Background: Metastasizing ovarian cancer (EOC) cells anchor in the submesothelial collagen matrix.
Results: Modeling EOC metastasis with cells in three-dimensional collagen culture down-regulates an inhibitor of Wnt signaling, Dickkopf-1.
Conclusion: Cell matrix adhesion can activate Wnt signaling in the absence of Wnt pathway mutations.
Significance: Cross-talk between adhesion-regulated signaling pathways may fine tune tissue invasive activity.
Keywords: Cancer Biology, Collagen, Extracellular Matrix, Integrins, Matrix Metalloproteinase (MMP), Wnt Signaling
Abstract
Cells respond to changes in the physical properties of the extracellular matrix with altered behavior and gene expression, highlighting the important role of the microenvironment in the regulation of cell function. In the current study, culture of epithelial ovarian cancer cells on three-dimensional collagen I gels led to a dramatic down-regulation of the Wnt signaling inhibitor dickkopf-1 with a concomitant increase in nuclear β-catenin and enhanced β-catenin/Tcf/Lef transcriptional activity. Increased three-dimensional collagen gel invasion was accompanied by transcriptional up-regulation of the membrane-tethered collagenase membrane type 1 matrix metalloproteinase, and an inverse relationship between dickkopf-1 and membrane type 1 matrix metalloproteinase was observed in human epithelial ovarian cancer specimens. Similar results were obtained in other tissue-invasive cells such as vascular endothelial cells, suggesting a novel mechanism for functional coupling of matrix adhesion with Wnt signaling.
Introduction
Anchorage-dependent cells in a tissue context reside within a three-dimensional extracellular matrix (ECM)2 environment that serves as a structural scaffold and provides guidance cues for various cellular processes (1, 2). Cells respond to changes in the physical properties of the three-dimensional ECM with altered behavior and gene expression; however, mechanisms that regulate these processes are largely unclear. Recent studies have demonstrated that the mechanical properties of a tissue can contribute actively to disease progression, highlighting the importance of understanding cellular responses to changes in matrix rigidity (3–7). Acquisition of a tissue-invasive phenotype occurs in normal development, for example migration of neuronal cells into the cortex (8) or endothelial cell migration during vasculogenesis (9). Malignant cancer cells also acquire tissue-invasive capacity to disseminate from the primary tumor and establish secondary lesions (10). Although the specific microenvironment of tissue-invasive cell types varies, a common feature often includes a three-dimensional collagen scaffold penetrated by invasive cells. Recent studies suggest that common mechanisms can be adopted by diverse cell types to invade three-dimensional collagen gels (11–15).
In this study, we utilized an in vitro model of epithelial ovarian cancer (EOC) metastasis to address the functional consequences of changes in gene expression that accompany penetration of three-dimensional collagen gels. Metastatic dissemination of EOC is initiated by exfoliation of cells from the primary tumor into the peritoneal cavity (see Fig. 1) wherein they exist as a non-adherent cell population. These metastatic cells induce retraction of peritoneal mesothelial cells and exposure of the underlying three-dimensional collagen matrix (see Fig. 1 and Refs. 16–18) to which EOC cells avidly adhere via integrin-mediated interactions. We have demonstrated previously that EOC cells show preferential β1 integrin-mediated adhesion to collagen I (19–22) and that following collagen I contact cells undergo morphologic alteration to a distinct invasive phenotype with altered expression of genes associated with invasion and motility including membrane type 1 matrix metalloproteinase (MT1-MMP), actinin-α4, and connective tissue growth factor (19, 23, 24).
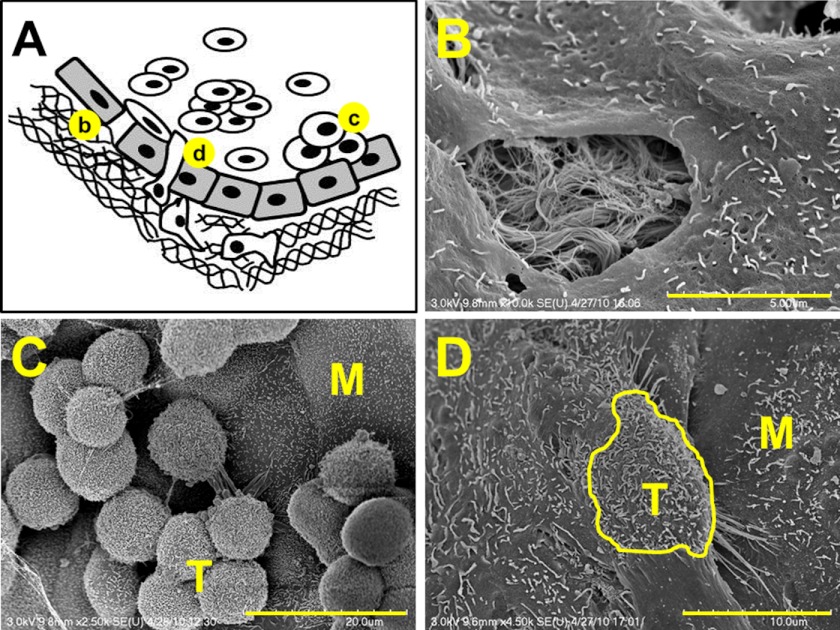
FIGURE 1.
Model of epithelial ovarian cancer metastasis. A, cells shed from the primary tumor circulate in ascites fluid, interact with peritoneal mesothelial cells, induce mesothelial cell retraction, and adhere avidly to the submesothelial three-dimensional collagen matrix wherein they anchor and proliferate to form secondary lesions. Scanning electron micrographs (SE) depicting events in metastasis (b, c, and d) are shown in the following panels. B, scanning electron micrographs of retracted mesothelial cells showing the underlying three-dimensional collagen matrix. Scale bar, 5 μm. C, scanning electron micrographs of EOC tumor cells (T) adherent to peritoneal tissue in a tissue explant. M designates mesothelial cells. Scale bar, 20 μm. D, scanning electron micrographs of EOC tumor cell (T; outlined in yellow) intercalated between peritoneal mesothelial cells (M) in a tissue explant. Scale bar, 10 μm.
Among the genes down-regulated by three-dimensional collagen I (CI) culture of EOC cells is the protein dickkopf-1 (DKK1), an inhibitor of the canonical Wnt signaling pathway (25). This pathway is activated by binding of a Wnt ligand to the frizzled receptor, association of the co-receptor LRP-5/6 with frizzled, and recruitment of disheveled and axin to the cytoplasmic face of the complex. Translocation of axin disrupts the cytoplasmic β-catenin degradation complex, enabling nuclear translocation of β-catenin (26). DKK1 functions as an inhibitor of Wnt signaling by binding the co-receptor LRP-5/6 and promoting internalization. When active, Wnt signaling regulates gene expression, cell adhesion, cell fate, and cell polarity during both embryogenesis and cancer progression (26, 27).
Constitutive activation of Wnt signaling has been observed in some cancers as a result of mutations in key pathway components. Although activating mutations of β-catenin (CTNNB1 mutations) are relatively rare in serous EOC and are restricted to the endometrioid histotype (28, 29), emerging data nevertheless link deregulated Wnt signaling to progression of serous ovarian cancer as well (30–33). Findings in the current study suggest that down-regulation of DKK1 occurs in response to three-dimensional CI with progressive loss observed in collagen gels of increasing mechanical rigidity. As a functional consequence of DKK1 down-regulation, Wnt signaling is activated, MT1-MMP expression is enhanced, and collagen invasion is increased. A similar loss of DKK1 expression was observed in both cortical neurons and endothelial cells in three-dimensional collagen culture, suggesting a common mechanism for activation of Wnt signaling by tissue-invasive cells.
EXPERIMENTAL PROCEDURES
Materials
The ovarian carcinoma cell lines of serous histotype DOV13, OVCA433, and OVCA429 were kindly provided by Dr. R. Bast, Jr. (M. D. Anderson Cancer Center, Houston, TX) and maintained as described previously (21). The OVCAR3 cell line was generously provided by Dr. A. Skubitz (University of Minnesota, Minneapolis, MN). High density primary cortical neuronal cultures were prepared from E18 rat embryos (under an animal protocol approved by the Northwestern University animal committee) as described (63) and maintained in Neurobasal medium supplemented with glutamine and B27 (Invitrogen). Human umbilical vein endothelial cells were obtained from ATCC, maintained in Medium 199 (Sigma), and supplemented with 2.5% fetal bovine serum, 10 mm HEPES, 2 mm glutamine, 30 μg/ml heparin, and 50 μg/ml endothelial mitogen (Biomedical Technologies) between passages 3 and 6. Cell lines MDA-MB231 (breast carcinoma) and ES2 (ovarian carcinoma of clear cell histotype) as well as ovarian carcinoma cell lines of serous histotype, SKOV-3 and Caov-3, were obtained from ATCC and maintained according to the manufacturer's suggestions. Rat tail type I collagen and Transwell 8.0-μm invasion chambers were obtained from BD Biosciences. Human collagens I and III, RGDS peptide, rabbit polyclonal anti-MT1-MMP antibodies (hinge region), mouse monoclonal anti-β-tubulin antibody, and bovine serum albumin (BSA) were purchased from Sigma. Mouse monoclonal anti-β-catenin (catalog number 610154) was obtained from BD Transduction Laboratories and Upstate (Lake Placid, NY), respectively. The broad spectrum inhibitor of matrix metalloproteinases GM6001 was obtained from Chemicon (Temecula, CA), the Src kinase inhibitor SU6656 was from Cayman Chemical, and the ERK inhibitor U0126 was from Cell Signaling Technology. Mouse anti-GAPDH antibody was from Research Diagnostics Inc. (Concord, MA). Rabbit polyclonal anti-histone H3 antibody was obtained from BioLegend (San Diego, CA). Four-arm polyethylene glycol (PEG)-acryl (10,000) was obtained from SunBio (Orinda, CA), and 2,2-dimethyl-2-phenylacetophenone was a generous gift from Ciba (Tarrytown, NY). DKK1-overexpressing plasmid (pCS2+/DKK1) was a generous gift from Dr. Xi He (Children's Hospital, Harvard Medical School). TOPFlash and FOPFlash reporter luciferase constructs as well as a construct expressing Renilla luciferase were kind gifts from Dr. Cara Gottardi (Northwestern University). Human recombinant DKK1 protein was purchased from R&D Systems. Polyclonal antibodies against DKK1 and control and DKK1 siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Flexercell 6-well tissue culture plates were purchased from Flexcell International Corp. (Hillsborough, NC). TissueScan real time ovarian cancer disease panel I was obtained from Origene (Rockville, MD).
Scanning Electron Microscopy
Sections of peritoneum (∼6 × 6 mm2) were removed from the ventral surface of female FVB mice and pinned with the mesothelial surface facing up to silastic resin immersed in PBS. For some sections, EOC cells were added to the ex vivo tissue section and allowed to incubate for 2–24 h prior to tissue fixation and preparation for scanning electron microscopy. Tissues were then fixed for 1 h in primary fixative solution containing 2% glutaraldehyde and 2% paraformaldehyde in 0.1 m cacodylate buffer, pH 7.35; washed in 2-ME buffer (0.1 m sodium cacodylate, 0.13 m sucrose, 0.01 m 2-mercaptoethanol, pH 7.35; 3 × 20 min); and fixed with 2% osmium tetroxide in cacodylate buffer using a microwave processing regimen. The tissues were rinsed with cacodylate buffer, washed (3 × 5 min) with ultrapure water, and dehydrated in a series of increasing concentrations of ethanol prior to critical point drying using an Autosampdri®-815 Series A dryer. After placing the samples on carbon stubs and applying Flash-DryTM silver paint, one cycle of platinum coating was performed using a platinum sputter coater machine. Samples were examined using a Hitachi S-4700 field emission scanning electron microscope.
Three-dimensional Matrix Models
To model early events in intraperitoneal EOC metastasis induced by cell interaction with a three-dimensional collagen I matrix (see Fig. 1), three-dimensional CI gels at 0.8 or 2 mg/ml were utilized as described previously (19). Additional control experiments used three-dimensional collagen III (CIII) gels at 0.25 mg/ml. Synthetic 5 and 10% PEG gels containing 0.3 mm RGDS were also used. Synthetic 10% four-arm PEG-acryl containing 0.3 mm RGDS was prepared by photocross-linking under ultraviolet light using 0.5% 2,2-dimethyl-2-phenylacetophenone in polyvinylpyrrolidone (600 mg/ml) as the photoinitiator. Collagen type I-conjugated polyacrylamide gels containing varying percentages of bisacrylamide from 0.03 to 0.3% were made using a procedure published previously (34). Cells were cultured atop three-dimensional matrices for various periods of time as described (19). Control cells were plated either on 10 μg/ml thin layer collagen I (indicated as two-dimensional CI throughout), 10 μg/ml planar CIII (two-dimensional CIII), or 0.3 mm unconjugated RGDS (two-dimensional). In control experiments, inhibitors of Src kinase (SU6656; 2 μm) or ERK (UO126; 25 μm) were added during the incubation. Physical properties of collagen and PEG gels (storage and loss moduli) were obtained using a standard rheology technique as described previously (35, 36).
Mechanical Strain
Ovarian carcinoma cells were cultured on collagen-coated Tissue Train 6-well culture plates. To evaluate the effect of mechanical strain on DKK1 expression, cells were subjected to 360 cycles of biaxial sinusoidal 0–20% mechanical stretch for 1 h using a Flexcell FX-4000T apparatus. Cells cultured in the area of the film subjected to stretch were collected, RNA was extracted, cDNA was synthesized, and expression of DKK1 was analyzed with real time RT-PCR. Control cells were cultured on collagen-coated Tissue Train 6-well culture plates without mechanical strain.
Quantitative Real Time PCR
Real time PCR was carried out with ABI Prizm (Applied Biosystems) according to the manufacturer's instructions as described before (23). Primers for mRNA detection of genes of interest were constructed according to requirements for oligonucleotide primers for real time RT-PCR using Primer3 software. Expression of DKK1 in human cells was evaluated using previously published sequences of primers (37); RPL-19 was used a housekeeping gene control (19). Expression of DKK1 in rat cortical neurons was evaluated using primers for rat DKK1 (forward, 5′-GACGAGTACTGCTCCAGT-3′; reverse, 5′-GAGGTAAATGGCTGTGGT-3′) and rat Rpl19 as a housekeeping control (forward, 5′-GTCAACAGATCAGGAAGCTG-3′; reverse, 5′-TACCCTTCCTCTTCCCTATG-3′). Relative quantification of gene expression between experimental (three-dimensional CI) and control (two-dimensional CI) samples was measured by normalization against endogenous RPL-19 using the ΔCt method. -Fold changes were quantified as 2−(ΔCt sample − ΔCt control) as described previously (38).
Real time RT-PCR was also used to detect the levels of DKK1 and MT1-MMP mRNAs in samples from tissues of ovary and ovarian carcinoma commercially available from Origene and assayed according to the manufacturer's suggestions. Ct values for DKK1, MT1-MMP, RPL-19, and ACTNB were obtained using ABI Prizm (Applied Biosystems) according to the manufacturer's instructions as described above under “Quantitative Real Time PCR.” RPL-19 and ACTNB were expressed in each sample as reported before (23). Data were analyzed using a Mann-Whitney U test for non-parametric statistical analysis of categorical data (Sigmaplot).
Immunohistochemistry
Immunohistochemical analysis of DKK1 expression was done retrospectively on tumor tissue microarrays prepared with Institutional Review Board approval by the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University and assembled from tissue originally taken for postoperative diagnostic purposes. The microarray tissue specimens included 17 paired primary and metastatic ovarian cancer tissues obtained during the same surgical procedure from patients who were not treated against ovarian cancer prior to the operation (15 serous and two endometroid). Samples were cut 3–4 μm thick and deparaffinized. The cores were 1 mm in diameter. Antigen retrieval was accomplished by heat induction at 99 °C for ∼45 min. Immunohistochemical staining with antibodies to DKK1 at a 1:100 dilution was done according to standard procedures at the Pathology Core of Northwestern University. Scoring of DKK1 was assigned according to the average overall intensity of the staining and was graded as follows: 0, no staining; 1, fine granular staining; 2, somewhat coarse staining but less than positive control tissue (human testis); 3, very coarse staining similar to positive control tissue. Staining of <10% of tumor cells, regardless of intensity, was considered negative. Staining of between 10 and 75% of tumor cells was considered focal positive, and staining of greater than 75% of tumor cells was considered diffuse positive. Immunohistochemical analysis of β-catenin expression in metastasis from ovarian carcinoma (38 serous, one mucinous, and one transitional histotype) was performed using tissue microarray OV808 (US Biomax, Rockville, MD) by the Pathology Core Facility at the University of Illinois at Chicago. Antigen retrieval was accomplished by heat induction at 90 °C for 15 min. Immunohistochemical staining with antibodies to β-catenin at a 1:50 dilution was done according to standard procedures. Analysis of tissue sections was done by light microscopy by an anatomic pathologist without prior knowledge of the clinical variables.
Immunofluorescent Staining
Cells were cultured atop three-dimensional CI and two-dimensional CI in glass chamber slides for 6 h followed by 20-min fixation with 4% paraformaldehyde. Membrane permeabilization and blocking for nonspecific immunoreactivity was achieved by incubation with 0.1% TritonX-100 and 3% BSA in PBS for 1 h at room temperature (22 °C). β-Catenin antibody (BD Transduction Laboratories) was used at a 1:50 dilution in a solution of 3% BSA in PBS for 1 h at room temperature. β-Catenin signal was visualized using goat anti-mouse Alexa Fluor 488-conjugated antibodies (incubation, 1 h; antibody dilution, 1:500). Images were collected with a Zeiss Axiovert fluorescence microscope.
Transient Transfections
Transient transfections were performed using the lipofection method with Lipofectamine 2000 (Invitrogen) as a vehicle. DKK1-specific and control siRNAs, pCS2+/DKK1, TOPFlash, FOPFlash, and the Renilla luciferase expression construct were transiently transfected into DOV13 cells according to the manufacturer's instructions.
Collagen Invasion and Motility Assays
Invasion assays were performed using Transwell chambers (0.8 μm; BD Biosciences) as described (19). Motility was assessed using a scratch wound assay. Briefly, DOV13 cells were cultured in standard conditions as described under “Materials” until 70–80% confluence following transient transfection with either DKK1 siRNA or control siRNA as indicated under “Transient Transfections.” Wounds were introduced into the confluent monolayers by a pipette tip. Wound closure was monitored over time and photographed using a Zeiss Axiovert microscope at 5× objective magnification. The distance between the monolayers was measured using Zeiss Axiovert software in 10 random places and averaged, and the percentage of wound healing at a given time compared with the initial wound width was calculated.
Cell Adhesion
Adhesion of cells transiently transfected with control, DKK1 siRNA, and pCS2+/DKK1 to collagen I (0.5 μg) was analyzed as described previously (35). Briefly, cells (3000) were seeded on collagen I deposited by passive adsorption in 96-well plates and allowed to attach for 1 and 3 h. Non-adherent cells were removed with the medium. Adherent cells were washed once with phosphate-buffered saline, fixed, stained using a Diff-Quik kit (Dade Behring), counted in five random fields, and averaged.
Subcellular Fractionation
Cells were cultured on two-dimensional CI and three-dimensional CI for 6 h; released with 0.05% trypsin, EDTA or 10 μg/ml collagenase, respectively; and collected by centrifugation for 5 min at 6000 rpm at 4 °C. Cells were resuspended in 50 mm Tris, pH 8.0 containing 150 mm NaCl, 1% Nonidet P-40, and phosphatase inhibitor mixture (Roche Applied Science); incubated on ice for 5 min; and spun down for 5 min at 12,000 rpm. Supernatant containing the cytoplasmic fraction was removed, and the pelleted nuclei were resuspended in 50 mm HEPES, pH 7.5 containing 150 mm NaCl, 10 mm EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor mixture (Roche Applied Science). Pellets were incubated for 2 min on ice, briefly vortexed, and returned to ice; the procedure was repeated four times; and then the samples were centrifuged as above. Supernatants were collected, protein concentration was measured with the Bradford protein assay, and samples were processed for Western blot.
Western Blotting
Cells incubated under various conditions were collected, and samples were prepared as described before (19). Cell lysates (20 μg) were electrophoresed on 9% SDS-polyacrylamide gels under reducing conditions (39), electroblotted to a polyvinylidene difluoride (PVDF) membrane (40), blocked with 5% skim milk in TBST (25 mm Tris, pH 7.5, 150 mm NaCl, 0.1% Tween 20) for 1 h at room temperature (20 °C). Membranes were incubated for 1–2 h at room temperature with antibodies against proteins of interest. The antibodies were used at the following dilutions: 1:200 for anti-human DKK1 polyclonal antibody in 3% bovine serum in TBST, 1:1000 for anti-β-tubulin monoclonal antibody in 5% skim milk in TBST, 1:1000 for anti-MT1-MMP polyclonal antibody in 3% BSA, 1:500 for anti-β-catenin monoclonal antibody in 5% skim milk in TBST, and 1:2000 for anti-GAPDH monoclonal antibody in 5% skim milk in TBST. Immunoreactive bands were visualized with anti-rabbit IgG-peroxidase or anti-mouse IgG-peroxidase (1:1000 in 5% skim milk in TBST) and enhanced chemiluminescence using a LAS3000 system (Fujifilm) and LAS3000 ImageReader software. Band intensities were determined using LAS3000 ImageGauge software according to the manufacturer's instructions. Levels of nuclear β-catenin were normalized to control blots containing TATA-binding protein.
RESULTS
Three-dimensional Collagen I Culture Down-regulates Dickkopf-1
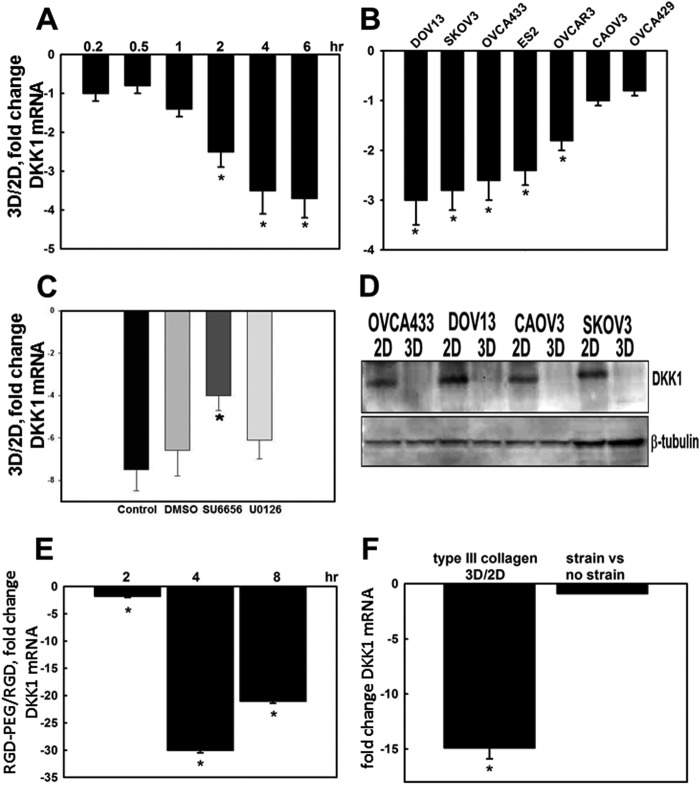
Following exfoliation from the primary tumor into the peritoneal cavity, disseminating ovarian cancer cells transition from a cell population that is free floating in ascites to adhesive, peritoneally anchored metastases (Fig. 1) (16, 18). Key events in this process are peritoneal mesothelial cell retraction and exposure of the underlying three-dimensional submesothelial matrix that is rich in interstitial collagens. Metastasizing cells establish β1 integrin-mediated interactions with the three-dimensional CI-rich submesothelial matrix. To evaluate changes in gene expression that accompany de novo collagen contacts established during metastatic implantation, a cDNA microarray was used for expression profiling of genes altered by three-dimensional CI culture. Our data revealed an unexpected strong down-regulation of the Wnt signaling inhibitor DKK1, suggesting a functional coupling of cell matrix adhesion and Wnt signaling (23, 41, 42). Wnt signaling controls many important cellular processes including expression of ECM-degrading proteases that promote invasion (43–49). Although activating mutations in this pathway are relatively rare in EOC, deregulated Wnt signaling has been implicated in EOC progression (30–33). We observed that three-dimensional CI culture led to a progressive sustained loss of DKK1 expression relative to cells cultured on planar (two-dimensional) collagen-coated surfaces. Significant sustained down-regulation of DKK1 mRNA was evident as early as 2 h of culture on three-dimensional CI (Fig. 2A). Multiple ovarian carcinoma cell lines responded to three-dimensional CI culture with down-regulation of DKK1 RNA and protein (Fig. 2, B and D). Inhibition of Src kinase activity partially restored DKK1 expression (Fig. 2C). Similar to results observed in collagen cultures, EOC cells grown in RGDS-supplemented three-dimensional PEG scaffolds also significantly down-regulated DKK1 (Fig. 2E) as did culture in three-dimensional type III collagen gels (Fig. 2F). However, alteration of cell shape through the application of mechanical strain to cells cultured on planar collagen I-coated Flexercell membranes had no effect on DKK1 expression (Fig. 2F).
FIGURE 2.
Three-dimensional collagen culture down-regulates DKK1 expression. A, DOV13 cells were cultured on three-dimensional CI (3D) or planar collagen (two-dimensional (2D)) for the indicated time followed by real time RT-PCR analysis of DKK1 mRNA expression. Shown is the -fold change in the ratio of DKK1 mRNA in three-dimensional relative to two-dimensional collagen. Ratios of DKK1 RNA expression were obtained using the 2−ΔΔCt method. An average of three independent experiments ±S.D. (error bars) is presented, and data were statistically evaluated by t test. * designates p < 0.05. B, multiple ovarian carcinoma cell lines as indicated were cultured on three-dimensional CI or planar collagen (two-dimensional) for 8 h followed by RNA extraction, cDNA synthesis, and real time RT-PCR to assess DKK1 mRNA levels. Shown is the -fold change in the ratio of DKK1 mRNA in three-dimensional CI relative to two-dimensional. * designates p < 0.05. C, DOV13 cells were cultured for 8 h on three-dimensional CI or planar collagen (two-dimensional) in the presence of DMSO, SU6656 (2 μm), or UO126 (25 μm) as indicated followed by analysis of DKK1 mRNA expression as in A. * designates p < 0.05. D, representative Western blot of DKK1 levels in whole cell lysates after 24-h culture on planar collagen (two-dimensional) and three-dimensional CI as indicated. Blots were developed using polyclonal anti-DKK1 (1:200) and anti-rabbit HRP-conjugated secondary antibodies (1:1000). Expression of β-tubulin was probed as a loading control. E, DOV13 cells were cultured atop three-dimensional RGDS-PEG gels or planar unconjugated RGDS for the indicated periods of time followed by RNA extraction, cDNA synthesis, and real time RT-PCR. Averaged ratios of DKK1 mRNA levels in cells cultured on three-dimensional versus two-dimensional substratum are plotted on the histogram as -fold down-regulation against time. * designates p ≤ 0.005. F, expression of DKK1 mRNA was analyzed in DOV13 cells cultured on three-dimensional gels prepared from type III collagen relative to cells cultured on planar (two-dimensional) collagen III. Additionally, expression was evaluated in cells subjected to mechanical strain (as described under “Experimental Procedures”) compared with control cells as indicated. Averaged ratios of DKK1 mRNA levels in cells cultured on three-dimensional CIII versus planar CIII (two-dimensional) and under conditions of strain versus controls are plotted on the histogram as -fold down-regulation. * designates p < 0.005.
Matrix Mechanical Properties Regulate DKK1 Expression
Cells respond to changes in the physical properties of the ECM with altered gene expression, and the mechanical properties of a tissue can contribute actively to disease progression (3, 4, 6, 7). To assess the effect of matrix rigidity on DKK1 expression, cells were cultured in collagen gels of varying density. DKK1 expression was decreased to a greater extent in more rigid three-dimensional collagen gels (formed from 2 mg/ml collagen) relative to those formed from more compliant collagen gels (0.8 mg/ml) (Fig. 3A). Because altering collagen concentration will also change the number of integrin binding sites, we used an alternative approach wherein gels were constructed with a constant amount of collagen (0.2 mg/ml) with varied polyacrylamide-bisacrylamide to test the effect of matrix elasticity on DKK1 expression (34, 50). Progressive down-regulation of DKK1 expression was dependent on the gel density (Fig. 3B). Control experiments used cells cultured on RGDS-PEG gels with constant RGDS and variable PEG content, demonstrating enhanced loss of DKK1 in higher PEG concentration gels (Fig. 3C; 10% PEG elastic modulus, 2760 pascals), indicating that matrix rigidity contributes to regulation of DKK1.
FIGURE 3.
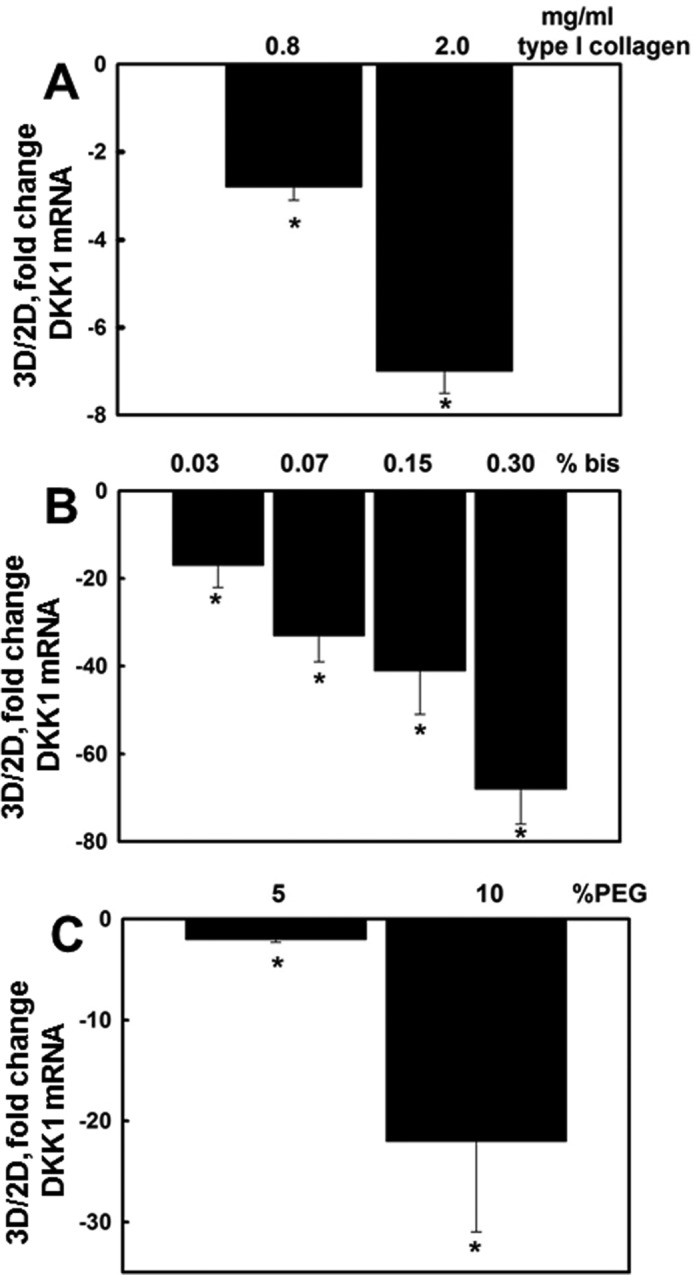
Matrix rigidity regulates DKK1 expression. A, DOV13 cells were cultured on three-dimensional (3D) collagen I gels formed using 0.8 or 2.0 mg/ml collagen I as indicated. Controls included cells cultured on planar (two-dimensional (2D)) collagen I. RNA was extracted, cDNA was synthesized, and real time RT-PCR was performed to detect DKK1 RNA expression. The ratio of DKK1 expression is depicted. An average of three independent experiments ±S.D. (error bars) is presented. p values were calculated using Student's t test. * designates p < 0.0005. B, DOV13 cells were cultured for 6 h on polyacrylamide gels containing a constant collagen concentration (0.2 mg/ml) with varied bisacrylamide to modulate gel stiffness. Cells were collected, total RNA was extracted, cDNA was synthesized, and DKK1 expression was detected using RT-PCR. * designates p < 0.05. C, DOV13 cells were cultured on 5 or 10% PEG gels with a constant RGDS concentration as indicated under “Experimental Procedures” for 8 h. Controls included cells cultured on a planar RGDS-coated tissue culture support. Total RNA was extracted, cDNA was synthesized, and real time RT-PCR was performed to detect DKK1 RNA expression. The ratio of DKK1 expression in three-dimensional versus two-dimensional culture conditions is depicted. An average of three independent experiments ±S.D. (error bars) is presented. p values were calculated using Student's t test. * designates p < 0.005.
Three-dimensional Collagen I Matrix Alters β-Catenin Dynamics
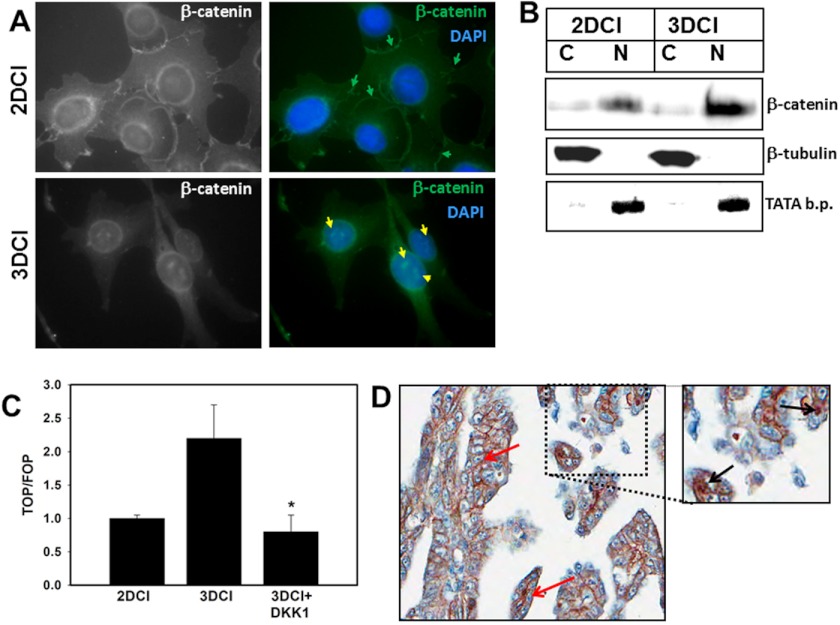
In contrast to cells cultured on planar collagen I that exhibit junctional β-catenin staining, three-dimensional CI culture alters β-catenin dynamics, leading to accumulation of nuclear β-catenin (Fig. 4, A and B). Densitometric analysis revealed a 2-fold increase in nuclear β-catenin in cells cultured on three-dimensional CI relative to two-dimensional. Transfection of cells with the TOPFlash Tcf/Lef-luciferase reporter construct was used to evaluate whether nuclear β-catenin was transcriptionally active. Increased TOPFlash activity was observed in cells on three-dimensional CI, and this was blocked by the addition of exogenous DKK1 (Fig. 4C). As metastasizing ovarian cancer cells encounter a three-dimensional CI-rich submesothelial matrix, expression and localization of β-catenin were examined in human metastatic ovarian carcinoma tissues. Consistent with previous findings (51, 52), our data indicate that 7.5% of cases display positive nuclear immunoreactivity for β-catenin (Fig. 4D), whereas 68% of cases exhibit β-catenin staining in the cytoplasm, the plasma membrane, or both. The apparent discrepancy between the number of cells positive for β-catenin in human tissues versus organotypic cell culture models can be attributed to the relatively transient nature of nuclear β-catenin localization as well as the lower fraction of tissue-associated cells that are actually in physical contact with the ECM.
FIGURE 4.
Three-dimensional CI modulates β-catenin dynamics. A, DOV13 cells were cultured for 6 h on three-dimensional (3D) CI or planar collagen (two-dimensional (2D)) as indicated followed by fixation and staining using anti-β-catenin and anti-mouse Alexa Fluor 488 antibodies at 1:50 and 1:500 dilutions, respectively. Nuclei are stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Immunofluorescence images were taken with a Zeiss Axiovert fluorescence microscope using a 63× objective. Green and yellow arrows show membranous and nuclear β-catenin, respectively. Nuclear β-catenin was detected in >70% of cells cultured on three-dimensional CI (evaluation of 100 cells per condition). Magnification, 400×. B, cells were cultured on three-dimensional CI or planar collagen I for 6 h and subjected to subcellular fractionation as indicated under “Experimental Procedures” to collect cytoplasmic (C) and nuclear (N) fractions. The lysates were analyzed by Western blot using anti-β-catenin at a 1:50 dilution, anti-β-tubulin at a 1:1000 dilution, and anti-histone H3 at a 1:50 dilution. β-Tubulin and TATA-binding protein (b.p.) expression served as indicators of successful separation of cytoplasmic or nuclear protein fractions, respectively. To provide semiquantitative analysis of β-catenin distribution, levels of nuclear β-catenin were quantified using densitometry and normalized relative to TATA-binding protein. A 2-fold increase in nuclear β-catenin was observed in cells cultured on three-dimensional CI relative to two-dimensional. C, cells were transiently transfected with TOPFlash (TOP) and FOPFlash (FOP) promoter-luciferase reporter constructs to evaluate β-catenin transcriptional activity in cells cultured on three-dimensional CI versus planar (two-dimensional) collagen I for 6 h. A Renilla luciferase-expressing plasmid was co-transfected to account for the efficiency of transfection. Cells were lysed, and the luciferase signal was measured using a Dual-Luciferase system. TOPFlash and FOPFlash signals were first normalized to the Renilla luciferase signal, and then ratios of TOPFlash to FOPFlash were calculated. Results represent the mean and S.D. of three experiments. * designates p < 0.05 relative to control cells on three-dimensional CI. D, immunohistochemical analysis of β-catenin expression in metastatic human ovarian carcinoma. Shown is a specimen of a metastatic serous papillary adenocarcinoma from ovary to omentum (core number 43 in OV808 tissue microarray, US Biomax). Pictures were taken with an Aperio Imagescope system at 20× magnification. The outlined region was magnified 1.5-fold. Red arrows indicate membranous and black arrows indicate nuclear β-catenin.
Loss of DKK1 Enables Metalloproteinase-dependent Collagen Invasion
Metastatic success of ovarian cancer cells requires localized penetration of and anchoring within a three-dimensional CI matrix (16, 18), and three-dimensional CI interaction induces a promigratory, invasive phenotype in ovarian cancer cells. Many invasion-associated genes are known Wnt targets including ECM-degrading proteinases such as the collagenolytic MT1-MMP, suggesting that modulation of Wnt signaling can regulate invasion (43–49). Indeed, siRNA silencing of DKK1 expression significantly enhanced invasion, whereas transfection with a DKK1 expression vector or addition of exogenous DKK1 dramatically reduced invasive activity (Fig. 5A andsupplemental Fig. 1). Neither motility nor adhesion to collagen were altered in DKK1-modified cells (supplemental Fig. 2). The interstitial collagenase MT1-MMP, an important driver of the collagen-invasive phenotype (11, 17, 53), is regulated downstream of β-catenin signaling (45, 48, 54). Three-dimensional CI culture increases MT1-MMP mRNA and protein levels (19), and this enhanced expression was blocked by the addition of exogenous DKK1 (Fig. 5, B–D). These data support a model for collagen-induced invasion via down-regulation of DKK1 and enhanced MT1-MMP activity.
FIGURE 5.
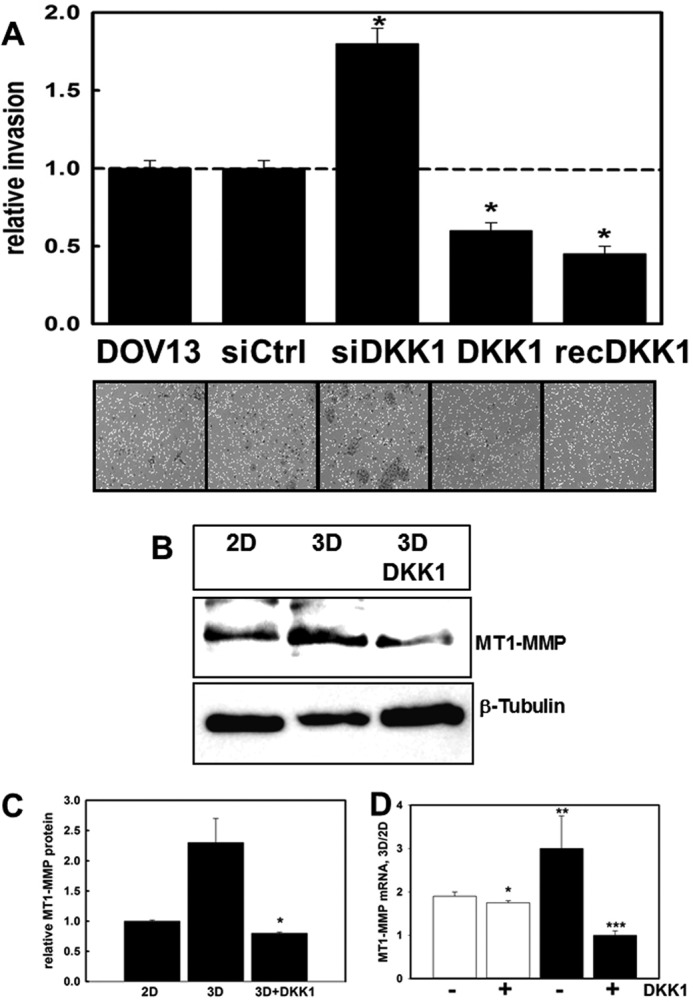
DKK1 regulates MT1-MMP-dependent collagen invasion. A, a modified Boyden chamber assay was used to evaluate invasion of cells through three-dimensional CI. Cells were transfected with control siRNA (siCtrl), DKK1-specific siRNA (siDKK1), or a DKK1 expression plasmid (pCS2+/DKK1; designated DKK1) or were incubated in the presence of 0.33 μg/ml recombinant DKK1 (rDKK1) as indicated. Cells invaded through the collagen gel (18 h) and attached to the lower surface of the filter. Following removal of non-invasive cells from the upper surface, the filter was stained, and invasive cells were quantified. Invasion of DOV13 cells was arbitrarily set as “1,” and other values were normalized to this value. An average of five independent experiments is presented. Error bars represent S.D. p values were obtained by Student's t test in comparison with non-treated DOV13 controls. * designates p < 0.0005. B and C, DOV13 cells were cultured on three-dimensional (3D) CI or planar (two-dimensional (2D)) collagen I in the presence or absence of 0.33 mg/ml exogenous DKK1 and subjected to Western blot to detect MT1-MMP expression (1:1000 antibody dilution). β-Tubulin was used as a protein loading control. The histogram (C) shows densitometric quantitation of MT1-MMP levels. Expression of MT1-MMP in cells on planar collagen I was arbitrarily set as 1. p values were obtained by Student's t test. * designates p < 0.005 relative to culture on three-dimensional CI. D, DOV13 cells were cultured on three-dimensional CI or planar (two-dimensional) collagen I in the presence or absence of 0.33 mg/ml exogenous DKK1 for 4 (open bars) or 8 h (solid bars). RNA was extracted, cDNA was synthesized, and real time RT-PCR was performed to detect MT1-MMP RNA expression. Ratios of MT1-MMP RNA expression were found using the 2−ΔΔCt method. p values were obtained by Student's t test. * designates p = 0.03 relative to the 4-h sample in the absence of DKK1, ** designates p = 0.002 relative to the 4-h sample in the absence of DKK1, and *** designates p = 0.001 relative to the 8-h sample in the absence of DKK1.
Inverse Correlation between DKK1 and MT1-MMP Expression in Human Ovarian Tumors
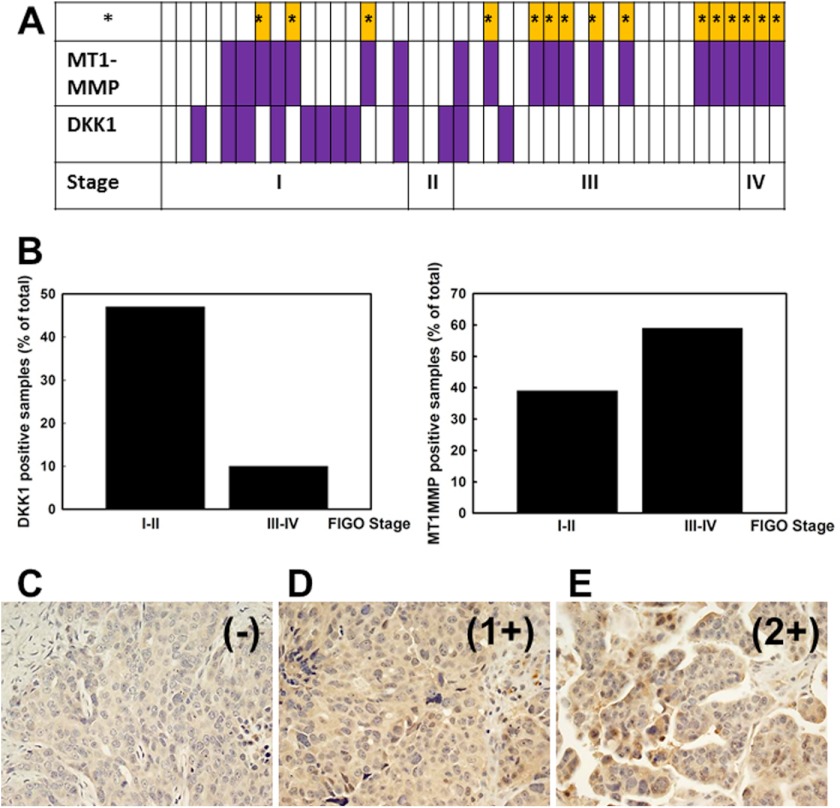
Real time RT-PCR was used to monitor DKK1 and MT1-MMP expression in mRNA samples from EOC patients. In samples from early stage (I-II) tumors, 47% expressed DKK1, whereas expression was retained in only 10% of late stage (III-IV) specimens (p = 0.004). In contrast, only 39% of early stage tumors were MT1-MMP-positive, whereas 60% of late stage EOC exhibited MT1-MMP expression (Fig. 6, A and B; p = 0.08). Coincident negative DKK1 and positive MT1-MMP expression was observed in 36.5% of the tested samples (Fig. 6A, yellow bars; p = 0.012). Similar results were obtained upon immunohistochemical examination of human tumor specimens (Fig. 6, C–E, and supplemental Table 1) (55).
FIGURE 6.
Analysis of DKK1 and MT1-MMP expression in human ovarian tumors. A, human ovarian carcinoma RNA extracts were tested for DKK1 and MT1-MMP expression using real time RT-PCR. Open rectangles represent specimens with negative DKK1 or MT1-MMP expression (defined as no signal or Ct < 35), and closed purple filled rectangles show positive DKK1 or MT1-MMP expression (defined as Ct ≥35). A total of 41 samples were tested. Results for samples from 1 to 41 are plotted from left to right. Samples from ovarian carcinoma International Federation of Gynecology and Obstetrics (FIGO) stages I, II, III, and IV are indicated as “I,” “II,” “III,” and “IV,” respectively. Cases where negative DKK1 coincided with positive MT1-MMP expression are indicated (*; yellow bars). B, histogram showing the percentage of DKK1- and MT1-MMP-positive samples as percentage of total. C–E, representative examples from immunohistochemical analysis of DKK1. Examples of DKK1 staining include negative (−) (C; case number 9 from supplemental Table 1), weakly positive 1+ (D; case number 8 from supplemental Table 1), and moderately positive 2+ (E; case number 15 from supplemental Table 1). Magnification, 20×.
Three-dimensional CI Culture Modulates DKK1 Expression in Multiple Invasive Cell Types
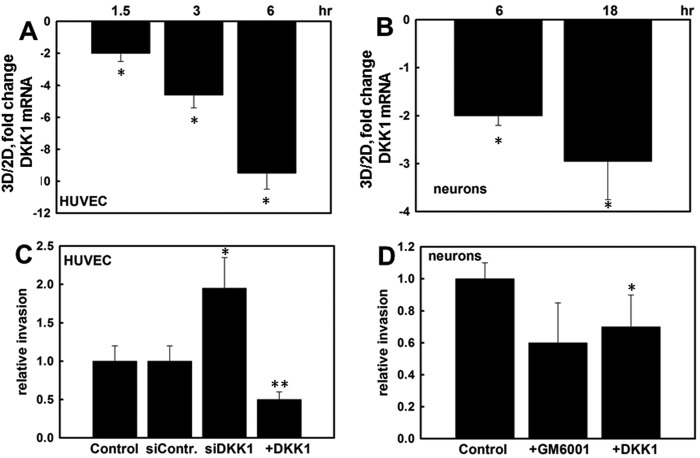
Matrix invasion is essential for a number of physiological and pathological processes. To determine whether other collagen-invasive cell types also respond to three-dimensional CI culture with DKK down-regulation, endothelial cells and cortical neurons were examined. Both cell types responded to three-dimensional CI culture with down-regulation of DKK1 expression (Fig. 7, A and B). Silencing of DKK1 expression using siRNA significantly increased endothelial cell invasion, whereas DKK1 overexpression down-regulated invasive activity (Fig. 7C). Similarly, three-dimensional CI invasion by cortical neurons was DKK1-dependent (Fig. 7D). Evaluation of invasion in the presence of the broad spectrum MMP inhibitor GM6001 suggests that MMPs also play a role in neuronal invasion of collagen type I (Fig. 7D).
FIGURE 7.
Three-dimensional CI regulates DKK1 expression and invasion in endothelial cells and primary cortical neurons. Human umbilical vein endothelial cells (HUVEC) (A) and E17 primary cortical neurons (B) were cultured on three-dimensional (3D) CI or planar (two-dimensional (2D)) collagen I for the indicated periods of time. RNA was extracted, cDNA was synthesized, and real time RT-PCR was performed to detect DKK1 RNA expression. Ratios of DKK1 RNA expression were found with the 2−ΔΔCt method. The averaged ratio of DKK1 expression in three-dimensional CI versus two-dimensional CI from three independent experiments is depicted in the histograms ±S.D. (error bars). * designates p < 0.0005. C, endothelial cells were transiently transfected with control siRNA (siContr), DKK1-specific siRNA (siDKK1), or a DKK1-expressing plasmid (+DKK1) as indicated followed by evaluation of the ability to invade three-dimensional CI gels. Invasion of untreated control cells was arbitrarily set as 1, and other values were calculated accordingly. The average of four independent experiments ±S.D. (error bars) is presented. * designates p < 0.05, and ** designates p < 0.005 relative to control. D, the ability of E17 cortical neurons to invade three-dimensional CI gels was assessed in the presence and absence of 10 μm GM6001 or 0.33 mg/ml exogenous DKK1 as indicated. The average of three independent experiments ±S.D. (error bars) is presented. * designates p < 0.05 relative to untreated controls. Note that comparison of invasion in the presence of GM6001 resulted in p = 0.06.
DISCUSSION
In addition to regulation of cell adhesion and behavior, the matrix microenvironment functions as an epigenetic regulator of gene expression (56, 57). Moreover, changes in matrix mechanical properties fine-tune these regulatory processes such that altered matrix deposition or de novo contact with a distinct matrix microenvironment contributes significantly to control of gene expression (3, 58). The current findings indicate that matrix rigidity may be a pivotal factor in regulating the activity of Wnt signaling in ovarian cancer cells lacking activating mutations in Wnt pathway components. β1 integrin clustering is robust on rigid substrates with high ligand density but is impaired on substrates that are highly compliant or have low ligand density (59). Similarly, our findings show that down-regulation of DKK1 is stronger in more rigid matrices, suggesting that the physical properties of the ECM in addition to matrix composition play a key role in fine-tuning gene expression. It should be noted that application of mechanical strain in the absence of a three-dimensional matrix did not alter DKK1 expression. Furthermore, we have recently used β1 integrin antibody-conjugated microspheres to mimic multivalent cell matrix engagement such as occurs when EOC cells contact three-dimensional CI. Integrin clustering resulted in loss of E-cadherin junctional integrity, nuclear translocation of β-catenin, and transcriptional activation of genes involved in Wnt signaling including the ligand Wnt5a and the co-receptor LRP-6 (33). Together with the current results, these data support a functional link between matrix rigidity, β1 integrin clustering, down-regulation of DKK1, and enhanced Tcf/Lef transcriptional activity. Similar results were observed using other tissue-invasive cells including endothelial cells and cortical neurons, suggesting a common matrix-modulated regulatory pathway used by tissue-invasive cells.
In our previous studies of cells contacting three-dimensional collagen matrices, activation of Src was observed as early as 10 min in three-dimensional CI culture and was sustained for 17 h (19). In the current study, inhibition of Src kinase activity partially restored DKK1 expression, suggesting that integrin-mediated Src activation plays a role in DKK1 down-modulation in response to three-dimensional matrix engagement. Furthermore, both integrin signaling through induction of Egr1 (19) and Wnt signaling through β-catenin/Tcf/Lef (45, 48) function to regulate expression of MT1-MMP. These data suggest the potential for complex cross-talk between adhesion-regulated signal transduction pathways to fine-tune pericellular proteolysis and ultimately control tissue invasion.
Down-regulation of DKK1 may lead to dramatic changes in gene expression as DKK1 functions as an inhibitor of Wnt signaling, which affects expression of numerous target genes that modulate ECM degradation, proliferation, apoptosis, transcription, and cell signaling (25, 60). In addition to MT1-MMP, it is interesting to speculate that dysregulation of additional genes that contribute to EOC progression, metastasis, and chemoresistance may result from loss of DKK1 expression. These findings may have significant implications for progression of ovarian carcinoma to metastasis. Metastatic ovarian carcinoma cells anchor in the submesothelial matrix of the peritoneum and omentum, a microenvironment composed predominantly of a three-dimensional matrix of interstitial (types I and III) collagens (61–64). The current data suggest that acquisition of this metastatic niche may activate Wnt signaling in the absence of Wnt pathway mutations (30–32). This activation may be transient and/or restricted to the subpopulation of metastatic cells engaged in matrix contact, leading to differential regulation of the target genes that regulate metastatic progression. As this subpopulation of metastatic EOC cells may impact successful clinical outcomes, a more detailed understanding of this regulatory mechanism is warranted.
Acknowledgments
We gratefully acknowledge Dr. Xi He for providing the DKK1 expression construct and Dr. Cara Gottardi for the gift of TOPFlash and FOPFlash constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants CA086984 and CA109545 (to M. S. S.). This work was also supported by the Ovarian Cancer Research Foundation Program of Excellence (to M. V. B.) and the Illinois Department of Public Health (to M. V. B.).

This article contains supplemental Figs. 1 and 2 and Table 1.
- ECM
- extracellular matrix
- EOC
- epithelial ovarian cancer
- DKK1
- dickkopf-1
- MT1-MMP
- membrane type 1 matrix metalloproteinase
- CI
- collagen I
- CIII
- collagen III
- MMP
- matrix metalloproteinase.
REFERENCES
- 1. Bissell M. J., Barcellos-Hoff M. H. (1987) The influence of extracellular-matrix on gene-expression: is structure the message. J. Cell Sci. Suppl. 8, 327–343 [DOI] [PubMed] [Google Scholar]
- 2. Labat-Robert J., Bihari-Varga M., Robert L. (1990) Extracellular matrix. FEBS Lett. 268, 386–393 [DOI] [PubMed] [Google Scholar]
- 3. Butcher D. T., Alliston T., Weaver V. M. (2009) A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engler A. J., Humbert P. O., Wehrle-Haller B., Weaver V. M. (2009) Multiscale modeling of form and function. Science 324, 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gehler S., Baldassarre M., Lad Y., Leight J. L., Wozniak M. A., Riching K. M., Eliceiri K. W., Weaver V. M., Calderwood D. A., Keely P. J. (2009) Filamin A-β1 integrin complex tunes epithelial cell response to matrix tension. Mol. Biol. Cell 20, 3224–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L., Weaver V. M. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., Hammer D. A., Weaver V. M. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 [DOI] [PubMed] [Google Scholar]
- 8. Hatten M. E. (1999) Central nervous system neuronal migration. Annu. Rev. Neurosci. 22, 511–539 [DOI] [PubMed] [Google Scholar]
- 9. Beck L., Jr., D'Amore P. A. (1997) Vascular development: cellular and molecular regulation. FASEB J. 11, 365–373 [PubMed] [Google Scholar]
- 10. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 11. Hotary K. B., Allen E. D., Brooks P. C., Datta N. S., Long M. W., Weiss S. J. (2003) Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114, 33–45 [DOI] [PubMed] [Google Scholar]
- 12. Koh W., Sachidanandam K., Stratman A. N., Sacharidou A., Mayo A. M., Murphy E. A., Cheresh D. A., Davis G. E. (2009) Formation of endothelial lumens requires a coordinated PKCϵ-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J. Cell Sci. 122, 1812–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koh W., Stratman A. N., Sacharidou A., Davis G. E. (2008) In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 443, 83–101 [DOI] [PubMed] [Google Scholar]
- 14. Stratman A. N., Saunders W. B., Sacharidou A., Koh W., Fisher K. E., Zawieja D. C., Davis M. J., Davis G. E. (2009) Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 114, 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsukatani T., Fillmore H. L., Hamilton H. R., Holbrook E. H., Costanzo R. M. (2003) Matrix metalloproteinase expression in the olfactory epithelium. Neuroreport 14, 1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barbolina M. V., Moss N. M., Westfall S. D., Liu Y., Burkhalter R. J., Marga F., Forgacs G., Hudson L. G., Stack M. S. (2009) Microenvironmental regulation of ovarian cancer metastasis. Cancer Treat. Res. 149, 319–334 [DOI] [PubMed] [Google Scholar]
- 17. Barbolina M. V., Stack M. S. (2008) Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin. Cell Dev. Biol. 19, 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hudson L. G., Zeineldin R., Stack M. S. (2008) Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin. Exp. Metastasis 25, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbolina M. V., Adley B. P., Ariztia E. V., Liu Y., Stack M. S. (2007) Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J. Biol. Chem. 282, 4924–4931 [DOI] [PubMed] [Google Scholar]
- 20. Fishman D. A., Chilukuri K., Stack M. S. (1997) Biochemical characterization of primary peritoneal carcinoma cell adhesion, migration, and proteinase activity. Gynecol. Oncol. 67, 193–199 [DOI] [PubMed] [Google Scholar]
- 21. Moser T. L., Pizzo S. V., Bafetti L. M., Fishman D. A., Stack M. S. (1996) Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the α2β1 integrin. Int. J. Cancer 67, 695–701 [DOI] [PubMed] [Google Scholar]
- 22. Ellerbroek S. M., Fishman D. A., Kearns A. S., Bafetti L. M., Stack M. S. (1999) Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through β1 integrin. Cancer Res. 59, 1635–1641 [PubMed] [Google Scholar]
- 23. Barbolina M. V., Adley B. P., Kelly D. L., Fought A. J., Scholtens D. M., Shea L. D., Stack M. S. (2008) Motility-related actinin α-4 is associated with advanced and metastatic ovarian carcinoma. Lab. Invest. 88, 602–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barbolina M. V., Adley B. P., Shea L. D., Stack M. S. (2008) Wilms' tumor gene protein 1 is associated with ovarian cancer metastasis and modulates cell invasion. Cancer 112, 1632–1641 [DOI] [PubMed] [Google Scholar]
- 25. Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 [DOI] [PubMed] [Google Scholar]
- 26. Nelson W. J., Nusse R. (2004) Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schambony A., Kunz M., Gradl D. (2004) Cross-regulation of Wnt signaling and cell adhesion. Differentiation 72, 307–318 [DOI] [PubMed] [Google Scholar]
- 28. Shedden K. A., Kshirsagar M. P., Schwartz D. R., Wu R., Yu H., Misek D. E., Hanash S., Katabuchi H., Ellenson L. H., Fearon E. R., Cho K. R. (2005) Histologic type, organ of origin, and Wnt pathway status: effect on gene expression in ovarian and uterine carcinomas. Clin. Cancer Res. 11, 2123–2131 [DOI] [PubMed] [Google Scholar]
- 29. Wu R., Hendrix-Lucas N., Kuick R., Zhai Y., Schwartz D. R., Akyol A., Hanash S., Misek D. E., Katabuchi H., Williams B. O., Fearon E. R., Cho K. R. (2007b) Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/β-catenin and PI3K/Pten signaling pathways. Cancer Cell 11, 321–333 [DOI] [PubMed] [Google Scholar]
- 30. Gatcliffe T. A., Monk B. J., Planutis K., Holcombe R. F. (2008) Wnt signaling in ovarian tumorigenesis. Int. J. Gynecol. Cancer 18, 954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gamallo C., Palacios J., Moreno G., Calvo de Mora J., Suárez A., Armas A. (1999) β-Catenin expression pattern in stage I and II ovarian carcinoma. Am. J. Pathol. 155, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright K., Wilson P., Morland S., Campbell I., Walsh M., Hurst T., Ward B., Cummings M., Chenevix-Trench G. (1999) β-Catenin mutation and expression analysis in ovarian cancer. Int. J. Cancer 82, 625–629 [DOI] [PubMed] [Google Scholar]
- 33. Burkhalter R. J., Symowicz J., Hudson L. G., Gottardi C. J., Stack M. S. (2011) Integrin regulation of β-catenin signaling in ovarian carcinoma. J. Biol. Chem. 286, 23467–23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y. L., Pelham R. J., Jr. (1998) Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 298, 489–496 [DOI] [PubMed] [Google Scholar]
- 35. Raeber G. P., Lutolf M. P., Hubbell J. A. (2005) Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 89, 1374–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Velegol D., Lanni F. (2001) Cell traction forces on soft biomaterials. I. Microrheology of type I collagen gels. Biophys. J. 81, 1786–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zirn B., Hartmann O., Samans B., Krause M., Wittmann S., Mertens F., Graf N., Eilers M., Gessler M. (2006) Expression profiling of Wilms tumors reveals new candidate genes for different clinical parameters. Int. J. Cancer 118, 1954–1962 [DOI] [PubMed] [Google Scholar]
- 38. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 39. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 40. Matsudaira P. (1987) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262, 10035–10038 [PubMed] [Google Scholar]
- 41. Barbolina M. V., Adley B. P., Kelly D. L., Shepard J., Fought A. J., Scholtens D., Penzes P., Shea L. D., Stack M. S. (2009) Downregulation of connective tissue growth factor by three-dimensional matrix enhances ovarian carcinoma cell invasion. Int. J. Cancer 125, 816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barbolina MV, Burkhalter RJ, Stack MS. (2011) Diverse mechanisms for activation of Wnt signaling in the ovarian tumour microenvironment. Biochem. J. 437, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brabletz T., Jung A., Dag S., Hlubek F., Kirchner T. (1999) β-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 155, 1033–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crawford H. C., Fingleton B. M., Rudolph-Owen L. A., Goss K. J., Rubinfeld B., Polakis P., Matrisian L. M. (1999) The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 18, 2883–2891 [DOI] [PubMed] [Google Scholar]
- 45. Hlubek F., Spaderna S., Jung A., Kirchner T., Brabletz T. (2004) β-Catenin activates a coordinated expression of the proinvasive factors laminin-5 γ2 chain and MT1-MMP in colorectal carcinomas. Int. J. Cancer 108, 321–326 [DOI] [PubMed] [Google Scholar]
- 46. Mann B., Gelos M., Siedow A., Hanski M. L., Gratchev A., Ilyas M., Bodmer W. F., Moyer M. P., Riecken E. O., Buhr H. J., Hanski C. (1999) Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. U.S.A. 96, 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marchenko G. N., Marchenko N. D., Leng J., Strongin A. Y. (2002) Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem. J. 363, 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takahashi M., Tsunoda T., Seiki M., Nakamura Y., Furukawa Y. (2002) Identification of membrane-type matrix metalloproteinase-1 as a target of the β-catenin/Tcf4 complex in human colorectal cancers. Oncogene 21, 5861–5867 [DOI] [PubMed] [Google Scholar]
- 49. Wu B., Crampton S. P., Hughes C. C. (2007) Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 26, 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown X. Q., Bartolak-Suki E., Williams C., Walker M. L., Weaver V. M., Wong J. Y. (2010) Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J. Cell. Physiol. 225, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kildal W., Risberg B., Abeler V. M., Kristensen G. B., Sudbø J., Nesland J. M., Danielsen H. E. (2005) β-Catenin expression, DNA ploidy and clinicopathological features in ovarian cancer: a study in 253 patients. Eur. J. Cancer 41, 1127–1134 [DOI] [PubMed] [Google Scholar]
- 52. Lee C. M., Shvartsman H., Deavers M. T., Wang S. C., Xia W., Schmandt R., Bodurka D. C., Atkinson E. N., Malpica A., Gershenson D. M., Hung M. C., Lu K. H. (2003) β-Catenin nuclear localization is associated with grade in ovarian serous carcinoma. Gynecol. Oncol. 88, 363–368 [DOI] [PubMed] [Google Scholar]
- 53. Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., Friedl P. (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893–904 [DOI] [PubMed] [Google Scholar]
- 54. Liu P., Yang J., Pei J., Pei D., Wilson M. J. (2010) Regulation of MT1-MMP activity by β-catenin in MDCK non-cancer and HT1080 cancer cells. J. Cell. Physiol. 225, 810–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moss N. M., Barbolina M. V., Liu Y., Sun L., Munshi H. G., Stack M. S. (2009) Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: a potential role in i.p. metastatic dissemination. Cancer Res. 69, 7121–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roskelley C. D., Bissell M. J. (2002) The dominance of the microenvironment in breast and ovarian cancer. Semin. Cancer Biol. 12, 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roskelley C. D., Srebrow A., Bissell M. J. (1995) A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr. Opin. Cell Biol. 7, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sheetz M. P., Felsenfeld D. P., Galbraith C. G. (1998) Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 8, 51–54 [DOI] [PubMed] [Google Scholar]
- 59. Paszek M. J., Boettiger D., Weaver V. M., Hammer D. A. (2009) Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput. Biol. 5, e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moon R. T. (2005). Wnt/β-catenin pathway. Sci. STKE 2005, cm1. [DOI] [PubMed] [Google Scholar]
- 61. Puistola U., Risteli L., Risteli J., Kauppila A. (1990) Collagen metabolism in gynecologic patients: changes in the concentration of the aminoterminal propeptide of type III procollagen in serum. Am. J. Obstet. Gynecol. 163, 1276–1281 [DOI] [PubMed] [Google Scholar]
- 62. Risteli L., Kauppila A., Mäkilä U. M., Risteli J. (1988) Aminoterminal propeptide of type-III procollagen in serum—an indicator of clinical behavior of advanced ovarian carcinoma? Int. J. Cancer 41, 409–414 [DOI] [PubMed] [Google Scholar]
- 63. Risteli L., Risteli J., Puistola U., Tomás C., Zhu G. G., Kauppila A. (1992) Aminoterminal propeptide of type III procollagen in ovarian cancer. A review. Acta Obstet. Gynecol. Scand. Suppl. 155, 99–103 [DOI] [PubMed] [Google Scholar]
- 64. Zhu G. G., Risteli J., Puistola U., Kauppila A., Risteli L. (1993) Progressive ovarian carcinoma induces synthesis of type I and type III procollagens in the tumor tissue and peritoneal cavity. Cancer Res. 53, 5028–5032 [PubMed] [Google Scholar]