromycin. J Cell Biol. 1977 Aug;74(2):358–364. doi: 10.1083/jcb.74.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Rates of initiation of protein synthesis by two purified species of vesicular stomatitis virus messenger RNA. J Biol Chem. 1977 Dec 25;252(24):8804–8811. [PubMed] [Google Scholar]
- Lodish H. F., Jacobsen M. Regulation of hemoglobin synthesis. Equal rates of translation and termination of - and -globin chains. J Biol Chem. 1972 Jun 10;247(11):3622–3629. [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J., Johnson L. D., Lazzarini R. A. Cell killing by viruses. V. Transcribing defective interfering particles of vesicular stomatitis virus function as cell-killing particles. Virology. 1977 Oct 1;82(1):242–246. doi: 10.1016/0042-6822(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Marvaldi J. L., Lucas-Lenard J., Sekellick M. J., Marcus P. I. Cell killing by viruses. IV. Cell killing and protein synthesis inhibition by vesicular stomatitis virus require the same gene functions. Virology. 1977 Jun 15;79(2):267–280. doi: 10.1016/0042-6822(77)90354-3. [DOI] [PubMed] [Google Scholar]
- Marvaldi J., Sekellick M. J., Marcus P. I., Lucas-Lenard J. Inhibition of mouse L cell protein synthesis by ultraviolet-irradiated vesicular stomatitis virus requires viral transcription. Virology. 1978 Jan;84(1):127–133. doi: 10.1016/0042-6822(78)90224-6. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Lockard R. E., Vamvakopoulos N., Rieser L., RajBhandary U. L., Vournakis J. N. Secondary structure of mouse and rabbit alpha- and beta-globin mRNAs: differential accessibility of alpha and beta initiator AUG codons towards nucleases. Cell. 1980 Jan;19(1):91–102. doi: 10.1016/0092-8674(80)90391-8. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Francoeur A. M., Lam T. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell. 1977 Jun;11(2):273–281. doi: 10.1016/0092-8674(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P., Breindl M., Holland J. J. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry. 1976 Apr 20;15(8):1663–1667. doi: 10.1021/bi00653a012. [DOI] [PubMed] [Google Scholar]