Background: Spinal cord injury causes irreversible neuronal damage.
Results: Human neural stem cells can integrate into chick neural tubes and regenerate neurons with extended axons in injured adult rats.
Conclusion: Grafted cells can overcome injury-induced inhibitory barriers to reintegrate into the host CNS.
Significance: It is feasible to generate new neuronal circuitry with grafted stem cells in a damaged CNS.
Keywords: Cell Differentiation, Neurodegeneration, Neurodifferentiation, Regeneration, Stem Cells, Neural Stem Cell, Spinal Cord Injury, Transplantation
Abstract
Spinal cord injury (SCI) results in devastating motor and sensory deficits secondary to disrupted neuronal circuits and poor regenerative potential. Efforts to promote regeneration through cell extrinsic and intrinsic manipulations have met with limited success. Stem cells represent an as yet unrealized therapy in SCI. Recently, we identified novel culture methods to induce and maintain primitive neural stem cells (pNSCs) from human embryonic stem cells. We tested whether transplanted human pNSCs can integrate into the CNS of the developing chick neural tube and injured adult rat spinal cord. Following injection of pNSCs into the developing chick CNS, pNSCs integrated into the dorsal aspects of the neural tube, forming cell clusters that spontaneously differentiated into neurons. Furthermore, following transplantation of pNSCs into the lesioned rat spinal cord, grafted pNSCs survived, differentiated into neurons, and extended long distance axons through the scar tissue at the graft-host interface and into the host spinal cord to form terminal-like structures near host spinal neurons. Together, these findings suggest that pNSCs derived from human embryonic stem cells differentiate into neuronal cell types with the potential to extend axons that associate with circuits of the CNS and, more importantly, provide new insights into CNS integration and axonal regeneration, offering hope for repair in SCI.
Introduction
Adult CNS neurons are critical for motor, sensory, and autonomic functions but have a limited potential to regenerate following injury. Numerous studies have identified factors responsible for inhibiting axonal regeneration or capable of promoting growth, isolating such molecules as Nogo, MAG, OMgp, NT3, BDNF, cAMP, and PTEN (1–5). However, translation of these findings into therapeutic applications can be difficult and has not yet yielded the desired outcome of complete recovery after spinal cord trauma. These approaches attempt to modify existing conditions within the injured spinal cord and are limited by the intrinsic properties of their targets. Embryonic stem cells (ESCs)5 and induced pluripotent stem cells represent an alternative strategy to re-establish lost neural connections by introducing cells with the capacity for neuronal differentiation (6). Whether transplanted ESCs can integrate into the damaged host environment and form connections remains largely unknown.
Previous studies involving the transplant of stem cells into an injured CNS have met with poor results. The in vivo environment presents conditions in which it is difficult for transplanted stem cells to survive. Furthermore, cell that do survive transplantation become glial cells rather than neuronal (7, 8). In a recent study with successful transplantation of neurospheres derived from induced pluripotent stem cells, 50% of the grafted cells differentiated in neurons; however, the majority of these neurons were GABAergic in nature (9). Deriving neurospheres from induced pluripotent stem cells also carries an increased risk for tumorigenicity. A means to address survival, multipotency, and tumorigenicity may be through the use of primitive neural stem cells (pNSCs) derived from human ESCs.
During early embryonic development, primitive neuroepithelial cells differentiate from ectodermal layers and give rise to the CNS. Neuroepithelial cells progress to pNSCs and are eventually specified into NSCs (10–12). Although it is thought that pNSCs represent a transient stage through which ESCs progress to becomes NSCs, we recently identified novel chemical combinations to induce and maintain pNSCs from human ESCs (13). A distinguishing characteristic between pNSCs and NSCs is the dependence on a trophic factor: pNSCs require leukemia inhibitory factor, whereas NSCs require basic fibroblast growth factor and epidermal growth factor in rodents (12, 14, 15). Such differences in growth factor dependence and culture purity of less restricted pNSCs may improve transplant survival and neuronal integration into the injured CNS.
We investigated whether human ESC-derived pNSCs could integrate into either the early developing CNS or the injured adult CNS. Because of their experimental accessibility, relevance, and extensive use for CNS regeneration, we used the chick embryo and rodent spinal cord injury models to assess CNS regeneration and integration (16–19). Significantly, we demonstrate that pNSCs integrate into the CNS and extend long distance axons in the injured spinal cord.
EXPERIMENTAL PROCEDURES
Human NSC Culture
Human embryonic stem cells at passages 20–25 were used to derive pNSCs as described previously (13). To monitor cell survival, differentiation, and process outgrowth after transplantation, pNSCs were transduced with a lentivirus expressing GFP under the control of the elongation factor-1α promoter.
Chick Embryonic Neural Tube Transplantation and Immunohistochemical Analysis
To prepare a cell suspension, pNSCs were fully dissociated with Accutase (Invitrogen) into single cells and resuspended in Ca2+- and Mg2+-free PBS containing 10% trypan blue at a final concentration of 5 × 104 cells/μl. Fertilized chicken eggs were incubated at 38 °C in a humidified incubator until stage 13 according to Hamburger-Hamilton criteria (20). A small amount of neutral red solution was overlaid on the embryo to paint the transparent embryo, and the vitelline membrane above was removed. A small longitudinal incision at the dorsal midline of the neural tube was made, and dissociated pNSCs were injected into the neural tube from the posterior direction with a glass micropipette. After cell injection, the eggs were tightly sealed and incubated for 1–5 days before analysis. Following transplantation, ∼50–70% of the embryos survived the first day, and 20–50% survived 5 days later. Only embryos with normal gross morphology were included for further analyses. They were removed from the eggs and immediately observed under a fluorescence microscope to assess the presence of GFP-expressing cells. After the initial inspection, embryos were immediately fixed overnight in 4% paraformaldehyde and cryoprotected in 30% sucrose solution. Embryos were then embedded in frozen optimal cutting temperature medium, and a cryostat was used to process transverse sections with a 15-μm thickness. For immunohistochemistry, sections were blocked with 3% BSA and 0.1% Triton X-100 in PBS. Subsequently, sections were incubated overnight at 4 °C with primary antibodies, including anti-GFP (Invitrogen), anti-N-cadherin (BD Biosciences), anti-promyelocytic zinc finger protein (Calbiochem), and anti-Tuj1 and anti-nestin (Covance). After washes with PBS, appropriate Alexa Fluor-conjugated secondary antibodies (Invitrogen) were applied for 60 min and counterstained with Hoechst 33342. Neural tube sections were mounted and imaged under a confocal microscope.
Rat Spinal Cord Transplantation and Immunohistochemical Analysis
Adult athymic nude rats (180–200 g; n = 4) were used and subjected to the care and experimental procedures as described previously (21). Briefly, the dissociated pNSCs were harvested and resuspended at a concentration of 200,000 cells/μl in fibrin matrices containing growth factors to support their survival. The graft mixture in a volume ∼2 μl was microinjected into the lesion cavity of C4, which was created 2 weeks earlier, in adult nude rats using a PicoSpritzer II microinjector (General Valve Corp., Fairfield, NJ). The subjects were allowed to survive for another 4 weeks before being killed. Animals were perfused with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.2). Spinal cords were dissected, post-fixed overnight at 4 °C, and transferred to 30% sucrose for 72 h. 1.5-cm-long sagittal sections of spinal cords containing the lesion/graft site were sectioned on a cryostat set at 30-μm thickness. Free-floating sections were stained with various antibodies to reveal the distribution, identity, and morphology of transplanted cells. The primary antibodies used in this study were anti-serotonin (5-hydroxytryptamine (5-HT); ImmunoStar); anti-tyrosine hydroxylase, anti-choline acetyltransferase, anti-βIII-tubulin (Tuj1), anti-GAP43, and anti-human synaptophysin (Millipore); anti-PSD-95 (Cell Signaling Technology); and anti-GFP (Invitrogen). After overnight incubation at 4 °C, the sections were washed, followed by staining with Alexa Fluor 488-, Alexa Fluor 555-, or Alexa Fluor 647-conjugated secondary antibodies for 2.5 h at room temperature.
RESULTS
pNSC Graft Integrates into the Neural Tube of Chick Embryo
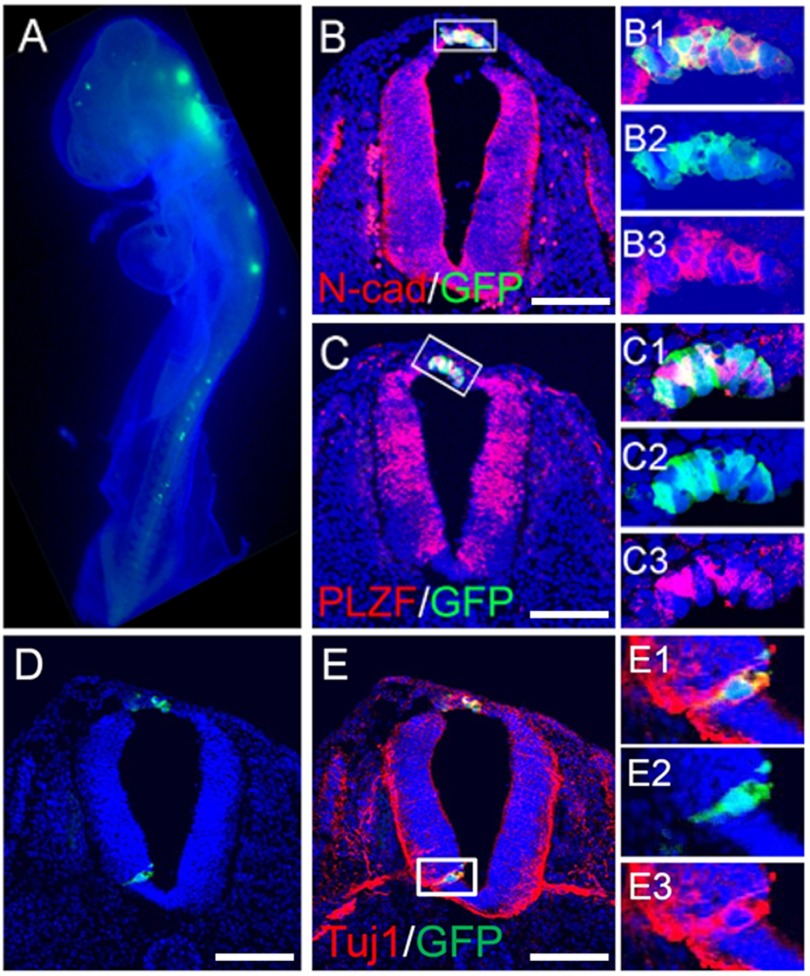
To evaluate the ability of human pNSCs to differentiate and integrate into the developing CNS, we took advantage of the accessibility of the developing nervous system of chick embryo for transplantation. The dissociated GFP-expressing pNSCs were injected through a dorsal midline longitudinal cut into the neural tube stage of chick embryos (E2, Hamburger-Hamilton stage 13). Subsequently, we examined whether these transplanted human pNSCs could survive and integrate into the host developing neural tube at both the E3 (n = 25; Hamburger-Hamilton stage 15/16) and E5 (n = 16; Hamburger-Hamilton stage 25/26) stages. At E3, we found that the grafted GFP-expressing pNSCs were readily observed as clusters along the neural tube (Fig. 1A) and integrated along the dorsal longitudinal incision, suggesting that pNSCs were integrated into the host tissue as a consequence of neural tube disruption and reclosure. Similar to host neural tube cells, the integrated pNSCs also expressed neural epithelium markers such as N-cadherin and promyelocytic zinc finger protein (also known as ZBTB16 (zinc finger- and BTB domain-containing protein 16)), and when ventrally integrated, the grafted cells also expressed the differentiated neuronal marker Tuj1 (Fig. 1, B–E).
FIGURE 1.
Integration of transplanted human pNSCs into chick neural tube. Dissociated pNSCs expressing enhanced GFP were injected into the posterior neural tube of a chick embryo at E2, and their distribution was monitored 24 h after transplantation (A). In a cross-section, pNSCs integrated into the dorsal spinal cord, with expression of N-cadherin (B) and promyelocytic zinc finger protein (C). B1–B3 show merged (B1), GFP (B2), and N-cadherin (B3) signals in the boxed area in B. Likewise, C1–C3 show merged (C1), GFP (C2), and N-cadherin (C3) signals in the boxed area in C. D and E, ventral integration of human pNSCs. GFP-expressing pNSCs were found in the ventral neural tube (D) with co-labeling of Tuj1 and GFP (E). Enlarged images of the boxed area in E are shown in E1–E3. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars = 100 μm.
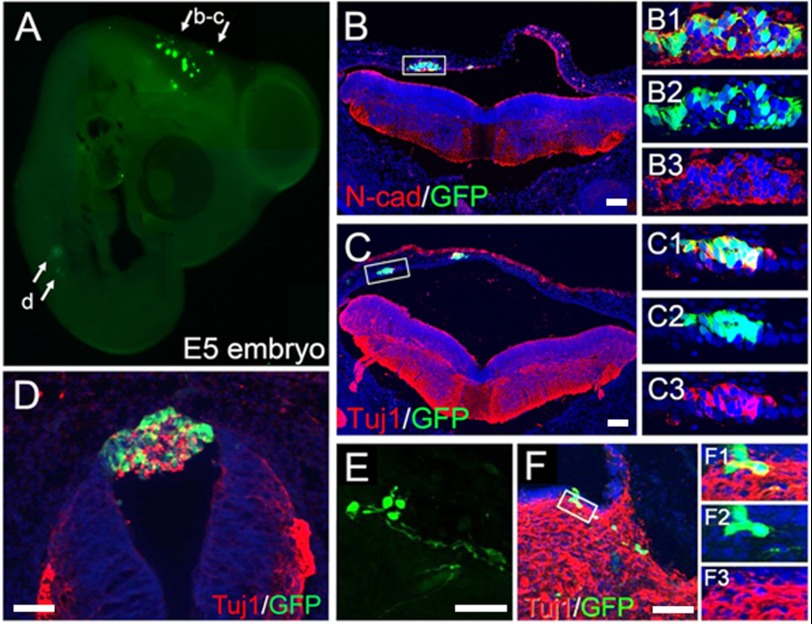
At E5, 3 days post-transplantation, GFP-expressing pNSCs were seen along the dorsal neural tube at the mesencephalic level (Fig. 2A). pNSCs assimilated into the lumen of the myelocoele wall and expressed both N-cadherin and Tuj1 (Fig. 2, B and C). The grafted cells formed Tuj1-expressing cell clusters (Fig. 2D), suggesting possible incomplete integration with the host neural tube. GFP-expressing cells were also found in the ventral aspect of mesencephalic tissues and expressed Tuj1 (Fig. 2, E and F).
FIGURE 2.
A, transplanted human pNSCs at E5. Many pNSCs formed clusters in the dorsal wall of the mesencephalon and expressed N-cadherin (N-cad; B) and Tuj1 (C). Enlarged images of the boxed areas in B and C are shown in B1–B3 and C1–C3, respectively. D, pNSCs in the dorsal spinal cord also formed cell clusters with Tuj1 expression. E and F, transplanted cells integrated into the ventral mesencephalon. Cells exhibited long neurites (E) and expressed Tuj1 (F). Enlarged images of the boxed area in F are shown in F1–F3. Scale bars = 100 μm.
pNSCs Survive and Integrate into an Injured Adult Rat CNS
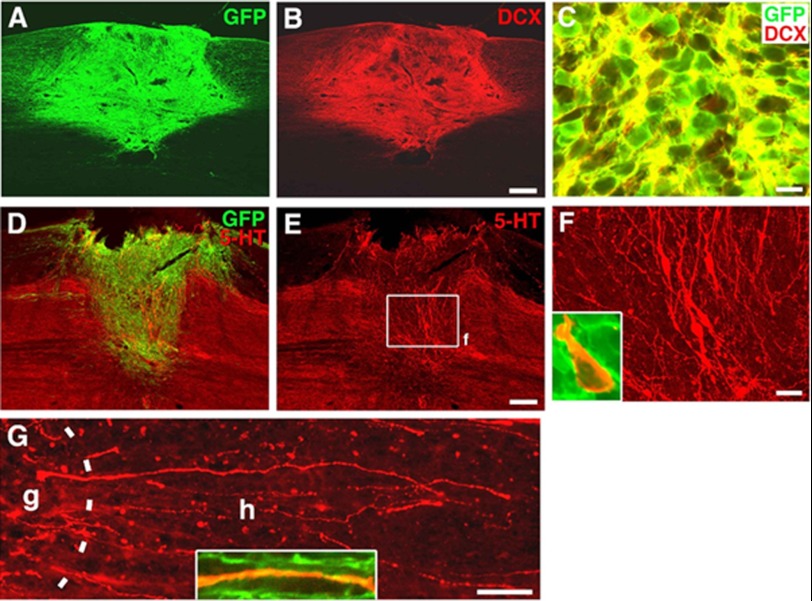
The chick embryonic neural tube represents a permissive environment for the integration of transplanted human pNSCs. The developing neural tissue is known to be rich in developmentally regulated growth factors and to lack inhibitory extracellular matrix molecules and myelin-associated proteins. We then asked whether human pNSCs are able to differentiate and integrate into a post-developed and mature adult neural tissue. To address this question, we used adult rats with lesioned spinal cords as a model to evaluate the survival, integration, and axonal growth and connection of human pNSCs following surgical transplantation into the injury sites of adult rat spinal cords. We chose athymic nude rats (T cell-deficient) to allow for evaluation of human pNSC integration in the absence of T cell-mediated immune reactions, which have a profound inhibitory effect on survival and integration of grafted cells. 4 weeks following transplantation, GFP-expressing cells filled and persisted within the lesion cavity (Fig. 3A), indicating the continued survival of grafted pNSCs in a mature mammalian CNS. To assess the neuronal differentiation potential of pNSCs in vivo, we performed fluorescent immunolabeling for detection of cells expressing both GFP and doublecortin, an early neuronal marker. Robust doublecortin staining signal occupied the majority of pNSC grafts (Fig. 3B) and co-localized with GFP-positive grafted cells (Fig. 3C), indicating that the majority of transplanted pNSCs were differentiating into a neuronal cell fate as opposed to a glial cell fate. To further analyze the neuronal phenotypes, we performed immunohistochemistry for several enzymes and neurotransmitters, including 5-HT (marker for serotonergic neurons), tyrosine hydroxylase (marker for dopaminergic neurons), and choline acetyltransferase (marker for cholinergic neurons). We detected abundant 5-HT-expressing neuronal somas and processes within the graft site (Fig. 3, D–F), which are rarely found in intact and injured rodent spinal cords (22). Additionally, these 5-HT-expressing neurons coexpressed GFP (Fig. 3F), confirming derivation from the transplanted human pNSCs. Furthermore, many 5-HT neurons extended linear processes across the graft-host interface and into the host spinal cord, and some of them were located ectopically within the dorsal column (Fig. 3G). In contrast, tyrosine hydroxylase- or choline acetyltransferase-positive neurons were not detected within the pNSC graft (data not shown), suggesting that pNSCs prefer to differentiate into 5-HT neurons.
FIGURE 3.
Survival and neuronal differentiation of human pNSCs after transplantation to the lesion site of an adult rat spinal cord. Immunohistochemical analysis revealed excellent survival of transplanted pNSCs expressing GFP (A) that co-localized with the early neuronal marker doublecortin (DCX) (B and C). GFP and 5-HT immunolabeling demonstrated 5-HT-positive neurons and their processes within the graft site (D–F). F is a higher magnification of the boxed area in E. The inset shows co-localization of a 5-HT neuron with GFP expression, confirming derivation from graft cells. Many linear 5-HT fibers extended into the host spinal cord from the graft site (G). The dashed line indicates the graft (g)-host (h) interface. The inset shows co-localization of a 5-HT axon with GFP. Scale bars = 230 μm (A and B), 10 μm (C), 310 μm (D and E), 30 μm (F), and 110 μm (G).
Long Distance Axonal Growth and Connectivity of Grafted Human pNSCs
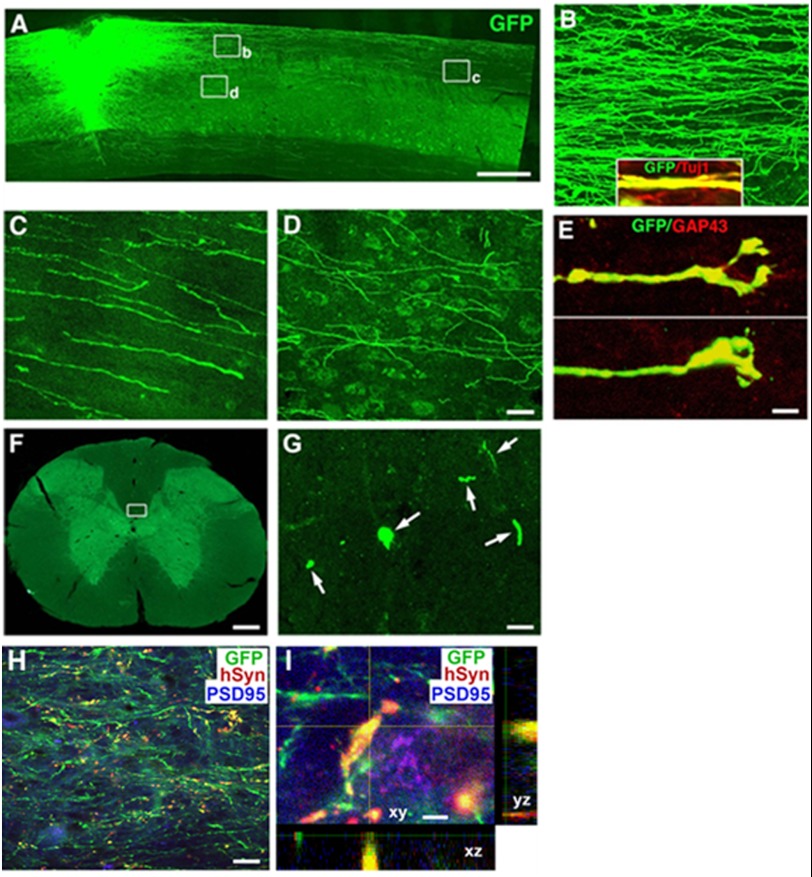
To determine whether grafted human pNSCs can differentiate into mature neurons and extend axons into the host spinal cord over long distances in a similar fashion as seen in cases of NSCs/progenitors derived from rat embryos and human ESCs (21), we characterized GFP-positive axons by immunolabeling. Transplanted pNSCs extended numerous GFP-positive processes beyond the graft-host interface for long distances in both the rostral and caudal directions (Fig. 4A). GFP-labeled processes from the graft were axons, as demonstrated by co-labeling with the axonal marker Tuj1 (Fig. 4B). These axons traveled primarily in host white matter (Fig. 4C). Some axons also innervated host gray matter and formed terminal-like structures (Fig. 4D). GFP-positive axons could be found to extend over 8 mm in both the rostral and caudal directions after a relatively short 4-week post-graft period (Fig. 4, F and G). The number of GFP-positive axons and the travel distance from the graft site are inversely related. Growth cone-like structures were found at many axon tips and often co-localized with the growth cone marker GAP43 (Fig. 4E), indicating active growth of these neurites. Furthermore, immunofluorescent labeling for GFP, human-specific synaptophysin, and PSD-95 showed that graft-derived axons with the human-specific synaptic marker were in direct contact with host neurons expressing the post-synaptic marker PSD-95, indicating sites of putative synapse formation between grafted human pNSCs and host neurons (Fig. 4, H and I). These results indicate that human pNSCs can survive, differentiate, and integrate into the injured CNS of an adult rat.
FIGURE 4.
Long distance axonal growth and integration of transplanted pNSCs. GFP-expressing pNSCs robustly extended axons into the host spinal cord caudal and rostral to the C3 dorsal column lesion site (rostral is shown) (A). Higher magnification views show that axons traveled mainly in host white matter (B and C) and that some axons innervated the gray matter (D). The inset in B shows that GFP-labeled projections arising from the graft expressed Tuj1, confirming an axonal identity. The tips of the growing axons featured growth cone-like structures (E) that co-localized with GAP43. Individual GFP-expressing axons can be detected at the C1 (shown) or C7 (F and G) spinal cord level, three segments rostral or caudal to the spinal cord lesion/graft site. G is a higher magnification of the boxed area in F showing individual axons in the dorsal column (arrows). GFP, human-specific synaptophysin (hSyn), and PSD-95 labeling revealed that numerous pNSC-derived axon terminals (green) in the host gray matter expressed synaptophysin (1 mm caudal to the lesion site) surrounding PSD-95-expressing host neurons (H). A high magnification z-stack image (I) triple-labeled for GFP, human-specific synaptophysin, and PSD-95 demonstrates co-localization of graft-derived axons with the human-specific synaptic marker in direct contact with host neurons expressing the post-synaptic marker PSD-95, indicating sites of putative synaptic connections. Scale bars = 650 μm (A), 32 μm (B–D), 5 μm (E), 300 μm (F), 8 μm (G), 30 μm (H), and 3 μm (I).
In addition, the progressive axonal extension from the lesion/graft site was serially examined over a time period of 4 weeks post-transplantation. Axons emerged from the lesion/graft site by the second day after transplantation and extended progressively. Documentation of axonal growth over time suggests the continuous extension of new axons and indicates that it is unlikely due to the cell fusion between the transplanted cells and host neurons.
DISCUSSION
In this study, we investigated whether human pNSCs derived from human ESCs possess the potential to integrate into the CNS and extend long distance axons. Remarkably, we found that transplanted pNSCs can do so in both the developing and injured adult CNS. In particular, their neural tissue integration and differentiation into serotonergic neurons have a broad implication for psychiatric and somatic therapies. Serotonergic fibers within the spinal cord regulate pain perception, locomotion, and autonomic functions (23–25). The most devastating consequence of spinal cord injury is the loss of function distal to the lesion. In these cases, even modest recovery of function such as bladder regulation may drastically improve quality of life (26) and decrease the incidence of potentially life-threatening complications such as autonomic dysreflexia and lower urinary tract infections (27, 28).
Previous studies have identified host factors that inhibit neuronal survival and axonal regeneration (1–5). It was believed that efficacy of integration is dependent on cellular interactions between the host and transplanted pNSCs (19, 29). It appears that the lesioned spinal cord produces undefined changes in the microenvironment that influence the degree of pNSC integration into neural tissue (16, 17, 19). We have shown that pNSCs possess intrinsic properties of regeneration and axonal extension that can overcome host inhibitory effects. Our results suggest that stem cell-based regeneration of neurons and their axonal outgrowth in the injured spinal cord are possible. This has implications for the treatment of neurodegenerative diseases and spinal cord injuries, when a majority of neurons undergo cell death, resulting in consequent irreversible loss of motor and sensory function. Our data suggest potential therapeutic options for replacing damaged CNS neurons and generating de novo neurons and connections in the spinal circuitry in the face of otherwise irreversible damage with grafts of human NSCs.
This work was supported, in whole or in part, by grants from the 973 Program (2013CB967504), NSFC (grant 81130017), National Institutes of Health Director's Transformative RO1 Program (Grant RO1 EY021374), National Eye Institute (Grant RO1 EY018660), a grant from the King Abdulazz City for Science and Technology-University of California San Diego Center of Excellence in Nanomedicine, the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, Korea Ministry of Education and Science (Grants 20110019409 and 20110019212), and the Veterans Administration.
- ESC
- embryonic stem cell
- pNSC
- primitive neural stem cell
- 5-HT
- 5-hydroxytryptamine
- E
- embryonic day.
REFERENCES
- 1. Park K. K., Liu K., Hu Y., Smith P. D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., He Z. (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Filbin M. T. (2003) Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713 [DOI] [PubMed] [Google Scholar]
- 3. Senut M. C., Tuszynski M. H., Raymon H. K., Suhr S. T., Liou N. H., Jones K. R., Reichardt L. F., Gage F. H. (1995) Regional differences in responsiveness of adult CNS axons to grafts of cells expressing human neurotrophin 3. Exp. Neurol. 135, 36–55 [DOI] [PubMed] [Google Scholar]
- 4. Hollis E. R., 2nd, Jamshidi P., Löw K., Blesch A., Tuszynski M. H. (2009) Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl. Acad. Sci. U.S.A. 106, 7215–7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hannila S. S., Filbin M. T. (2008) The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 209, 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thuret S., Moon L. D., Gage F. H. (2006) Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643 [DOI] [PubMed] [Google Scholar]
- 7. Hofstetter C. P., Holmström N. A., Lilja J. A., Schweinhardt P., Hao J., Spenger C., Wiesenfeld-Hallin Z., Kurpad S. N., Frisén J., Olson L. (2005) Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 8, 346–353 [DOI] [PubMed] [Google Scholar]
- 8. Cao Q. L., Zhang Y. P., Howard R. M., Walters W. M., Tsoulfas P., Whittemore S. R. (2001) Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 167, 48–58 [DOI] [PubMed] [Google Scholar]
- 9. Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., Toyama Y., Yamanaka S., Nakamura M., Okano H. (2011) Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. U.S.A. 108, 16825–16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elkabetz Y., Panagiotakos G., Al Shamy G., Socci N. D., Tabar V., Studer L. (2008) Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smukler S. R., Runciman S. B., Xu S., van der Kooy D. (2006) Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J. Cell Biol. 172, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hitoshi S., Seaberg R. M., Koscik C., Alexson T., Kusunoki S., Kanazawa I., Tsuji S., van der Kooy D. (2004) Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling. Genes Dev. 18, 1806–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li W., Sun W., Zhang Y., Wei W., Ambasudhan R., Xia P., Talantova M., Lin T., Kim J., Wang X., Kim W. R., Lipton S. A., Zhang K., Ding S. (2011) Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. U.S.A. 108, 8299–8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tropepe V., Hitoshi S., Sirard C., Mak T. W., Rossant J., van der Kooy D. (2001) Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30, 65–78 [DOI] [PubMed] [Google Scholar]
- 15. Tropepe V., Sibilia M., Ciruna B. G., Rossant J., Wagner E. F., van der Kooy D. (1999) Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol. 208, 166–188 [DOI] [PubMed] [Google Scholar]
- 16. Boulland J. L., Halasi G., Kasumacic N., Glover J. C. (2010) Xenotransplantation of human stem cells into the chicken embryo. J. Vis. Exp. 41, e2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pochampally R. R., Neville B. T., Schwarz E. J., Li M. M., Prockop D. J. (2004) Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion. Proc. Natl. Acad. Sci. U.S.A. 101, 9282–9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clarke D. L., Johansson C. B., Wilbertz J., Veress B., Nilsson E., Karlström H., Lendahl U., Frisén J. (2000) Generalized potential of adult neural stem cells. Science 288, 1660–1663 [DOI] [PubMed] [Google Scholar]
- 19. Sigurjonsson O. E., Perreault M. C., Egeland T., Glover J. C. (2005) Adult human hematopoietic stem cells produce neurons efficiently in the regenerating chicken embryo spinal cord. Proc. Natl. Acad. Sci. U.S.A. 102, 5227–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamburger V. (1992) The stage series of the chick embryo. Dev. Dyn 195, 273–275 [DOI] [PubMed] [Google Scholar]
- 21. Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E. S., Havton L. A., Zheng B., Conner J. M., Marsala M., Tuszynski M. H. (2012) Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubasak M. D., Jindrich D. L., Zhong H., Takeoka A., McFarland K. C., Muñoz-Quiles C., Roy R. R., Edgerton V. R., Ramón-Cueto A., Phelps P. E. (2008) OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain 131, 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray K. C., Nakae A., Stephens M. J., Rank M., D'Amico J., Harvey P. J., Li X., Harris R. L., Ballou E. W., Anelli R., Heckman C. J., Mashimo T., Vavrek R., Sanelli L., Gorassini M. A., Bennett D. J., Fouad K. (2010) Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nature medicine 16, 694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mason P. (1999) Central mechanisms of pain modulation. Curr. Opin. Neurobiol. 9, 436–441 [DOI] [PubMed] [Google Scholar]
- 25. Audero E., Coppi E., Mlinar B., Rossetti T., Caprioli A., Banchaabouchi M. A., Corradetti R., Gross C. (2008) Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science 321, 130–133 [DOI] [PubMed] [Google Scholar]
- 26. Anderson K. D. (2004) Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 27. Schottler J., Vogel L., Chafetz R., Mulcahey M. J. (2009) Patient and caregiver knowledge of autonomic dysreflexia among youth with spinal cord injury. Spinal Cord 47, 681–686 [DOI] [PubMed] [Google Scholar]
- 28. Rabchevsky A. G. (2006) Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog. Brain Res. 152, 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wichterle H., Lieberam I., Porter J. A., Jessell T. M. (2002) Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397 [DOI] [PubMed] [Google Scholar]