Background: The ghrelin receptor (GHS-R1a) has multiple biological functionalities, including the regulation of appetite and hedonic food intake.
Results: A novel GHS-R1a/5-HT2C heterodimer was identified, in addition to GHS-R1a/D1 and GHS-R1a/MC3.
Conclusion: Promiscuous GHS-R1a receptor heterodimerization attenuates downstream signaling and affects trafficking depending on dimer partner.
Significance: The existence of multiple GHS-R1a heterodimers has important consequences for the future development of therapeutics with enhanced specificity.
Keywords: Dopamine Receptors, G Protein-coupled Receptors (GPCR), Growth Hormone, Obesity, Serotonin, Ghrelin Receptor, Heterodimerization, Serotonin 2C Receptor
Abstract
G protein-coupled receptors (GPCRs), such as the ghrelin receptor (GHS-R1a), the melanocortin 3 receptor (MC3), and the serotonin 2C receptor (5-HT2C), are well known for their key role in the homeostatic control of food intake and energy balance. Ghrelin is the only known gut peptide exerting an orexigenic effect and has thus received much attention as an anti-obesity drug target. In addition, recent data have revealed a critical role for ghrelin in dopaminergic mesolimbic circuits involved in food reward signaling. This study investigates the downstream signaling consequences and ligand-mediated co-internalization following heterodimerization of the GHS-R1a receptor with the dopamine 1 receptor, as well as that of the GHS-R1a-MC3 heterodimer. In addition, a novel heterodimer between the GHS-R1a receptor and the 5-HT2C receptor was identified. Interestingly, dimerization of the GHS-R1a receptor with the unedited 5-HT2C-INI receptor, but not with the partially edited 5-HT2C-VSV isoform, significantly reduced GHS-R1a agonist-mediated calcium influx, which was completely restored following pharmacological blockade of the 5-HT2C receptor. These results combined suggest a potential novel mechanism for fine-tuning GHS-R1a receptor-mediated activity via promiscuous dimerization of the GHS-R1a receptor with other G protein-coupled receptors involved in appetite regulation and food reward. These findings may uncover novel mechanisms of significant relevance for the future pharmacological targeting of the GHS-R1a receptor in the homeostatic regulation of energy balance and in hedonic appetite signaling, both of which play a significant role in the development of obesity.
Introduction
The growth hormone secretagogue (GHS-R1a) receptor was initially described as an orphan receptor, activated by synthetic peptidyl growth hormone secretagogues (GHS),3 such as herexalin, growth hormone-releasing peptides (GHRP1, GHRP2, and GHRP6) and the nonpeptidyl ligand, MK0677, which were all shown to stimulate the release of growth hormone from the pituitary (1–3). Shortly thereafter, the gastric-derived peptide ghrelin was identified as the endogenous ligand for the GHS-R1a receptor by a reverse pharmacological approach (4), subsequently designating the GHS-R1a receptor as the ghrelin receptor (5). The GHS-R1a receptor is expressed in both the periphery and central nervous system, and when activated by ghrelin, it mediates a multitude of biological activities, including the secretion of growth hormone, as well as the stimulation of appetite and food intake, maintaining the body's energy homeostasis (for review see Ref. 6). Because of its orexigenic effect, the ghrelinergic system has received attention as a promising anti-obesity therapeutic target (6–14). In addition, recent studies identified a pivotal role for the ghrelinergic system in additional food intake behaviors, including reward signaling following ingestion of palatable food, as well as the motivational drive to eat (for review see Refs. 15–20). Expression of the GHS-R1a receptor in the extrahypothalamic neurocircuitry, regulating this nonhomeostatic feeding, including the ventral tegmental area, nucleus accumbens, hippocampus, and amygdala, is in line with the role of ghrelin in the hedonic aspects of food intake (21, 22).
The GHS-R1a receptor is a G protein-coupled receptor (GPCR) belonging to class I of GPCRs (23, 24). GPCRs, such as the GHS-R1a, have been found to cross-talk with other GPCRs and have been found to exist and function as dimers or even higher structure oligomeric complexes (25–28). Heterodimerization of the GHS-R1a receptor with other GPCRs involved in the homeostatic or hedonic regulation of food intake may be able to explain cross-talk between neuropeptide systems and could potentially serve to modulate specific GHS-R1a-mediated signaling pathways and functionalities. Recent studies support these notions by demonstrating the existence of heterodimers of the GHS-R1a receptor with the melanocortin 3 receptor (MC3) (29, 30), which is an important downstream signaling receptor in the homeostatic control of food intake and energy balance (31–34). In addition, the involvement of a dimer between the GHS-R1a and the dopamine D2 receptor in the regulation of appetite has recently been shown (35). Moreover, accumulating evidence supports dimerization of the dopamine (D1) receptor with the GHS-R1a receptor, leading to enhanced dopamine signaling (36). The rewarding and pleasurable aspects of palatable food are primarily mediated via neuronal dopamine release in the mesolimbic circuitry system (37). Interestingly, ghrelin has also been shown to enhance food reward (15, 20, 38), and this effect may well be mediated via dimerization with dopamine receptors.
The serotonin 2C (5-HT2C) receptor is another centrally expressed GPCR involved in satiety signaling (11–14, 39–42). Interestingly, interactions between the serotonin and ghrelin signaling pathways have been previously described. For example, pharmacological increases of brain serotonin levels and 5-HT2C receptor agonism were shown to inhibit the increase in plasma active ghrelin in response to an overnight fast in mice, suggesting the existence of a negative feedback mechanism (43). In addition, ghrelin has been shown to inhibit serotonin release in rat hypothalamic synaptosomes (44). Moreover, the 5-HT2C receptor has been identified in the regulation of reward-related behaviors (45, 46), demonstrating overlapping functionalities with the GHS-R1a receptor in the homeostatic and hedonic regulation of food intake. In addition, the neuronal 5-HT2C receptor (47, 48) has an overlapping expression profile to the neuronal circuits expressing the GHS-R1a receptor (21, 22, 49). A recent study demonstrated that an increase in serotonin (5-hydroxytryptamine, 5-HT) via direct administration effectively blocked the orexigenic actions of ghrelin in rats (50). In the same study, a similar attenuation of ghrelin-induced food intake was observed following administration of the 5-HT2 receptor agonist 5-dimethoxy-4-iodoamphetamine. This serotonin-mediated attenuation of ghrelin signaling may potentially involve dimerization of the GHS-R1a and the 5-HT2C receptor, which is a previously unexplored possibility under investigation here. Notably, serotonin receptors have been found to exist as oligomer complexes with other serotonin receptor family members (51), with metabotrophic glutamate receptors (52–54), and with dopamine receptors (55). However, oligomeric complex formation does not always translate into second messenger signaling effects, because downstream consequences were unaffected in a 5-HT2A/mGlu2 heterocomplex (54).
Further elucidating the promiscuous heterodimerization of the GHS-R1a receptor with candidate GPCRs might lead to a better understanding of the fine-tuning of GHS-R1a receptor activity, as well as cross-talk within neuronal circuits, which may ultimately lead to new therapeutic intervention strategies to reduce food intake. Therefore, the current study aims to investigate the potential of the GHS-R1a receptor to dimerize with the 5-HT2C receptor through analysis of co-localized expression in vitro in heterologous cells. Receptor co-localization is the primary requirement for the existence of potential receptor dimers, because without co-localized expression there would be no possibility for dimerization in normal physiology. In addition, GHS-R1a-mediated downstream calcium signaling and ligand-mediated receptor trafficking following co-expression of the GHS-R1a receptor with the MC3, the D1, and the 5-HT2C receptor will be analyzed.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Lentiviral Transduction
Human embryonic kidney cells (Hek293A) were maintained in high glucose DMEM (Invitrogen) supplemented with 10% heat-inactivated FBS and 1% nonessential amino acids in an atmosphere of 95% air and 5% CO2 at 37 °C. The cells were maintained to a confluence of >85%, after which the cells were passaged to a lower density. Hek293A cells were transfected with a plasmid construct expressing the human GHS-R1a receptor (Genecopeia, X0963; accession code U60179.1), the unedited 5-HT2C-INI receptor (Genecopeia, H3309; accession code NM_000868), or the partly edited 5-HT2C-VSV receptor isoform (Genecopeia, T0336; accession code AF208053.1), each including a C-terminal EGFP tag, using Lipofectamine LTX Plus reagent (Invitrogen), according to the manufacturer's instructions. The unedited 5-HT2C-INI receptor contains the amino acids isoleucine, asparagine, isoleucine on positions 156, 158 and 160, respectively. The partly edited 5-HT2C-VSV isoform, one of the principal expressed isoforms in the human brain, contains valine, serine and valine on these amino acid positions (70). Constructs contained the neomycin resistance marker, and cells stably expressing the GPCR-EGFP fusion proteins were selected using Geneticin (G418; Merck) as a selection antibiotic. The cells with the highest CMV promoter-mediated expression of the insert gene tagged with the C-terminal EGFP were further selected using flow-assisted cell sorting, after which they were maintained in complete DMEM, supplemented with 300 ng/μl G418 as a maintenance antibiotic. In addition, HEK293A cells stably expressing the GHS-R1a-EGFP, the 5-HT2C-EGFP, or the 5-HT2C-VSV-EGFP were transduced using in-house-generated lentiviral vectors to co-express the GHS-R1a, 5-HT2C, 5-HT2C-VSV, D1, or MC3 constructs with a red fluorescent protein (RFP) tag. Transduction was performed using a third generation packaging, gene delivery, and viral vector production system developed by Naldini and co-workers (56–60). All of the GPCR gene constructs were cloned into a HIV-based, replication deficient, lentiviral expression plasmid pHR-SIN-BX-RFP. This vector was generated from a pHR-SIN-BX-IRES-EmGFP vector (kind gift of Adrian Thrasher, Institute of Child Health, London, UK), which was modified in our lab to exclude the shRNA U6 promoter and contain a RFP-amplified from the pTagRFP-N vector (FP142; Evrogen) instead of an IRES-EmGFP. HIV-based lentivector particles, pseudotyped with the vesicular stomatitis virus coat protein G expressing the GPCR constructs from a spleen focus-forming virus promoter in conjunction with the RFP sequence were produced using 293T-17 cells, following transient co-transfection of the cloned expression construct, pHR-GPCR-RFP; the packaging construct, pCMVΔR8.91; and the envelope construct, pMD.G-VSV-G. The cells were transduced with the GPCR-RFP expressing lentiviral vectors diluted in transduction media, consisting of DMEM with 2% heat-inactivated FBS, 1% nonessential amino acids and an additional 8 μg/ml Polybrene® (Sigma; H9268). Fluorescence was monitored using flow cytometry as indicator of receptor expression.
Receptor Ligands
Endogenous agonists ghrelin (ghrl, 1465; Tocris), MK0677 (SP960334C; NeoMPS), 5-HT (H9523; Sigma), 6-amino-5,6,7,8-tetrahydronaphthalene-2,3-diol (6,7-ADTN) hydrobromide (Asc-150; Ascent Scientific), [Nle4,d-Phe7]-α-MSH (3013; Tocris), and the peptide [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (SP analog, 1946; Tocris) were prepared in assay buffer (1× Hanks' balanced salt solution supplemented with 20 mm HEPES). The 5-HT2C specific antagonist RS10222 (1050, Tocris) was prepared in Me2SO as 10 mm stock solutions before diluting into assay buffer.
Co-localization and Internalization of GHS-R1a, D1, MC3, and 5-HT2C Receptors
Transgenic Hek293A cells stably overexpressing the GHS-R1α-EGFP receptor were transduced with lentiviral vectors lvDR1-tagRFP, lvMC3-tagRFP, lv5-HT2C-INI-tagRFP, or lv5-HT2C-VSV-tagRFP to assess co-localized expression. In addition, transgenic Hek293A cells stably overexpressing the 5-HT2C-INI-EGFP or the edited isoform 5-HT2C-VSV-EGFP were transduced with lvGHS-R1a-tagRFP. The cells were transduced with viral vectors as described and seeded at 50000 cells in a total volume of 70 μl to 100 μl on poly-l-lysine-coated (P4707; Sigma) borosilicate glass slides (631-0150; VWR International) in 24-well plates. After 3 h of incubation at 37 °C, 5.0% CO2, wells were flooded with DMEM, containing 10% FBS, 1% nonessential amino acids and penicillin/streptomycin, and incubated further. Co-localization was assessed using laser scanning confocal fluorescent microscopy (FV 1000 Confocal System; Olympus). To determine whether GPCRs also co-internalize, receptors were ligand-activated, and cell fluorescent translocation was monitored on an inverted microscope (CKX41; Olympus) setup with a sensitive XM10 camera (C-BUN-F-XM10-BUNDLE) with an infrared cut filter, mercury burner (USH-103OL), and fluorescence condenser (CKX-RFA; Olympus) converting the microscope to a 1.4 megapixel cooled monochrome CCD digital microscope. Both the green and red fluorescence of the cells were captured and merged for analysis using Cell̂F software. Ligand-mediated co-internalization of the receptor pairs was quantified using the Image J software (1.45 s). In each merged image, five individual cells co-expressing the GHS-R1a and the partner GPCR were selected, and fluorescence intensity of plasma membrane versus perinuclear receptor expression was determined. The single highest intracellular pixel was compared with membrane pixel intensity along a straight line axis in each selected cell. The average pixel intensity ratio of each treatment was expressed as the mean ± S.E.
Calcium Mobilization Assay
Receptor-mediated changes in intracellular calcium (Ca2+) were monitored on a Flex station II multiplate fluorometer (Molecular Devices, Sunnyvale, CA). Stably transfected cells were seeded in black 96-well microtiter plates at a density of 2.5 × 105 cells/ml (2.5 × 104 cells/well) and maintained for ∼24 h at 37 °C in a humidified atmosphere containing 5% CO2. After removal of the growth medium, the cells were incubated with 25 μl of assay buffer (1× Hanks' balanced salt solution supplemented with 20 mm HEPES buffer) and 25 μl of Ca4 dye (R8141; Molecular Devices) according to the manufacturer's protocol. The addition of ligand prepared in assay buffer (25 μl/well) was performed by the Flexstation II, and fluorescent readings were taken for 160 s in flex mode with excitation wavelength of 485 nm and emission wavelength of 525 nm. The relative increase in cytosolic calcium [Ca2+] was calculated as the difference between the maximum and base-line fluorescence (Vmax − Vmin; the treatment-associated emission subtracted with the unstimulated base-line emission), corrected for background fluorescence, and depicted as percentage relative fluorescent units (RFU) compared with the maximum response (100%). Each agonist dose-response curve was constructed using GraphPad Prism software (PRISM 4.0) using nonlinear regression analysis with variable slope. The values resulting from obvious incorrect pipetting by the Flexstation were excluded from the analysis.
Statistical Analysis
Statistical differences were analyzed using one-way or two-way analysis of variance (ANOVA) where appropriate. The statistically significant differences were subsequently depicted as follows: *, indicating p < 0.05; **, indicating p < 0.01; and ***, indicating p < 0.001, or as indicated in figure legends.
RESULTS
Co-localization of the GHS-R1a Receptor with Candidate GPCRs
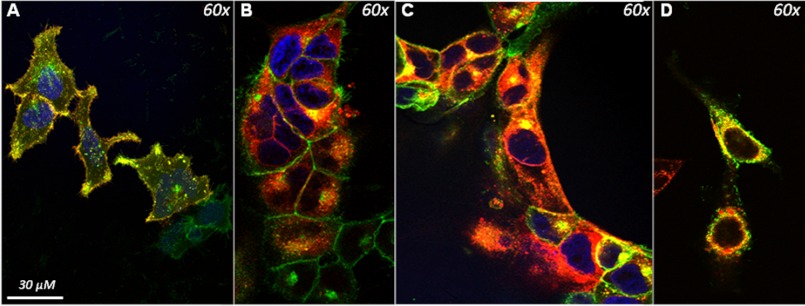
Human embryonic kidney (HEK293A) cells, generated to stably express a functional human GHS-R1a receptor as an EGFP fusion protein, were transduced with lentiviral vectors to co-express several other GPCRs involved in appetite and satiety regulation, expressed as RFP fusion proteins. Consequently, all cells expressed the GHS-R1a receptor, whereas most, but not all, cells expressed the candidate GPCR. Successful lentiviral transduction and RFP-tagged receptor expression was confirmed using flow cytometry (data not shown). Using fluorescent laser scanning confocal microscopy, co-localization could be demonstrated between the GHS-R1a receptor and the D1 receptor (Fig. 1A), between the GHS-R1a and the MC3 receptor (Fig. 1B), and between the GHS-R1a and two distinct isoforms of the 5-HT2C receptor, the unedited variant 5-HT2C-INI (Fig. 1C) and a partially edited isoform, 5-HT2C-VSV (Fig. 1D). Co-localization of the GHS-R1a with the MC3 receptor was shown to be mostly intracellular, whereas GHS-R1a and D1 co-localization was ubiquitous. Co-localization of other receptor pairs following in vitro overexpression (e.g., the 5-HT2C receptor with the MC3 receptor) was also investigated but failed to show overlapping expression (data not shown).
FIGURE 1.
Co-localization of the GHS-R1a receptor with candidate GPCRs in Hek293A cells. Hek293a cells stably expressing the GHS-R1a receptor as an EGFP fusion protein were transduced with lentiviral vectors expressing candidate GPCRs as RFP fusion proteins. Co-localization of fluorescence was analyzed using confocal microscope and is indicated by yellow color overlap. A, co-localization (yellow) of the D1 receptor (red) with the GHS-R1a receptor (green) was observed. B, co-localization of the MC3 receptor (red) with the GHS-R1a (green) receptor was only observed intracellularly. C, co-localization of the 5-HT2C receptor (red) with the GHS-R1a receptor (green) was demonstrated both intracellularly and on the membrane. D, the edited 5-HT2C isoform, 5-HT2C-VSV receptor (red), also demonstrated co-localization with the GHS-R1a receptor mainly in the intracellular space.
Co-internalization of GHS-R1a Heterodimers
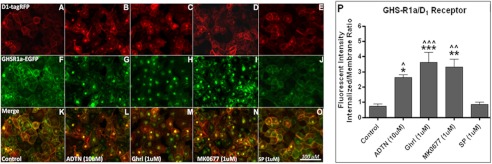
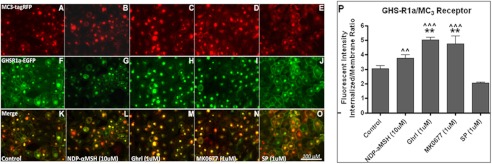
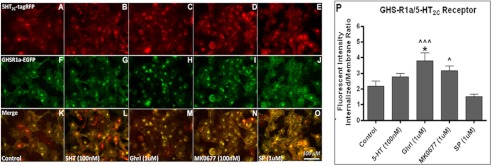
The GHS-R1a receptor was demonstrated to co-localize with the D1 receptor (Fig. 2), the MC3 receptor (Fig. 3), and the 5-HT2C receptor (Fig. 4). In addition, receptor co-internalization was shown following agonist treatment for all receptor pairs. Agonist-mediated receptor trafficking was subsequently quantified for all receptor pairs, and statistically significant co-internalization was in line with the visual observations (Figs. 2P, 3P, and 4P).
FIGURE 2.
Co-internalization of the GHS-R1 receptor with the D1 receptor. Hek293A cells stably expressing GHS-R1a-EGFP were transduced with viral vectors expressing lvDR1-RFP. A–O, expression of the D1 receptor (red), the GHS-R1a receptor (green), and co-localization (yellow) could be observed 48 h after transduction (A, F, and K). The cells were incubated for 60 min with 10 μm of the specific D1 receptor agonist, 6,7-ADTN hydrobromide (B, G, and L), with 1 μm ghrelin (C, H, and M), with 1 μm MK0677 (D, I, and N), or with 1 μm of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (E, J, and O). The images were made using a CKX41 inverted microscope (Olympus) with a XM10 camera and Cell̂Fv3.3 software. P, quantified agonist-mediated co-internalization is depicted. Statistical significance was analyzed using ANOVA followed by Bonferroni multiple comparison test; the statistical significance of agonist-mediated co-internalization compared with control is notated as follows: ***, p < 0.001; **, p < 0.01; and *, p < 0.05. Statistically significant difference compared with [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P is notated as follows: ^^^, p < 0.001; ^^, p < 0.01; and ^, p < 0.05.
FIGURE 3.
Co-internalization of the GHS-R1 receptor with the MC3 receptor. Hek293A cells stably expressing GHS-R1a-EGFP were transduced with viral vectors expressing lvMC3-RFP. A–O, expression of the MC3 receptor (red), the GHS-R1a receptor (green), and co-localization (yellow) could be observed 48 h after transduction (A, F, and K). The cells were incubated for 60 min with 10 μm of the specific MC3-agonist, [Nle4,d-Phe7]-α-MSH (B, G, and L), with 1 μm ghrelin (C, H, and M), with 1 μm MK0677 (D, I, and N), or with 1 μm of [-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (SP; E, J, and O). Images were made using a CKX41 inverted microscope (Olympus) with a XM10 camera and Cell̂Fv3.3 software. P, quantified agonist-mediated co-internalization is depicted. Statistical significance was analyzed using ANOVA followed by Bonferroni multiple comparison test; statistical significance of agonist-mediated co-internalization compared with control is notated as follows: ***, p < 0.001; **, p < 0.01; and *, p < 0.05. Statistically significant difference compared with [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P is notated as follows: ^^^, p < 0.001; ^^, p < 0.01; and ^, p < 0.05.
FIGURE 4.
Co-internalization of the GHS-R1 receptor with the 5-HT2C receptor. Hek293A cells stably expressing GHS-R1a-EGFP were transduced with viral vectors expressing lv5-HT2C-RFP. A–O, expression of the 5-HT2C receptor (red), the GHS-R1a receptor (green), and co-localization (yellow) could be observed 48 h after transduction (A, F, and K). The cells were incubated for 60 min with 100 nm of serotonin (5-HT) (B, G, and L), with 1 μm ghrelin (C, H, and M), with 100 nm MK0677 (D, I, and N), or with 1 μm of [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (SP; E, J, and O). The images were made using a CKX41 inverted microscope (Olympus) with a XM10 camera and Cell̂Fv3.3 software. P, quantified agonist-mediated co-internalization is depicted. Statistical significance was analyzed using ANOVA followed by Bonferroni multiple comparison test; statistical significance of agonist-mediated co-internalization compared with control is notated as follows: ***, p < 0.001; **, p < 0.01; and *, p < 0.05. Statistically significant difference compared with [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P is notated as follows: ^^, p < 0.001; ^^, p < 0.01; and ^, p < 0.05.
Clear co-internalization of the GHS-R1a/D1 receptor pair was observed following treatment with the D1 agonist 6,7-ADTN hydrobromide (Fig. 2G), which was shown to be significant (p < 0.05) compared with control and the GHS-R1a receptor-specific inverse agonist, [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (SP-analog) (Fig. 2P). Exposure to the SP-analog alone had no effect on receptor distribution compared with the control (Fig. 2P). Moreover, the endogenous GHS-R1a agonist ghrelin and the synthetic agonist MK0677 demonstrated not only the internalization of the GHS-R1a receptor (Fig. 2, H and I) but also the D1 receptor was co-internalized into the endosomes (Fig. 2, C and D). Overlay images confirmed co-localized expression of both receptors (Fig. 2, K–O). Co-internalization of the GHS-R1a/D1 receptor pair was significant in response to both ghrelin (p < 0.001) and MK0677 (p < 0.01), respectively (Fig. 2P). This reinforces the concept of heterodimerization of the GHS-R1a receptor with the D1 receptor, because the individual receptors are normally not responsive toward agonists activating the partnering GPCR.
Next, co-internalization was observed for the GHS-R1a/MC3 pair (Fig. 3), which was shown to be significant compared with control following treatment with both ghrelin (p < 0.01) and MK0677 (p < 0.01) but not upon treatment with the MC3 agonist, [Nle4,d-Phe7]-α-MSH (Fig. 3P). Noteworthy, however, was the high basal cytosolic localization of the GHS-R1a/MC3 receptor pair (Fig. 3, A, F, and K). This demonstrates an altered cellular localization upon co-expression, considering the GHS-R1a receptor is expressed on the cell membrane when expressed on its own (data not shown) and under basal conditions upon co-expression with other GPCRs (Figs. 2F and 4F). Co-localization of both receptors in the cytosol was observed after merging of the obtained images (Fig. 3, K–O). The strong cytosolic presence of both receptors suggests heterodimerization of the GHS-R1a receptor with the MC3 receptor. Treatment with the SP-analog (Fig. 3, E, J, and O), although not significant, slightly reduced the high intracellular expression, leading to a significant different co-localization of the GHS-R1a/MC3 pair following exposure to [Nle4,d-Phe7]-α-MSH (p < 0.01), when compared with SP-analog (Fig. 3P).
Finally, co-localization and co-internalization of the GHS-R1a/5-HT2C receptor pair was investigated (Fig. 4). Treatment with 5-HT (Fig. 4, B, G, and L), with ghrelin (Fig. 4, C, H, and M) or with MK0677 (Fig. 4, D, I, and N) caused internalization of the GHS-R1a/5-HT2C receptor pair, whereas exposure to the GHS-R1a inverse agonist [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (SP-analog) caused an increase of membrane-bound GHS-R1a and 5-HT2C receptor (Fig. 4, E, J, and O). However, statistical analysis revealed that only co-internalization following ghrelin exposure was significant (p < 0.05) (Fig. 4P). Treatment with MK0677 and 5-HT, although showing an elevated internalized intensity, did not reach statistical significance (Fig. 4P). However, these cells also had elevated internalized expression under control conditions (compare Fig. 2P with Fig. 4P), explaining the absence of significance. When comparing the MK0677-mediated receptor co-internalization to cells exposed to SP-analog, statistical significance was reached (p < 0.05), and significance for ghrelin-mediated co-internalization was potentiated (p < 0.001). These data support the existence of a novel heterodimer between the GHS-R1a and the 5-HT2C receptor.
Functional Interaction of the GHS-R1a Receptor with the D1 and MC3 Receptor
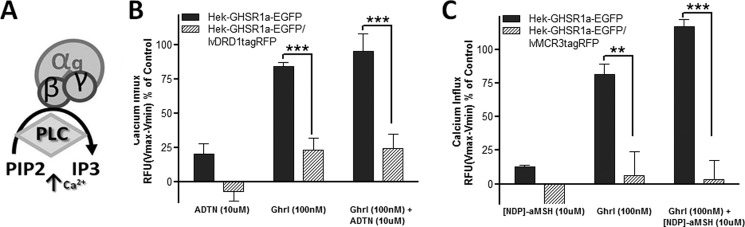
Both the MC3 and the D1 receptors are coupled to the G protein Gαs, and receptor ligand binding subsequently activates adenylyl cyclase, leading to increasing intracellular concentrations of the second messenger cAMP. Cell lines transduced with either the D1 or MC3 constructs demonstrated increased intracellular cAMP upon exposure to the agonists 6,7-ADTN hydrobromide or [Nle4,d-Phe7]-α-MSH, respectively, which was used to validate functional activity of the generated viral vectors (data not shown). In contrast, activation of the GHS-R1a receptor results in a Gq α-subunit mediated increase in phospholipase C, which subsequently elevates intracellular calcium levels (Fig. 5A). The intracellular calcium increase resulting from ghrelin-mediated GHS-R1a receptor activation was reduced in cells co-expressing the D1 receptor (Fig. 5B). Similarly, the GHS-R1a receptor-mediated intracellular calcium influx was completely blocked with MC3 receptor co-expression (Fig. 5C). The addition of the D1 receptor agonist, 6,7-ADTN hydrobromide, or the MC3 agonist, [Nle4,d-Phe7]-α-MSH, alone did not cause any significant GHS-R1a receptor-mediated influx of calcium.
FIGURE 5.
Co-expression of D1 or MC3 attenuates GHS-R1a mediated signaling in Hek293A cells. Ghrelin-mediated intracellular calcium increase (A) is significantly reduced when the D1 receptor (B) or the MC3 receptor (C) is co-expressed with the GHS-R1a receptor in Hek293A cells. The cells were incubated for 30 min with 100 nm ghrelin, 10 μm 6,7-ADTN hydrobromide, [Nle4,d-Phe7]-α-MSH, or a combination of both, and intracellular calcium influx was measured. Intracellular calcium increase was depicted as a percentage of maximal calcium increase as elicited by control (3% FBS). The data are representative figures from three independent experiments depicting the means ± S.E. with each concentration point performed in triplicate. Statistical significance was analyzed using a two-way ANOVA followed by Bonferroni multiple comparison test; statistical significance is notated as follows: ***, p < 0.001; and **, p < 0.01.
Functional Interaction of the GHS-R1a Receptor with the 5-HT2C Receptor
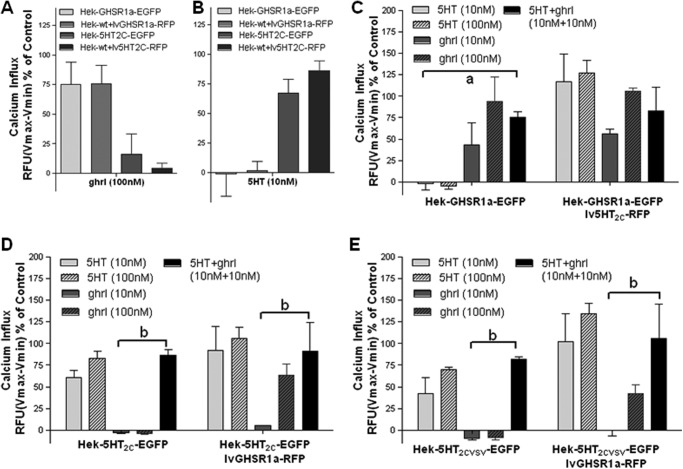
Heterologous cells co-expressing the GHS-R1a receptor and the 5-HT2C receptor were analyzed for downstream signaling consequences. Both the GHS-R1a receptor and the 5-HT2C receptor couple to the Gq protein, which leads to an increase in intracellular calcium. First, the EGFP and RFP constructs used for transfection and transduction were compared for their ability to elicit a calcium response in HEK293A cells (Fig. 6, A and B). Calcium responses were found to be independent of fluorescent tag. In addition, no 5-HT-mediated calcium response was observed in Hek-GHSR1a-EGFP cells (Fig. 6, A–C), and neither were the Hek-5-HT2C-EGFP cells responsive in the presence of ghrelin (Fig. 6, A, B, D, and E). Furthermore, cells co-expressing both receptors were shown not to demonstrate an additive or synergistic calcium influx when treated with a combination of 10 nm agonists (5-HT+ghrl) compared with when treated with either 10 nm 5-HT (Fig. 6, D and E) or 10 nm ghrelin alone (Fig. 6C).
FIGURE 6.
No synergistic or additive effects on intracellular calcium signaling in cells co-expressing the GHS-R1a and the 5-HT2C receptor. A and B, co-expression of the GHSR1a receptor (A) or the 5-HT2C receptor (B) as EGFP or RFP fusion proteins yields similar ligand-mediated calcium increases. C–E, no synergistic or additive effects are observed in cells co-expressing the GHS-R1a receptor and the 5-HT2C-RFP receptor (C), cells stably expressing the unedited 5-HT2C-INI (D), or the partially edited 5-HT2C-VSV (E) receptor transduced with the GHS-R1a-RFP receptor, following treatment with 10 or 100 nm 5-HT (light gray bars), with 10 or 100 nm ghrl (dark gray bars), or with a combination of 10 nm both (black bars). The data represent the means ± S.E. of an experiment performed in triplicate for each concentration point with the intracellular calcium increase depicted as a percentage of maximal calcium increase as elicited by control (3% FBS). Statistical significance within the 10 nm data sets was analyzed using two-way ANOVA followed by Bonferroni multiple comparison test; statistically significant effects of combination treatment (10 nm serotonin, 5-HT, + 10 nm ghrl) were compared with 10 nm 5-HT alone at p < 0.01 (a) or with 10 nm ghrl alone at p < 0.001 (b).
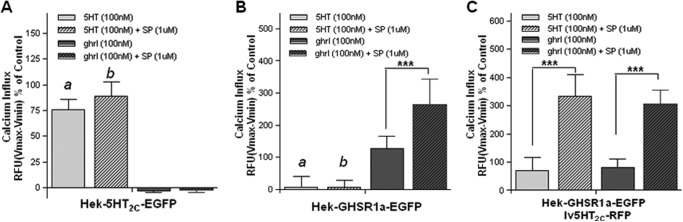
The GHS-R1a receptor has very high ligand-independent constitutive activity (61). Reducing this constitutive activity using an inverse agonist increases receptor expression on the membrane and sensitizes receptor signaling (62, 63). Thus, we set out to determine downstream signaling consequences following GHS-R1a receptor sensitization. To this end, GHS-R1a constitutive, ligand-independent activity was blocked using the GHS-R1a inverse agonist SP-analog. A significant (p < 0.001) ghrelin-induced enhancement of Gq-mediated calcium influx was observed following pretreatment with SP-analog in cells stably expressing the GHS-R1a receptor (Fig. 7B), but not in cells solely expressing the 5-HT2C (Fig. 7A). A similar increase in GHS-R1a-mediated calcium influx was observed in cells co-expressing both the GHS-R1a receptor and the 5-HT2C receptor (Fig. 7C). Interestingly, a significant (p < 0.001) 5-HT-induced enhancement of 5-HT2C receptor-mediated calcium influx after SP pretreatment was observed in GHS-R1a co-expressing cell lines (Fig. 7C), whereas the SP-analog was not able to increase the 5-HT-induced calcium influx in cells solely expressing the 5-HT2C receptor (Fig. 7A). Similar results were obtained in cells co-expressing the GHS-R1a receptor with the partially edited 5-HT2C-VSV isoform (data not shown). In addition, cells stably expressing the 5-HT2C receptor transduced to co-express the GHS-R1a receptor also demonstrated an increase in 5-HT-mediated 5-HT2C receptor signaling following pretreatment with SP-analog, whereas SP-analog pretreatment had no effect on 5-HT-mediated signalling in cells solely expressing the 5-HT2C receptor (data not shown). These results strongly suggest the potential existence of GHS-R1a-5-HT2C heterodimer.
FIGURE 7.
Enhanced 5-HT2C mediated signaling when GHS-R1a ligand-independent activity is blocked. Cells stably expressing the GHS-R1a receptor were transduced with viral vectors expressing the 5-HT2C receptor and treated with 100 nm 5-HT (light gray bars), 100 nm ghrl (dark gray bars) with or without pretreatment of GHS-R1a inverse agonist, peptide [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (1 μm), and intracellular calcium influx was measured. A and B, substance P (SP) has no effect on 5-HT2C-mediated signaling (A), but a significant increase in GHS-R1a-mediated Gq activation is observed following SP pretreatment (B). C, interestingly, 5-HT2C receptor-mediated calcium influx is enhanced after SP pretreatment when the GHS-R1a receptor is co-expressed. Intracellular calcium increase is depicted in RFU as a percentage of maximal calcium increase as elicited by control in each separate experiment (3% FBS). The data represent the means ± S.E. of two independent experiments performed in triplicate for each concentration point. ANOVA followed by a Bonferroni multiple comparison test; statistical significance is notated as follows: a, p < 0.001, 5-HT compared with ghrl; b, p < 0.001, 5-HT+SP compared with ghrl+SP; and ***, p < 0.001, treatment with SP compared with no SP.
Interestingly, we observed that the maximal calcium influx obtained with ghrelin was consistently lower in cells that co-expressed both the GHS-R1a and the 5-HT2C receptor. This suggests that a 5-HT2C receptor mediated attenuation of GHS-R1a receptor signaling (maximal relative fluorescent units obtained with FBS control for GHS-R1a/5-HT2C-expressing cells (13,340 RFU) was ∼35% of that in cells expressing GHS-R1a receptor alone (36,082 RFU)). To investigate this further, we analyzed the ligand-mediated calcium influx relative to the ghrelin-mediated calcium response following pharmacological blockade of the 5-HT2C receptor, using RS102221 (64, 65). Pretreatment with RS102221 (1 μm) was shown to strongly attenuate 5-HT-mediated calcium increase in heterologous cells stably expressing either the unedited 5-HT2C receptor or the partially edited 5-HT2C-VSV isoform (data not shown). A clear attenuation of MK0677- and ghrelin-induced GHS-R1a-mediated signaling became apparent when comparing the ligand-mediated calcium increase in cells solely expressing the GHS-R1a receptor or co-expressing the unedited 5-HT2C receptor or the partially edited 5-HT2C-VSV isoform (Fig. 8). Suppression of GHS-R1a constitutive activity by pretreatment with the GHS-R1a inverse agonist peptide [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (1 μm) increased overall GHS-R1a-mediated signaling but was only able to restore attenuated GHS-R1a activation in cells co-expressing the partially edited 5-HT2C-VSV receptor isoform (Fig. 8B). Full GHS-R1a activity in cells co-expressing the unedited 5-HT2C-VSV isoform was only restored following pharmacological blockade of the 5-HT2C receptor, achieved by pretreatment with the 5-HT2C specific antagonist RS102221.
FIGURE 8.
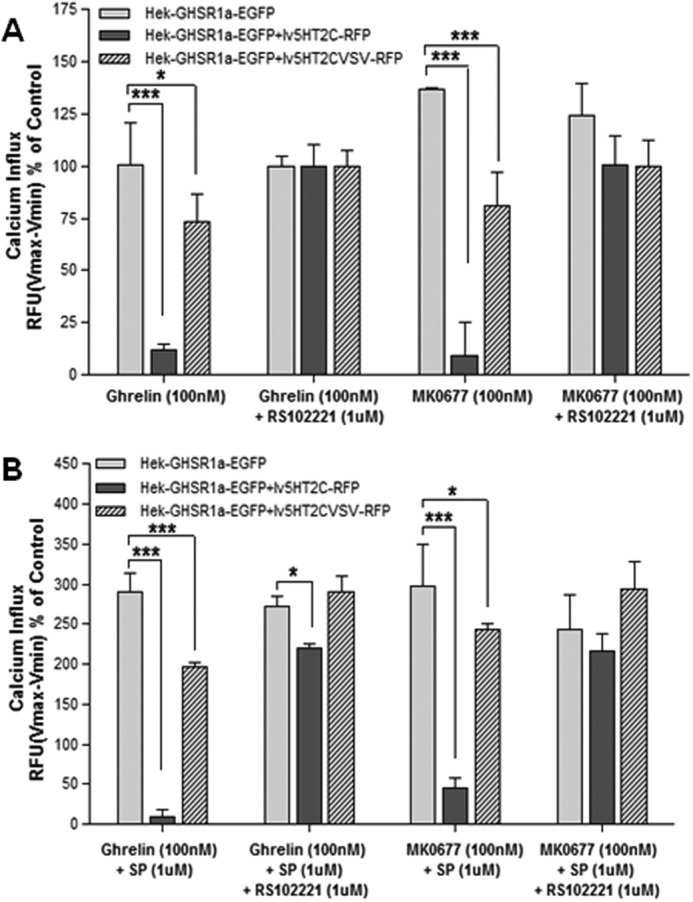
Co-expression of the 5-HT2C receptor attenuates GHS-R1a-mediated signaling. Cells stably expressing the GHS-R1a receptor (light gray bars) were transduced with viral vectors expressing the 5-HT2C receptor (dark gray bars) or the partially edited isoform 5-HT2C-VSV (hatched bars) and treated with 100 nm ghrl or 100 nm MK0677 and with or without (1 μm) RS10221 exposure. A, intracellular calcium increase was reduced in cells co-expressing the 5-HT2C receptor, which was rescued after RS102221 treatment. B, the GHS-R1a inverse agonist, peptide [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (SP; 1 μm), increased total calcium influx but was not able to restore full GHS-R1a-mediated signaling. Intracellular calcium increase was depicted in RFU as a percentage of maximal calcium increase as elicited by ghrelin or MK0677 response in presence of RS102221. Intracellular calcium increase was depicted as a percentage of maximal calcium increase as elicited by control. The data represent the means ± S.E. of a representative experiment of three independent transductions with each concentration point performed in triplicate. Two-way ANOVA followed by Bonferroni multiple comparison test; statistical significance is notated as follows: ***, p < 0.001; and **, p < 0.01.
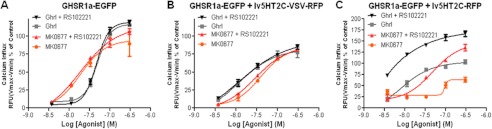
Co-expression of the edited 5-HT2C-VSV isoform was only partially able to attenuate GHS-R1a-mediated signaling (Fig. 8). To further investigate the potential of the 5-HT2C receptor to attenuate GHS-R1a-mediated signaling and to analyze the effect of 5-HT2C receptor editing, dose-response curves were performed. Cell lines co-expressing the GHS-R1a receptor and the unedited 5-HT2C-INI receptor or the partially edited 5-HT2C-VSV receptor were pretreated with substance P-analog alone or in combination with the 5-HT2C specific antagonist, RS102221 (Fig. 9). Next, calcium influx was analyzed following exposure to the endogenous GHS-R1a agonist, ghrelin, or the synthetic agonist, MK0677. Pharmacological blockade of the 5-HT2C receptor had no effect on ghrelin- or MK0677-mediated calcium signaling in cells that only expressed the GHS-R1a receptor (Fig. 9A) or in cells co-expressing the partially edited 5-HT2C-VSV isoform in parallel to the GHS-R1a receptor (Fig. 9B). However, ghrelin- and MK0677-induced calcium levels in cells co-expressing the partially edited 5-HT2C-VSV isoform were comparable with cells solely expressing the GHS-R1a receptor, albeit with a slightly reduced efficacy. Cells co-expressing the unedited 5-HT2C receptor isoform attenuated GHS-R1a-mediated calcium signaling, which was fully restored following exposure to the 5-HT2C specific antagonist, RS10221 (Fig. 9C).
FIGURE 9.
Attenuation of GHS-R1a-mediated signaling depends on specific 5-HT2C isoform co-expression. Cells co-expressing the GHS-R1a receptor and the 5-HT2C receptor or the partially edited isoform, 5-HT2C-VSV were treated with ghrl or MK0677 in a dose-response manner (300 to 3.7 nm, 3-fold serial dilution), following pretreatment with the GHS-R1a inverse agonist, peptide [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P (1 μm). A, no effect of 5-HT2C receptor blockade in cells solely expression the GHS-R1a receptor. B and C, RS102221 rescues GHS-R1a-induced calcium influx in GHS-R1/5-HT2C-expressing cells (B), but there was no change in GHS-R1a/5-HT2C-VSV-expressing cells (C). Intracellular calcium increase was depicted as a percentage of maximal calcium increase as elicited by control in each separate experiment (3% FBS in presence of SP-analog). The data represent the means ± S.E. of a representative experiment of three independent transductions with each concentration point performed in triplicate.
DISCUSSION
The realization that GPCRs do not exclusively exist or function as monomeric units has become increasingly evident from heterologous expression systems demonstrating that GPCRs can both traffic and signal as oligomeric structures (66–69). However, evidence for heterodimerization of centrally expressed GPCRs involved in appetite and satiety regulation is only beginning to emerge and not yet abundant. This study demonstrated promiscuous heterodimerization of the GHS-R1a receptor with both the centrally expressed MC3 receptor and the D1 receptor. Even though heterodimerization of the GHS-R1a receptor with these two GPCRs is not novel (29, 36), analysis of downstream signaling revealed a previously unreported attenuation of GHS-R1a receptor-mediated calcium signaling upon co-expression compared with GHS-R1a receptor signaling alone. This may suggest a novel molecular mechanism to attenuate the GHS-R1a-mediated orexigenic signaling or a dimerization-dependent modulation of the dopaminergic food reward signaling, which has recently been shown to involve the GHS-R1a receptor (15–20). Interestingly, we show for the first time significant changes in trafficking of the GHS-R1a/D1 and GHS-R1a/MC3 receptor pairs following ligand exposure, suggesting distinct dimer-dependent mechanism in the attenuation of GHS-R1a receptor activation. Ghrelin-mediated calcium signaling in the GHS-R1a/MC3 co-expressing cells is likely inhibited following attenuation of the receptor pair in the cytosol. Receptor trafficking in cells co-expressing the GHS-R1a/D1 is not affected, indicating a different, as-yet unidentified mechanism of GHS-R1a signaling inhibition.
Furthermore, we show evidence for a novel dimer between the GHS-R1a and the 5-HT2C receptor following ligand-mediated co-internalization in cells heterologously expressing this receptor pair. We found further evidence for a GHS-R1a/5-HT2C receptor dimer when SP-analog, a potent specific inverse agonist of the GHS-R1a receptor, significantly increased 5-HT2C-mediated signaling in heterologous cells co-expressing the GHS-R1a/5-HT2C receptor dimer, whereas SP-analog had no effect on the Gq-mediated calcium increase in cells expressing the 5-HT2C receptor alone. This inverse GHS-R1a agonist has not only been reported to suppress ligand-independent basal activity of the GHS-R1a receptor (61) but has also been shown to cause a significant increase of expression of the GHS-R1a receptor on the cell membrane, both in our lab and in reported studies (63). Following GHS-R1a dimerization with the 5-HT2C receptor, pretreatment with SP sensitizes the 5-HT2C receptor, as well as the GHS-R1a receptor leading to an increased membrane receptor co-recruitment of the GHS-R1a/5-HT2C receptor pair. The most interesting finding was that co-expression of the unedited 5-HT2C-INI receptor, but not the partially edited 5-HT2C-VSV isoform, in cells stably expressing the GHS-R1a receptor attenuated GHS-R1a-mediated intracellular calcium influx following ghrelin and MK0677 treatment. This is in line with the general consensus that increased editing of the 5-HT2C receptor leads to a decreased receptor functioning (42, 70–73). This suggests that alteration of the 5-HT2C receptor editing profile in vivo is likely to also have an impact on GHS-R1a receptor signaling.
Finally, we hypothesized, that if the 5-HT2C receptor functions to attenuate the GHS-R1a-mediated signaling, blockade of the 5-HT2C receptor should restore GHS-R1a-mediated calcium influx. Indeed, upon 5-HT2C receptor blockade using the 5-HT2C specific antagonist, RS102221, ghrelin- and MK0677-induced calcium signaling was restored in cells co-expressing the GHS-R1a receptor and the unedited 5-HT2C-INI receptor. This suggests a novel mechanism of 5-HT2C-mediated attenuation of the GHS-R1a receptor. Considering the differential functional isoforms arising from 5-HT2C receptor editing, this could have important biological consequences for GHS-R1a receptor function.
This is the first time, to our knowledge, that functional heterodimerization between the GHS-R1a receptor and the 5-HT2C receptor is demonstrated, suggesting that a potential relevant interaction in vivo may exist. Given that both of these GPCRs are known to regulate feeding behavior, it is tempting to speculate that such heterodimerization may play a role in satiety signaling. Further investigations into the extent of dimerization in vivo are now warranted. The observed serotonin-mediated attenuation of the orexigenic effect of ghrelin in rats, as reported by Currie et al. (50), is in line with our findings and supports a potentially significant relevance of GHS-R1a/5-HT2C receptor dimerization in appetite regulation in vivo.
Nevertheless, it is currently unclear how the intracellular calcium influx mediated by both GHS-R1a receptor and 5-HT2C receptor activation could have opposing effects on food intake in vivo. It has been shown that ghrelin signaling can be mediated via differential tissue-specific G protein coupling. For example, the GHS-R1a receptor has been shown to couple to Gαi2 in pancreatic islet β-cells, mediating the suppression of glucose-induced Ca2+ signaling resulting in attenuated insulin release (74). In addition, in NPY neurons the GHS-R1a-mediated Ca2+ mobilization is achieved through the Gs-cAMP-PKA signaling pathway (75). These and other alternate downstream signaling pathways may be able to explain the opposing effects on food intake and should be further investigated.
In conclusion, these observations combined are important in strengthening the case for dimerization of the GHS-R1a receptor with several other GPCRs, including the D1, MC3, and 5-HT2C receptors, and behavioral studies are now warranted to validate the functional relevance of each heterodimer in vivo. The promiscuous GHS-R1a dimerization with candidate GPCRs, such as the MC3, the D1, and the 5-HT2C receptor may play a key role in the modulation and fine-tuning of GHS-R1a-mediated downstream signaling and subsequent satiety and appetite signaling, as well as the reward and motivational aspects of food intake. The implications of GHS-R1a receptor heterodimerization will fundamentally impact current knowledge of the structure, activation, and desensitization processes of this receptor. In addition, novel heterodimerization of GPCRs involved in energy homeostasis and reward, as well as further elucidating of the downstream signaling pathways, will lead to a better understanding of not only homeostatic regulation of food intake but also the incentive motivational value and rewarding aspects of food. This may ultimately have dramatic impacts on drug development and screening and lead to novel ghrelin-targeted therapies with increased selectivity and reduced side effects. Heterodimer complexes of the GHS-R1a receptor provide unique pharmacological targets to control a subset of functions within a broader ghrelin-mediated signaling spectrum of feeding behaviors, including homeostatic appetite signaling, as well as the hedonic pathways in response to food and drugs of abuse. The potential role for the GHS-R1a/5-HT2C dimer in the ghrelin-mediated effects on the rewarding properties of food warrants further investigation.
Acknowledgment
We thank Suzanne Crotty for technical assistance with confocal microscopy in the BioSciences Imaging Centre (University College Cork, Ireland).
The work was supported by Enterprise Ireland under Grant CC20080001 and by Science Foundation Ireland Grants 02/CE/B124 and 07/CE/B1368.
- GHS
- growth hormone secretagogue
- GPCR
- G protein-coupled receptor
- GHS-R1a
- ghrelin receptor
- 5-HT2C
- serotonin 2c receptor
- MC3
- melanocortin 3 receptor
- 5-HT
- 5-hydroxytryptamine
- D1
- dopamine 1 receptor
- EGFP
- enhanced green fluorescent protein
- RFP
- red fluorescent protein
- RFU
- relative fluorescence unit(s)
- ANOVA
- analysis of variance
- 6-Amino-5,6,7,8-tetrahydronaphthalene-2,3-diol
- ADTN
- SP-analog
- [d-Arg1,d-Phe5,d-Trp7,9,Leu11]-substance P
- ghrl
- ghrelin.
REFERENCES
- 1. Howard A. D., Feighner S. D., Cully D. F., Arena J. P., Liberator P. A., Rosenblum C. I., Hamelin M., Hreniuk D. L., Palyha O. C., Anderson J., Paress P. S., Diaz C., Chou M., Liu K. K., McKee K. K., Pong S. S., Chaung L. Y., Elbrecht A., Dashkevicz M., Heavens R., Rigby M., Sirinathsinghji D. J., Dean D. C., Melillo D. G., Patchett A. A., Nargund R., Griffin P. R., DeMartino J. A., Gupta S. K., Schaeffer J. M., Smith R. G., Van der Ploeg L. H. (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273, 974–977 [DOI] [PubMed] [Google Scholar]
- 2. Muccioli G., Baragli A., Granata R., Papotti M., Ghigo E. (2007) Heterogeneity of ghrelin/growth hormone secretagogue receptors. Toward the understanding of the molecular identity of novel ghrelin/GHS receptors. Neuroendocrinology 86, 147–164 [DOI] [PubMed] [Google Scholar]
- 3. Muccioli G., Tschöp M., Papotti M., Deghenghi R., Heiman M., Ghigo E. (2002) Neuroendocrine and peripheral activities of ghrelin. Implications in metabolism and obesity. Eur. J. Pharmacol. 440, 235–254 [DOI] [PubMed] [Google Scholar]
- 4. Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 [DOI] [PubMed] [Google Scholar]
- 5. Davenport A. P., Bonner T. I., Foord S. M., Harmar A. J., Neubig R. R., Pin J. P., Spedding M., Kojima M., Kangawa K. (2005) International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol. Rev. 57, 541–546 [DOI] [PubMed] [Google Scholar]
- 6. Schellekens H., Dinan T. G., Cryan J. F. (2010) Lean mean fat reducing “ghrelin” machine. Hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology 58, 2–16 [DOI] [PubMed] [Google Scholar]
- 7. Castañeda T. R., Tong J., Datta R., Culler M., Tschöp M. H. (2010) Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 31, 44–60 [DOI] [PubMed] [Google Scholar]
- 8. Chollet C., Meyer K., Beck-Sickinger A. G. (2009) Ghrelin. A novel generation of anti-obesity drug. Design, pharmacomodulation and biological activity of ghrelin analogues. J. Pept. Sci. 15, 711–730 [DOI] [PubMed] [Google Scholar]
- 9. Soares J. B., Roncon-Albuquerque R., Jr., Leite-Moreira A. (2008) Ghrelin and ghrelin receptor inhibitors. Agents in the treatment of obesity. Expert Opin. Ther. Targets 12, 1177–1189 [DOI] [PubMed] [Google Scholar]
- 10. Clifton P. G., Kennett G. A. (2006) Monoamine receptors in the regulation of feeding behaviour and energy balance. CNS Neurol. Disord. Drug Targets 5, 293–312 [DOI] [PubMed] [Google Scholar]
- 11. Dutton A. C., Barnes N. M. (2006) Anti-obesity pharmacotherapy. Future perspectives utilising 5-HT2C receptor agonists. Drug Discov. Today 3, 577–583 [Google Scholar]
- 12. Garfield A. S., Heisler L. K. (2009) Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. 587, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halford J. C., Harrold J. A., Boyland E. J., Lawton C. L., Blundell J. E. (2007) Serotonergic drugs. Effects on appetite expression and use for the treatment of obesity. Drugs 67, 27–55 [DOI] [PubMed] [Google Scholar]
- 14. Miller K. J. (2005) Serotonin 5-HT2C receptor agonists. Potential for the treatment of obesity. Mol. Interv. 5, 282–291 [DOI] [PubMed] [Google Scholar]
- 15. Schellekens H., Finger B. C., Dinan T. G., Cryan J. F. (2012) Ghrelin signalling and obesity. At the interface of stress, mood and food reward. Pharmacol. Ther. 135, 316–326 [DOI] [PubMed] [Google Scholar]
- 16. Skibicka K. P., Hansson C., Egecioglu E., Dickson S. L. (2012) Role of ghrelin in food reward. Impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict. Biol. 17, 95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perelló M., Zigman J. M. (2012) The role of ghrelin in reward-based eating. Biol. Psychiatry 72, 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skibicka K. P., Dickson S. L. (2011) Ghrelin and food reward. The story of potential underlying substrates. Peptides 32, 2265–2273 [DOI] [PubMed] [Google Scholar]
- 19. Egecioglu E., Skibicka K. P., Hansson C., Alvarez-Crespo M., Friberg P. A., Jerlhag E., Engel J. A., Dickson S. L. (2011) Hedonic and incentive signals for body weight control. Rev. Endocr. Metab. Disord. 12, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dickson S. L., Egecioglu E., Landgren S., Skibicka K. P., Engel J. A., Jerlhag E. (2011) The role of the central ghrelin system in reward from food and chemical drugs. Mol. Cell. Endocrinol. 340, 80–87 [DOI] [PubMed] [Google Scholar]
- 21. Guan X. M., Yu H., Palyha O. C., McKee K. K., Feighner S. D., Sirinathsinghji D. J., Smith R. G., Van der Ploeg L. H., Howard A. D. (1997) Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 48, 23–29 [DOI] [PubMed] [Google Scholar]
- 22. Zigman J. M., Jones J. E., Lee C. E., Saper C. B., Elmquist J. K. (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 494, 528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delgado M., Ganea D. (2008) Anti-inflammatory neuropeptides. A new class of endogenous immunoregulatory agents. Brain Behav. Immun. 22, 1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J. V., Ren P. G., Avsian-Kretchmer O., Luo C. W., Rauch R., Klein C., Hsueh A. J. (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 310, 996–999 [DOI] [PubMed] [Google Scholar]
- 25. Kaupmann K., Malitschek B., Schuler V., Heid J., Froestl W., Beck P., Mosbacher J., Bischoff S., Kulik A., Shigemoto R., Karschin A., Bettler B. (1998) GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 [DOI] [PubMed] [Google Scholar]
- 26. Kent T., McAlpine C., Sabetnia S., Presland J. (2007) G-protein-coupled receptor heterodimerization. Assay technologies to clinical significance. Curr. Opin. Drug Discov. Devel. 10, 580–589 [PubMed] [Google Scholar]
- 27. Luttrell L. M. (2008) Reviews in molecular biology and biotechnology. Transmembrane signaling by G protein-coupled receptors. Mol. Biotechnol. 39, 239–264 [DOI] [PubMed] [Google Scholar]
- 28. Panetta R., Greenwood M. T. (2008) Physiological relevance of GPCR oligomerization and its impact on drug discovery. Drug Discov. Today 13, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 29. Rediger A., Tarnow P., Bickenbach A., Schaefer M., Krude H., Gruters A., Biebermann H. (2009) Heterodimerization of hypothalamic G-protein-coupled receptors involved in weight regulation. Obesity Facts 2, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rediger A., Piechowski C. L., Yi C. X., Tarnow P., Strotmann R., Grüters A., Krude H., Schöneberg T., Tschöp M. H., Kleinau G., Biebermann H. (2011) Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J. Biol. Chem. 286, 39623–39631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adan R. A., Tiesjema B., Hillebrand J. J., la Fleur S. E., Kas M. J., de Krom M. (2006) The MC4 receptor and control of appetite. Br. J. Pharmacol. 149, 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cone R. D., Lu D., Koppula S., Vage D. I., Klungland H., Boston B., Chen W., Orth D. N., Pouton C., Kesterson R. A. (1996) The melanocortin receptors. Agonists, antagonists, and the hormonal control of pigmentation. Recent Prog. Horm. Res. 51, 287–318 [PubMed] [Google Scholar]
- 33. Kishi T., Aschkenasi C. J., Lee C. E., Mountjoy K. G., Saper C. B., Elmquist J. K. (2003) Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 457, 213–235 [DOI] [PubMed] [Google Scholar]
- 34. Yang Y. (2011) Structure, function and regulation of the melanocortin receptors. Eur. J. Pharmacol. 660, 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kern A., Albarran-Zeckler R., Walsh H. E., Smith R. G. (2012) Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 73, 317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang H., Betancourt L., Smith R. G. (2006) Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol. Endocrinol. 20, 1772–1785 [DOI] [PubMed] [Google Scholar]
- 37. Kenny P. J. (2011) Reward mechanisms in obesity. New insights and future directions. Neuron 69, 664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perello M., Sakata I., Birnbaum S., Chuang J. C., Osborne-Lawrence S., Rovinsky S. A., Woloszyn J., Yanagisawa M., Lutter M., Zigman J. M. (2010) Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry 67, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Somerville E. M., Horwood J. M., Lee M. D., Kennett G. A., Clifton P. G. (2007) 5-HT2C receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fos immunoreactivity in mice. Eur. J. Neurosci. 25, 3115–3124 [DOI] [PubMed] [Google Scholar]
- 40. Lam D. D., Heisler L. K. (2007) Serotonin and energy balance. Molecular mechanisms and implications for type 2 diabetes. Expert Rev. Mol. Med. 9, 1–24 [DOI] [PubMed] [Google Scholar]
- 41. Lam D. D., Przydzial M. J., Ridley S. H., Yeo G. S., Rochford J. J., O'Rahilly S., Heisler L. K. (2008) Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149, 1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schellekens H., Clarke G., Jeffery I. B., Dinan T. G., Cryan J. F. (2012) Dynamic 5-HT2C receptor editing in a mouse model of obesity. PLoS One 7, e32266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nonogaki K., Ohashi-Nozue K., Oka Y. (2006) A negative feedback system between brain serotonin systems and plasma active ghrelin levels in mice. Biochem. Biophys. Res. Commun., 341, 703–707 [DOI] [PubMed] [Google Scholar]
- 44. Brunetti L., Recinella L., Orlando G., Michelotto B., Di Nisio C., Vacca M. (2002) Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur. J. Pharmacol. 454, 189–192 [DOI] [PubMed] [Google Scholar]
- 45. Alex K. D., Pehek E. A. (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 113, 296–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Higgins G. A., Fletcher P. J. (2003) Serotonin and drug reward. Focus on 5-HT2C receptors. Eur. J. Pharmacol. 480, 151–162 [DOI] [PubMed] [Google Scholar]
- 47. Clemett D. A., Punhani T., Duxon M. S., Blackburn T. P., Fone K. C. (2000) Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39, 123–132 [DOI] [PubMed] [Google Scholar]
- 48. Leysen J. E. (2004) 5-HT2 receptors. Curr. Drug Targets CNS Neurol. Disord. 3, 11–26 [DOI] [PubMed] [Google Scholar]
- 49. Cowley M. A., Smith R. G., Diano S., Tschöp M., Pronchuk N., Grove K. L., Strasburger C. J., Bidlingmaier M., Esterman M., Heiman M. L., Garcia-Segura L. M., Nillni E. A., Mendez P., Low M. J., Sotonyi P., Friedman J. M., Liu H., Pinto S., Colmers W. F., Cone R. D., Horvath T. L. (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 [DOI] [PubMed] [Google Scholar]
- 50. Currie P. J., John C. S., Nicholson M. L., Chapman C. D., Loera K. E. (2010) Hypothalamic paraventricular 5-hydroxytryptamine inhibits the effects of ghrelin on eating and energy substrate utilization. Pharmacol. Biochem. Behav. 97, 152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Derangeon M., Bozon V., Defamie N., Peineau N., Bourmeyster N., Sarrouilhe D., Argibay J. A., Hervé J. C. (2010) 5-HT4 and 5-HT2 receptors antagonistically influence gap junctional coupling between rat auricular myocytes. J. Mol. Cell. Cardiol. 48, 220–229 [DOI] [PubMed] [Google Scholar]
- 52. González-Maeso J., Ang R. L., Yuen T., Chan P., Weisstaub N. V., López-Giménez J. F., Zhou M., Okawa Y., Callado L. F., Milligan G., Gingrich J. A., Filizola M., Meana J. J., Sealfon S. C. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fribourg M., Moreno J. L., Holloway T., Provasi D., Baki L., Mahajan R., Park G., Adney S. K., Hatcher C., Eltit J. M., Ruta J. D., Albizu L., Li Z., Umali A., Shim J., Fabiato A., MacKerell A. D., Jr., Brezina V., Sealfon S. C., Filizola M., González-Maeso J., Logothetis D. E. (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147, 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delille H. K., Becker J. M., Burkhardt S., Bleher B., Terstappen G. C., Schmidt M., Meyer A. H., Unger L., Marek G. J., Mezler M. (2012) Heterocomplex formation of 5-HT(2A)-mGlu(2) and its relevance for cellular signaling cascades. Neuropharmacology 62, 2184–2191 [DOI] [PubMed] [Google Scholar]
- 55. Lukasiewicz S., Polit A., Kdracka-Krok S., Wdzony K., Makowiak M., Dziedzicka-Wasylewska M. (2010) Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta 1803, 1347–1358 [DOI] [PubMed] [Google Scholar]
- 56. Follenzi A., Naldini L. (2002) HIV-based vectors. Preparation and use. Methods Mol. Med. 69, 259–274 [PubMed] [Google Scholar]
- 57. Follenzi A., Naldini L. (2002) Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 346, 454–465 [DOI] [PubMed] [Google Scholar]
- 58. Naldini L. (1998) Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr. Opin. Biotechnol. 9, 457–463 [DOI] [PubMed] [Google Scholar]
- 59. Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 [DOI] [PubMed] [Google Scholar]
- 60. Vigna E., Naldini L. (2000) Lentiviral vectors. Excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gene Med. 2, 308–316 [DOI] [PubMed] [Google Scholar]
- 61. Holst B., Cygankiewicz A., Jensen T. H., Ankersen M., Schwartz T. W. (2003) High constitutive signaling of the ghrelin receptor. Identification of a potent inverse agonist. Mol. Endocrinol. 17, 2201–2210 [DOI] [PubMed] [Google Scholar]
- 62. Els S., Beck-Sickinger A. G., Chollet C. (2010) Ghrelin receptor. High constitutive activity and methods for developing inverse agonists. Methods Enzymol. 485, 103–121 [DOI] [PubMed] [Google Scholar]
- 63. Liu G., Fortin J. P., Beinborn M., Kopin A. S. (2007) Four missense mutations in the ghrelin receptor result in distinct pharmacological abnormalities. J. Pharmacol. Exp. Ther. 322, 1036–1043 [DOI] [PubMed] [Google Scholar]
- 64. Bonhaus D. W., Rocha C. L., Dawson M. W., Eglen R. M. (1998) Absorption and brain penetration of a high affinity, highly selective 5-HT2C receptor antagonist, RS-102221. Ann. N.Y. Acad. Sci. 861, 269. [DOI] [PubMed] [Google Scholar]
- 65. Bonhaus D. W., Weinhardt K. K., Taylor M., DeSouza A., McNeeley P. M., Szczepanski K., Fontana D. J., Trinh J., Rocha C. L., Dawson M. W., Flippin L. A., Eglen R. M. (1997) RS-102221. A novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36, 621–629 [DOI] [PubMed] [Google Scholar]
- 66. González-Maeso J. (2011) GPCR oligomers in pharmacology and signaling. Mol. Brain 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rozenfeld R., Devi L. A. (2010) Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 31, 124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rozenfeld R., Devi L. A. (2011) Exploring a role for heteromerization in GPCR signalling specificity. Biochem. J. 433, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Angers S., Salahpour A., Bouvier M. (2002) Dimerization. An emerging concept for G protein-coupled receptor ontogeny and function. Annu. Rev. Pharmacol. Toxicol. 42, 409–435 [DOI] [PubMed] [Google Scholar]
- 70. Berg K. A., Clarke W. P., Cunningham K. A., Spampinato U. (2008) Fine-tuning serotonin 2C receptor function in the brain. Molecular and functional implications. Neuropharmacology 55, 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burns C. M., Chu H., Rueter S. M., Hutchinson L. K., Canton H., Sanders-Bush E., Emeson R. B. (1997) Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387, 303–308 [DOI] [PubMed] [Google Scholar]
- 72. Niswender C. M., Copeland S. C., Herrick-Davis K., Emeson R. B., Sanders-Bush E. (1999) RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 274, 9472–9478 [DOI] [PubMed] [Google Scholar]
- 73. Olaghere da Silva U. B., Morabito M. V., Canal C. E., Airey D. C., Emeson R. B., Sanders-Bush E. (2010) Impact of RNA editing on functions of the serotonin 2C receptor in vivo. Front. Neuropharmacol. 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dezaki K., Kakei M., Yada T. (2007) Ghrelin uses Gα2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells. Novel signal transduction of ghrelin. Diabetes 56, 2319–2327 [DOI] [PubMed] [Google Scholar]
- 75. Kohno D., Gao H. Z., Muroya S., Kikuyama S., Yada T. (2003) Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus. Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 52, 948–956 [DOI] [PubMed] [Google Scholar]