Background: Neurokinin-1 receptor is known to promote tumor cell proliferation.
Results: Neurokinin-1 receptor activates ERK, JNK, and Akt through the Gq-phospholipase C pathway, up-regulating MMP-2 and MT1-MMP and subsequently enhancing glioma cell migration.
Conclusion: Neurokinin-1 receptor mediates glioma cell migration by the up-regulation of MMP-2 and MT1-MMP.
Significance: This study shows for the first time that neurokinin-1 receptor is directly involved in tumor cell migration.
Keywords: Akt, MAP Kinases (MAPKs), Matrix Metalloproteinase (MMP), Migration, Transcription Factors, Neurokinin-1 Receptor
Abstract
Neurokinin-1 receptor (NK1R) occurs naturally on human glioblastomas. Its activation mediates glioma cell proliferation. However, it is unknown whether NK1R is directly involved in tumor cell migration. In this study, we found human hemokinin-1 (hHK-1), via NK1R, dose-dependently promoted the migration of U-251 and U-87 cells. In addition, we showed that hHK-1 enhanced the activity of MMP-2 and the expression of MMP-2 and MT1-matrix metalloproteinase (MMP), which were responsible for cell migration, because neutralizing the MMPs with antibodies decreased cell migration. The involved mechanisms were then investigated. In U-251, hHK-1 induced significant calcium efflux; phospholipase C inhibitor U-73122 reduced the calcium mobilization, the up-regulation of MMP-2 and MT1-MMP, and the cell migration induced by hHK-1, which meant the migration effect of NK1R was mainly mediated through the Gq-PLC pathway. We further demonstrated that hHK-1 boosted rapid phosphorylation of ERK, JNK, and Akt; inhibition of ERK and Akt effectively reduced MMP-2 induction by hHK-1. Meanwhile, inhibition of ERK, JNK, and Akt reduced the MT1-MMP induction. hHK-1 stimulated significant phosphorylation of p65 and c-JUN in U-251. Reporter gene assays indicated hHK-1 enhanced both AP-1 and NF-κB activity; inhibition of ERK, JNK, and Akt dose-dependently suppressed the NF-κB activity; only the inhibition of ERK significantly suppressed the AP-1 activity. Treatment with specific inhibitors for AP-1 or NF-κB strongly blocked the MMP up-regulation by hHK-1. Taken together, our data suggested NK1R was a potential regulator of human glioma cell migration by the up-regulation of MMP-2 and MT1-MMP.
Introduction
Malignant gliomas are the most frequent primary brain tumors in adults and children (1). Their aggressive invasion into adjacent brain tissue made thorough surgical resection impossible (2, 3). Standard radiation and chemotherapy failed to improve the survival of patients with gliomas (4, 5). The depressing results were due to the biological characteristics of the malignant tumor cells. To get better curative effects, researchers have been looking for a deeper understanding of glioma biology to find new strategies to block tumor development.
In the past decade, tachykinin receptor neurokinin-1 (NK1R)2 has received a lot of attention as a promising new target in malignant glioma therapy (6–8). NK1R belongs to class A of the G-protein-coupled receptor family. Both substance P and the newly found mammalian tachykinin hemokinin-1 (HK-1) are identified as the endogenous ligands of NK1R with high affinities (9, 47, 48). Studies demonstrated that malignant gliomas expressed functional NK1R at certain percentages, and the expression level seemed to be related to the malignant degree of tumors (10, 11). NK1R was also detected in many established glioma cell lines, such as U-251 MG, U-87 MG, DBTRG-05 MG, and SNB-19 (12–14), which were often used as the natural models to investigate NK1R biological functions. It was reported that NK1R activation induced the phosphorylation of Akt and mitogen-activated protein kinase (MAPK) family members (15–17), which subsequently stimulated different transcription factor activities to adjust target gene expression (18–20). These regulatory mechanisms were engaged by NK1R to stimulate DNA synthesis and cytokine secretion and to mediate the anti-apoptosis effect (15, 16, 18, 21).
Gliomas occur as a result of a multistep procedure, which involves several alterations in astrocyte physiology, including the deregulation of cell proliferation, apoptosis, and migration (22, 23). The majority of NK1R-related studies are focused on its role in glioma cell proliferation and cytokine secretion (12, 15, 16). Moreover, NK1R activation in endothelial cells regulated angiogenesis and encouraged vascular survival pro-angiogenesis, which is essential for supplying O2 and nutriments in the expansion of tumors (24–26). Moreover, NK1R is an important regulator of immune cell motility. NK1R induced natural killer cell migration in a dose-dependent manner (27). Substance P and its C-terminal fragments stimulated human monocytes and peripheral blood leukocyte chemotaxis via NK1R (28, 29). Such ability to modulate the immune system is important in NK1R-related tumor development. However, whether the activation of NK1R would influence the migration of the tumor cell itself was unclear. Recently, Meshki (30, 31) reported that substance P mediated membrane blebbing in both HEK-293-NK1R and U-373 MG cells via the NK1 receptor, which was not associated with apoptosis but possibly with migration. Considering the elevated expression level of NK1R and the high motility of glioma cells, we speculated that NK1R might be a new target to regulate glioma cell migration and invasion.
The invasion process of glioblastoma is complicated, involving a series of sequential steps. Matrix metalloproteinases (MMPs) play a crucial role in the process because of their activities to degrade local extracellular matrices, enabling tumor cells to infiltrate into surrounding areas (32, 33). Various MMPs, such as MMP-2 and MMP-9, have been tightly associated with the glioblastoma progression and malignancy (34, 35). The activities of these enzymes are delicately regulated at several levels, including gene expression, the activation of proenzyme, and the inhibition of active enzymes by their specific inhibitors (tissue inhibitor of metalloproteinases) (34–36). MT1-MMP is a membrane-type MMP (also named MMP-14), which forms a complex with TIMP-2 as a specific receptor for latent MMP-2. The complex cleaves newly synthesized pro-MMP-2 on the cell surface into active MMP-2 (35, 37). As mentioned above, NK1R has been implicated to promote cell movement in different cell types; however, the detailed molecular mechanisms are hardly known. It will be interesting to investigate whether and how NK1Rs are directly involved in glioma cell migration.
Previously, we reported that hHK-1 and its derivatives selectively activated Gs and Gq signaling pathways in CHO cells expressing NK1R (38); among them, hHK-1 and hHK-1(4–11) have been demonstrated to stimulate the migration of freshly isolated human umbilical vein endothelial cells and to promote angiogenesis in the chick embryo chorioallantoic membrane model (26). In this study, we showed for the first time that by activating NK1R, hHK-1 induced significant up-regulation of MMP-2 and MT1-MMP that were responsible for the enhancement of glioma cell migration.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
Dulbecco's modified Eagle's medium (DMEM), Opti-MEM medium, fetal bovine serum (FBS), cell dissociation buffer, Fluo-4 AM, Pluronic F-127, and transfection reagent Lipofectamine2000 were from Invitrogen. PD98059, LY294002, cAMP-Glo assay kit, and luciferase assay system were from Promega (Madison, WI). The enhanced chemiluminescence (ECL) detection system and BCA protein assay kit were from Pierce. Probenecid, SP600125, forskolin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), gelatin, L-732138, U-73122, 3-isobutyl-1-methylxanthine, and Ro 20-1724 were from Sigma. Curcumin, tanshinone IIA, and caffeic acid phenethyl ester (CAPE) were from Sangon (Shanghai, China). Protease inhibitor mixture and phosphatase inhibitor mixtures were from Roche Applied Science. Pyrrolidinedithiocarbamic acid (PDTC), 5× Laemmli buffer, and RIPA lysis buffer were from Beyotime (Jiangsu, China). Antibodies against phospho-ERK1/2, ERK1/2, phospho-SAPK/JNK, SAPK/JNK, pAkt, phospho-p65, phospho-c-JUN, GAPDH, and HRP-conjugated secondary antibody were purchased from Cell Signaling Technology Inc. (Danvers, MA). Antibodies against MMP-2 and MT1-MMP were from Abcam (Cambridge, UK). PCR primers, RNAiso Plus, and PrimeScript RT reagents were from Takara Biotechnology (Dalian, China).
Cell Culture and Peptide Synthesis
Human glioblastoma cell line U251 MG was purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin) in a humidified atmosphere of CO2/air (5:95%) at 37 °C.
Human hemokinin-1 (TGKASQFFGLM-NH2) was synthesized using the Fmoc (N-(9-fluorenyl)methoxycarbonyl) method on a solid-phase peptide synthesis system, as described previously (38). The identity of the peptide was confirmed using ESI-TOF mass spectrometry. Peptides were determined to be >95% pure by reversed-phase high performance liquid chromatography using a C18 column as the solid phase and an H2O/acetonitrile gradient as the solution phase.
Migration Assays
In vitro migration assays were performed using Millicell Hanging Cell Culture inserts (8 μm pore size; Millipore, Billerica, MA) in 24-well plates. U-251 cells were digested with cell dissociation buffer containing no trypsin. Approximately 4 × 104 cells in 0.1 ml of serum-free DMEM were seeded in the upper chamber, and 0.6 ml of the same medium with or without hHK-1 was placed in the lower chamber. After incubating the plates at 37 °C for 24 h, cells were fixed with 90% EtOH for 30 min and then stained with 0.1% crystal violet in PBS for 15 min. The nonmigrant cells were removed from the upper face of the transwell membrane with a cotton swab. The stained cells were subsequently photographed and then extracted with 10% acetic acid for 15 min. The absorbance values were determined at 600 nm on a plate reader (Infinite M200, Tecan, Switzerland). For the inhibitory assays, cells were pretreated with different inhibitors for 30 min. The migration fold of the cells in each experiment was adjusted by the cell viability assay to correct for proliferation or cytotoxic effects of different chemical reagents treatment.
Intracellular cAMP Accumulation
The intracellular cAMP level was measured as described previously using the commercially available cAMP-Glo assay kit (38). Briefly, 5,000 U-251 cells were seeded in a 96-well plate with DMEM containing 10% FBS and incubated in 37 °C for 24 h. After removing the medium, 20 μl of treatment buffer (PBS containing 0.5 mm 3-isobutyl-1-methylxanthine and 0.1 mm Ro 20-1724, pH 7.4) with or without hHK-1, was added to the cells and incubated at 37 °C for 15 or 30 min. 20 μl/well of the cAMP-Glo Lysis buffer was added to the cells, and the buffer was shaken for 15 min at room temperature before being developed with the detection buffer and substrate supplied by the cAMP-Glo assay kit. Finally, luminescent signal was measured by a plate reader (Infinite M200, Tecan, Switzerland). The potent adenylate cyclase activator, forskolin, was used as a positive control.
Intracellular Calcium Release
U-251 cells were seeded in a 96-well plate at a density of 20,000/well and cultured for 24 h. The cells were rinsed three times with assay buffer (130 mm NaCl, 5 mm KCl, 10 mm HEPES, 8 mm d-glucose, 1.2 mm MgCl2, and 1.5 mm CaCl2, pH 7.4). The cells were then incubated with this buffer supplemented with the organic anion transport inhibitor probenecid (2.5 mm), 1 μm Fluo 4-AM, and 0.1% Pluronic F-127 for 60 min at 37 °C. Before the measurement, cells were rinsed three times with assay buffer and then placed in a FLEXstation II plate reader (Molecular Devices Corp., Palo Alto, CA) at 37 °C. The fluorescence emission at 525 nm following excitation at 480 nm was measured as hHK-1 was added. For inhibitory assays, cells were pretreated with different concentrations of the inhibitors for 30 min. The peak fluorescent value was used as an index of intracellular calcium release.
Whole Cell Lysate Preparations and Western Blotting Analysis
U251 cells were seeded in 12-well plates at a density of 250,000/well. At the end of cell treatment, the cells were lysed in RIPA lysis buffer containing protease inhibitor mixture and phosphatase inhibitor mixtures. The lysates were centrifuged at 15,000 × g for 10 min at 4 °C. The supernatants were collected and detected by BCA reagent to determine protein concentration. A total amount of 30 μg of protein from each sample was loaded and separated on a 10% SDS-polyacrylamide gel. After electrophoresis, the samples were transferred onto a PVDF membrane. The membranes were probed with the specific primary antibodies as indicated, followed by the incubation with horseradish peroxidase-conjugated secondary antibodies. The signal was detected by an enhanced chemiluminescence detection system and visualized by Kodak film (Eastman Kodak, Rochester, NY). The untreated cells were used as control in all experiments.
Measurements of Cell Viability
Cell viability was determined by the MTT assay. U251 cells were seeded in a 96-well plate at a density of 10,000/well. The cells were treated with various compounds for 24 h. After the incubation, MTT (0.5 mg/ml) was added for 4 h at 37 °C. The medium was removed, and the cells were dissolved in dimethyl sulfoxide and shaken for 15 min. The absorbance value at 570 nm was measured on a plate reader.
Luciferase Reporter Assays for NF-κB and AP-1 Activity
The luciferase reporter gene assay was measured as described previously (38, 43). Briefly, U-251 cells were seeded in a 60-mm dish to reach 80–90% confluence overnight. 8 μg of reporter plasmid pNF-κB-luc or pAP-1-luc plasmid of high purity was transfected into cells with Lipofectamine 2000 following the instructions of the manufacturer. 6 h later, the transfected cells were trypsinized and seeded in a 96-well plate at a density of 30,000 and grown for another 24 h with DMEM containing 10% FBS. Then the cells were exposed to 1 μm hHK-1 for 8 h at 37 °C. Untreated cells were used as control. For the inhibitory assays, the cells were pretreated with different concentrations of inhibitors for 30 min followed by the treatment of 1 μm hHK-1. Then the cells were lysed, and the luciferase activities were measured with the luciferase assay system on the plate reader. The untreated control was defined as 1.0. The luciferase activity was expressed as fold induction relative to untreated control.
Semi-quantitative Reverse Transcriptase-PCR
U-251 MG cells were lysed with RNAiso Plus, and total RNA was isolated per the manufacturer's instructions. 1 μg of total RNA was reverse-transcribed into cDNA by PrimeScript RT reagent according to the manufacturer. Levels of MMP-2, MT1-MMP, and β-actin mRNA were determined by PCR using oligonucleotide primers as follows: MMP-2, 5′-CCCACACTGGGCCCTGTCACT-3′, and 5′- TGGGCTTGTCACGTGGCGTC-3′; MT1-MMP, 5′-CGCTACGCCATCCAGGGTCTCAAA-3′, and 5′-CGGTCATCATCGGGCAGCACAAAA-3′; and β-actin, 5′- AGCGAGCATCCCCCAAAGTT-3′, and 5′- GGGCACGAAGGCTCATCATT-3′. For MT1-MMP, the PCR protocol was 30 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 2 min. For MMP-2 and β-actin, the PCR protocol was 30 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min. Amplified products were resolved by 2% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light.
Gelatin Zymography
U-251 MG cells were incubated with serum-free medium. Conditioned media were collected and concentrated. The samples were prepared in nondenaturating conditions in 5× Laemmli buffer without DTT. Samples were loaded in 10% SDS-polyacrylamide gel containing 1 mg/ml gelatin. The gels were rinsed three times in 2.5% Triton X-100 to remove SDS and then washed three times in the developing buffer (50 mm Tris-HCl, 0.2 m NaCl, 5 mm CaCl2, 0.02% Brij 35, pH 7.5). Subsequently, the gel was incubated in the developing buffer at 37 °C for 24 h. Gels were stained with 0.5% Coomassie Brilliant Blue and destained with double distilled H2O overnight; the clear zones within the blue background indicate proteinolytic activity.
Statistical Analysis
Curve-fitting and statistical analysis was conducted by use of GraphPad Prism 5.01 software (GraphPad Software Inc.). Statistical significance of the differences between more than two groups was calculated by one-way analysis of variance, followed by Tukey's post test.
RESULTS
hHK-1-induced Glioma Cell Migration via NK1R
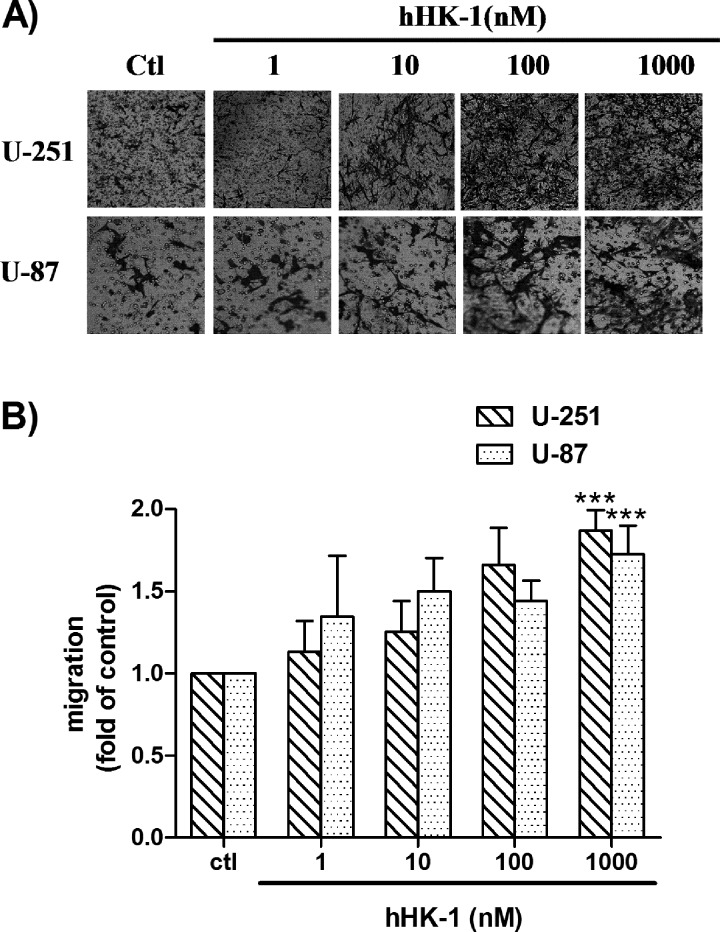
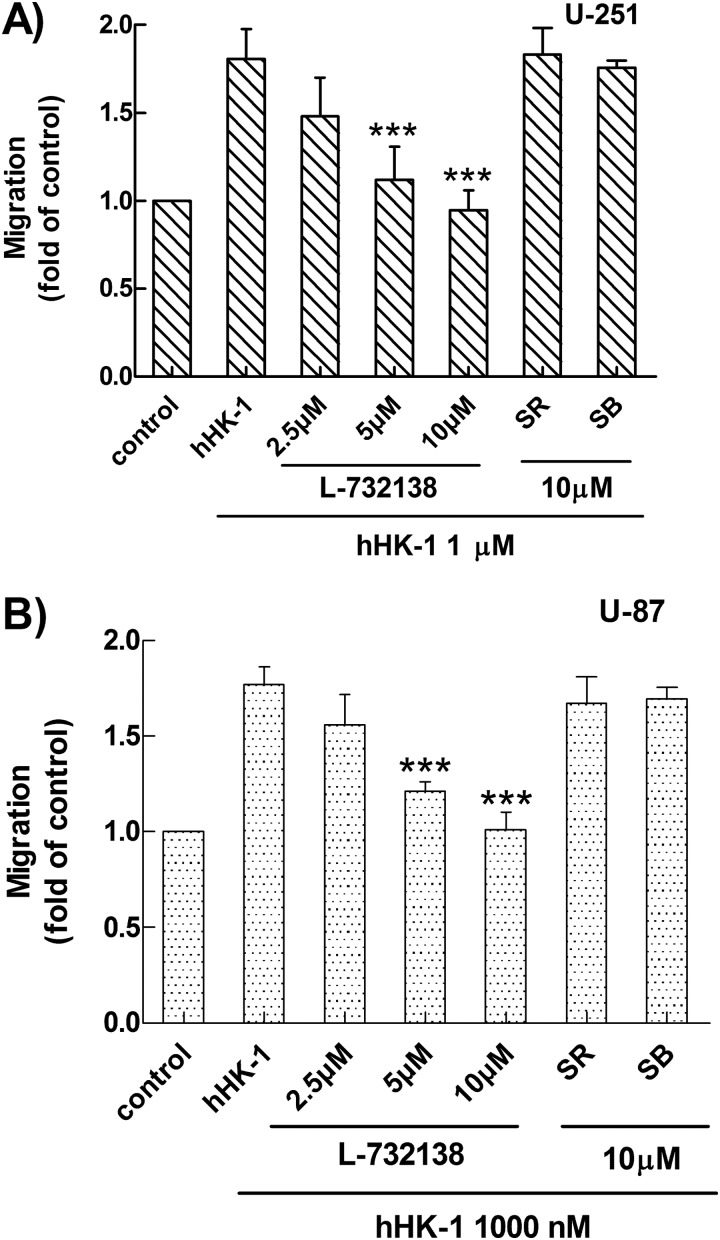
In this study, a transwell assay was used to evaluate NK1R-mediated glioma cell migration. hHK-1 caused dose-dependent cell migration in human glioma cell U-251 and U-87 (Fig. 1, A and B).Although hHK-1 was a highly selective agonist for the NK1 receptor, many studies showed that hHK-1 was also functional for the NK2 and NK3 receptor (48, 49). To further confirm the NK receptors involved in the glioma cell migration, we used the selective antagonists L-732138 (NK1, 2.5–10 μm), SR48968 (NK2, 10 μm), and SB22200 (NK3, 10 μm). hHK-1-induced cell migration of U-251 was abolished by the pretreatment of L-732138 at different concentrations but was not affected by pretreatment of SR48968 and SB22200 (Fig. 2A). These antagonists showed similar effects in U-87 cells (Fig. 2B). The increase in the amount of migrating cells was corrected by cell viability data from MTT assays. These data suggested that hHK-1 induced U-251 and U-87 cell migration via NK1R.
FIGURE 1.
hHK-1 induced dose-dependent migration of U-251 and U-87 as determined by a transwell assay after 24 h. A, migrant cells at the bottom side of the filter were stained with crystal violet and photographed under a phase contrast microscope. B, extraction of stained cells was measured at 600 nm. Using untreated cells as control (Ctl), data were presented as the means ± S.E. for five independent experiments. ***, p < 0.001 versus untreated controls.
FIGURE 2.
Selective antagonists (L-732138 for NK1, SR48968 for NK2, and SB22200 for NK3 receptor) were used to identify the receptor subtype responsible for the observed U-251 cell migration. A, effect of L-732138, SR48968, and SB22200 on the cell migration of U-251 induced by hHK-1. B, effect of L-732138, SR48968, and SB22200 on the cell migration of U-87 induced by hHK-1. The extraction of stained cells was measured at 600 nm. Using untreated cells as control, data were presented as the means ± S.E. for five independent experiments. ***, p < 0.001 versus hHK-1 controls.
hHK-1 Up-regulated the Activity of MMP-2 and Increased the Expression of MMP-2 and MT1-MMP in U-251 MG Cells
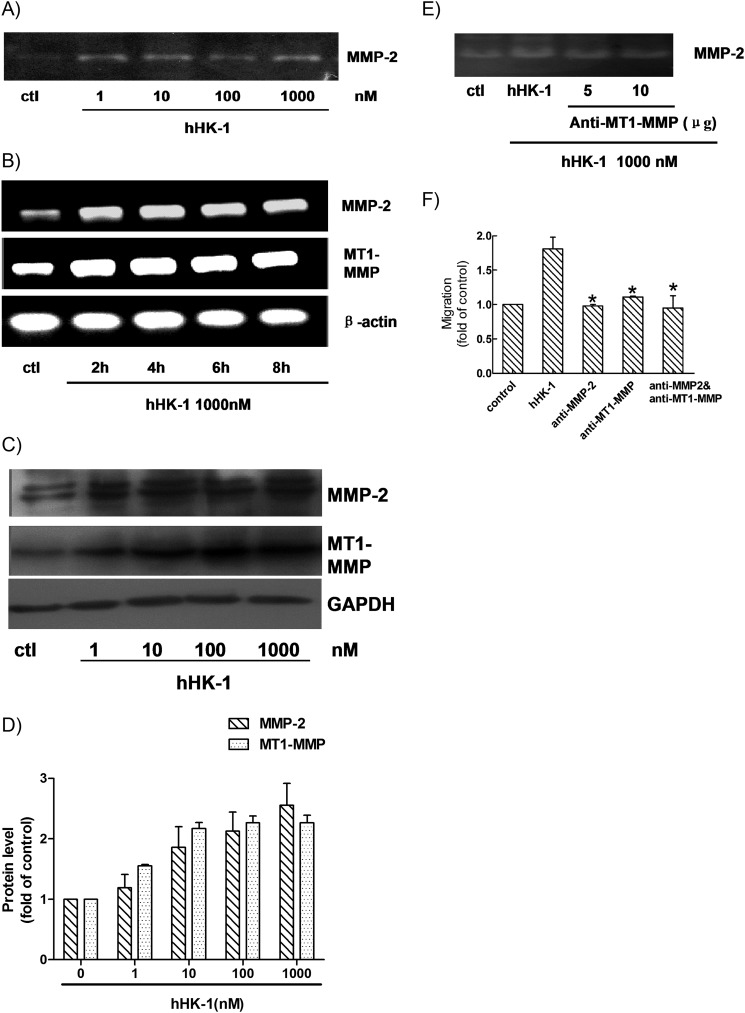
The migration and tumor grade of glioma cells are strongly associated with the expression and activity of the MMP family (32–35). To investigate the possible mechanism involved in NK1R-mediated glioma cell migration, we used gelatin zymography to analyze the MMPs activities in the supernatant of U-251 cultures. As shown in Fig. 3A, treatment with hHK-1 for 24 h significantly enhanced MMP-2 secretion, suggesting that the augmentation of MMP-2 activity possibly played a key role in the hHK-1-induced cell migration. To test this hypothesis, we examined MMP-2 expression level in U-251 cells. MT1-MMP was reported to play a key role in processing pro-MMP-2 into active MMP-2. We also examined the expression level of MT1-MMP in the same samples. Semi-quantitative RT-PCR analysis showed that the RNA levels of both MMP-2 and MT1-MMP were significantly augmented when U-251 cells were treated with hHK-1 for 2 h, and this effect lasted for at least 8 h (Fig. 3B). Western blotting analysis showed that hHK-1 markedly increased the protein level of MMP-2 and MT1-MMP compared with the untreated control (Fig. 3, C and D).
FIGURE 3.
Up-regulation of MMP-2 and MT1-MMP induced by hHK-1. A, U-251 cells were stimulated with hHK-1 (1–1000 nm) for 24 h. Then the conditioned medium was collected for gelatin zymography analysis to detect the secretion of MMP-2. B, semi-quantitative RT-PCR analysis of total RNA from U-251 MG cells treated with hHK-1 (1000 nm) for the indicated time points to detect the mRNA level of MMP-2 and MT1-MMP. C, Western blotting analysis of whole cell lysates from U-251 MG treated with hHK-1 at different concentrations for 24 h to detect the protein level of MMP-2 and MT1-MMP. D, densitometric analysis of MMP-2 and MT1-MMP protein expression relative to GAPDH. Results are means ± S.E. for three independent experiments. E, antibody against MT1-MMP was capable of inhibiting MMP-2 secretion detected by gelatin zymography. F, antibody for MMP-2 and/or MT1-MMP was capable of inhibiting the migration activity induced by hHK-1. Using untreated cells as control, data were presented as the means ± S.E. for five independent experiments. *, p < 0.001 versus hHK-1 controls.
To confirm the role of MT1-MMP in the procession of pro-MMP-2 in U-251 cells, U-251 cells were preincubated with anti-MT1-MMP antibody followed by hHK-1 treatment. Gelatin zymography analysis showed that the activity of MMP-2 was reduced (Fig. 3E). To further ensure the roles of MMP-2 and MT1-MMP in hHK-1-induced U-251 cell migration, we preincubated the cells with antibodies neutralizing MMP-2 and/or MT1-MMP (6 μg, respectively). Such pretreatments significantly inhibited the migration of U-251 cells induced by hHK-1 (Fig. 3F). These data suggested that U-251 cell migration induced by hHK-1 may at least partly be mediated by MMP-2 and MT1-MMP.
NK1R Led to U-251 Cell Migration by Activating Gq but Not by Gs Protein
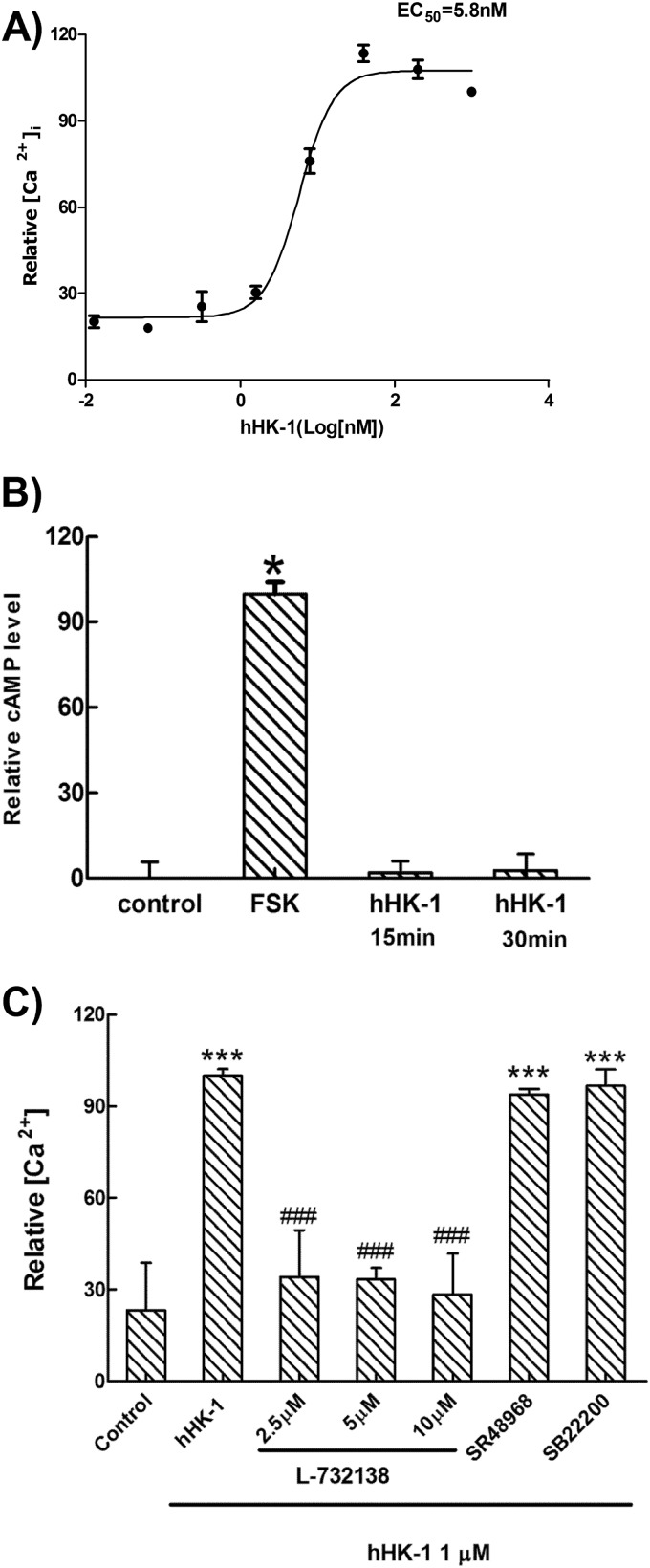
NK1R couples to two distinct signaling pathways as follow: a Gs pathway that activates adenylate cyclase inducing intracellular cAMP accumulation, and a Gq pathway that activates PLC initiating inositol phosphate formation and intracellular calcium release (39). To elucidate the molecular mechanism of NK1R-mediated U-251 cell migration, we monitored both Gs and Gq activation induced by hHK-1. Fig. 4A shows that hHK-1 promoted significant intracellular calcium release with an EC50 value of 5.8 nm. However, hHK-1 had almost no influence on the intracellular cAMP level compared with forskolin, the activator of adenylate cyclase (Fig. 4B). We also examined the effect of H89 on ERKs phosphorylation induced by hHK-1 in Western blotting analysis, indicating that the inhibition of H89 on PKA did not decrease the phosphorylation of ERKs (data not shown). hHK-1-induced intracellular calcium release was blocked by L-732138 but not by SR48968 or SB22200 (Fig. 4C).
FIGURE 4.
hHK-1 induced intracellular calcium mobilization but had no effect on cAMP levels in U-251 MG cells. A, dose-response curve for hHK-1 induced intracellular calcium release in U-251 MG cells (EC50 = 5.8 nm). B, hHK-1 almost had no effect on the intracellular cAMP levels compared with forskolin. C, antagonist of NK1 receptor L-732138 was able to block the Ca2+ signaling induced by hHK-1. The antagonist for NK2 (SR48968) and NK3 (SB22200) had no effect on the Ca2+ signaling. All data were presented as the means ± S.E. for three independent experiments. ***, p < 0.001 versus untreated controls; ###, p < 0.001 versus hHK-1-treated cells.
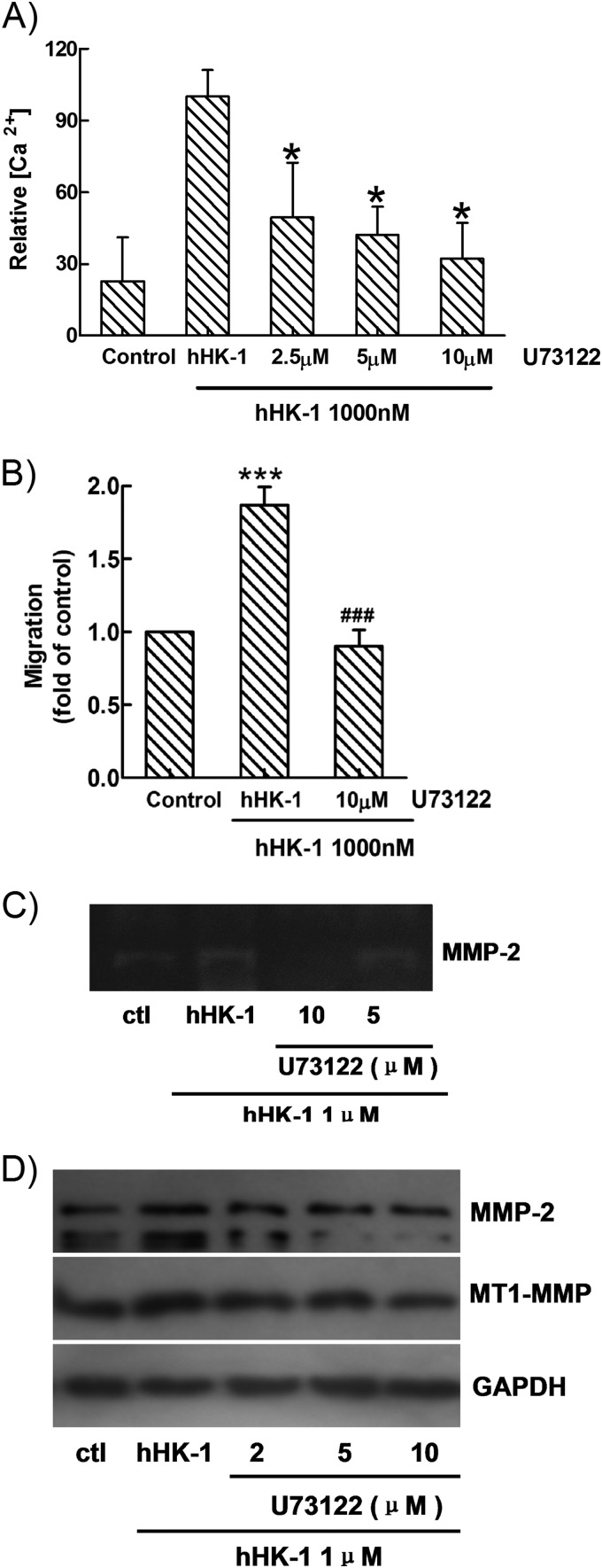
Pretreatment of U-73122, the inhibitor of PLC, showed significant inhibition of calcium mobilization (Fig. 5A) as well as the cell migration induced by hHK-1 (Fig. 5B). Then U-251 cells were pretreated with U-73122 following hHK-1 administration; we found that the activity of MMP-2 in the supernatant was significantly inhibited (Fig. 5C), and the protein levels of both MMP-2 and MT1-MMP were dose-dependently reduced (Fig. 5D). These results suggested that in U-251 cells, interaction of hHK-1-NK1R mainly activated the Gq protein-mediated PLC pathway, which was responsible for the up-regulation of MMP-2 and MT1-MMP and the cell migration induced by hHK-1.
FIGURE 5.
U-73122 significantly inhibited. A, Ca2+ signaling induced by hHK-1. B, migration of U-251 cells induced by hHK-1. C, MMP-2 activity induced by hHK-1. D, protein expression of MMP-2 and MT1-MMP induced by hHK-1. All data were presented as the means ± S.E. for three independent experiments. *, p < 0.001 versus untreated controls (ctl); ###, p < 0.001 versus hHK-1-treated cells.
Involvement of ERK, JNK, p38, and Akt Signaling Pathways in hHK-1-induced MMP Up-regulation
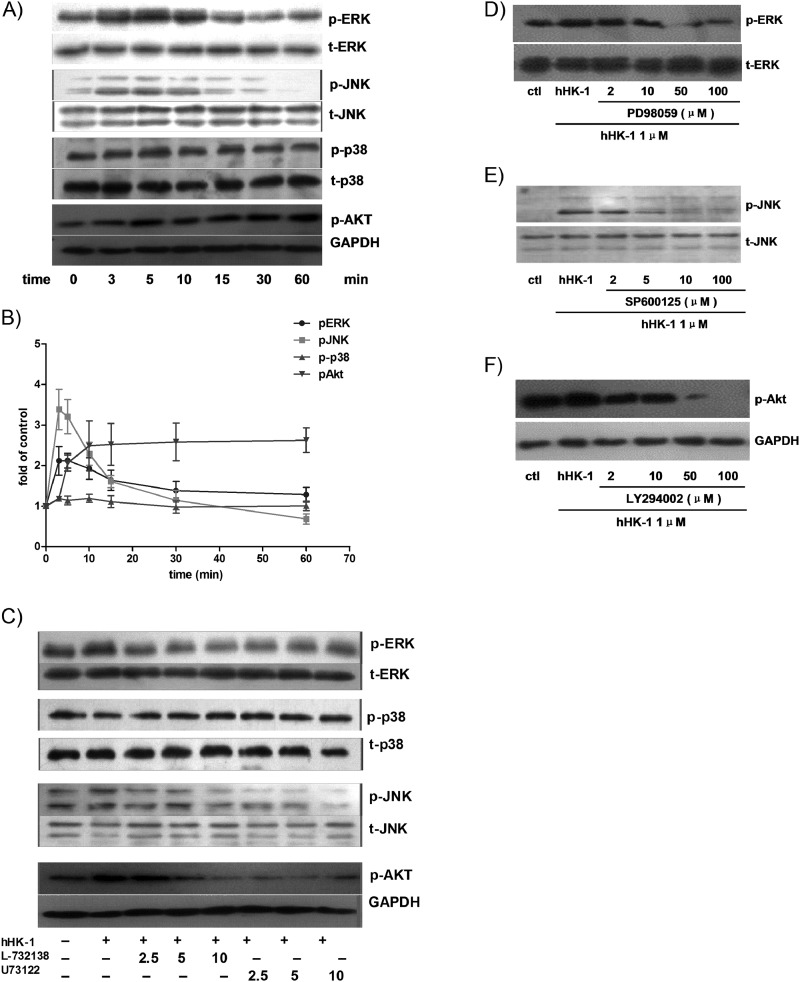
To elucidate in detail the signaling mechanisms involved in MMP up-regulation induced by hHK-1, we next examined the effect of hHK-1 on MAPKs and Akt signaling in U-251 cells. As shown in Fig. 6, A and B, treatment of the cells with hHK-1 significantly boosted ERK, JNK, and Akt phosphorylation in a time-dependent manner but induced almost no change in the phosphorylation of p38. In consistency with the results of the intracellular calcium release assays, L-732138 and U-73122 blocked the phosphorylation of ERK, JNK, and Akt while having little effect on phosphorylated-p38 (Fig. 6C).
FIGURE 6.
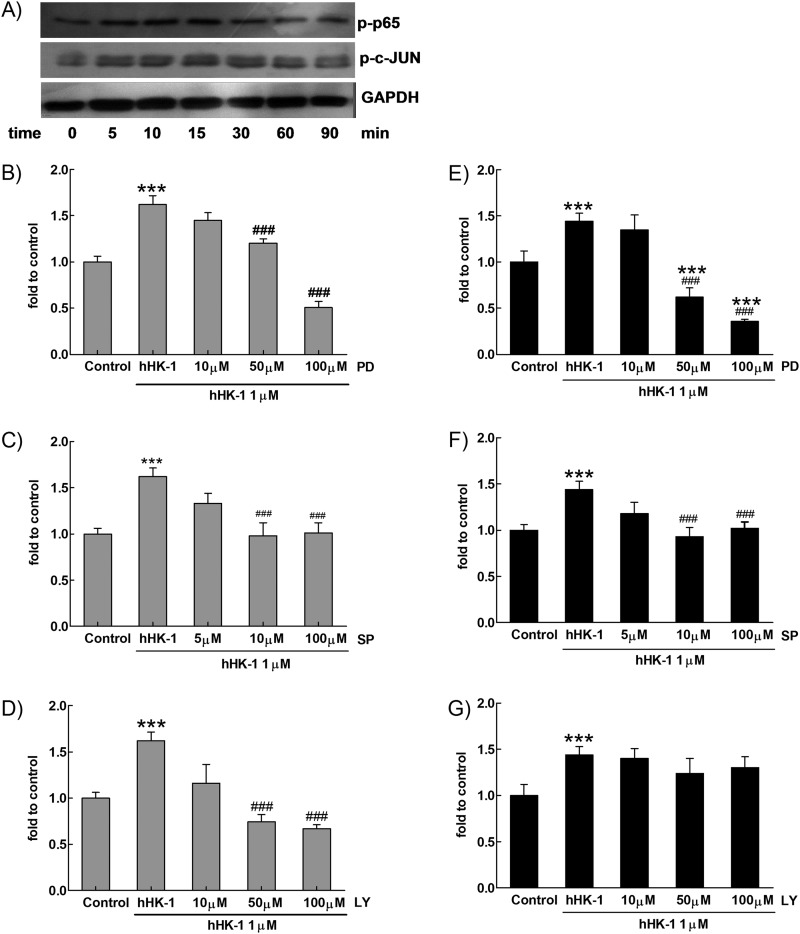
Effect of hHK-1 on the phosphorylation of ERK, JNK, p38, and Akt in U-251. A, hHK-1 stimulated time-dependent phosphorylation of ERK, JNK, and Akt in U-251 but had no influence on p38 phosphorylation. B, densitometric analysis of phosphorylated ERK relative to ERK, phosphorylated JNK relative to JNK, phosphorylated p38 relative to p38, and phosphorylated Akt relative to GAPDH. Results are means ± S.E. for three independent experiments. C, pretreatment of U251 with L-732138 or U-73122 for 30 min dose-dependently blocked the phosphorylation of ERK, JNK, and Akt, but the phosphorylation of p38 was not affected. D, PD98059 dose-dependently inhibited the phosphorylation of ERK. E, SP600125 dose-dependently inhibited the phosphorylation of JNK. F, LY294002 dose-dependently inhibited the phosphorylation of Akt. ctl, control.
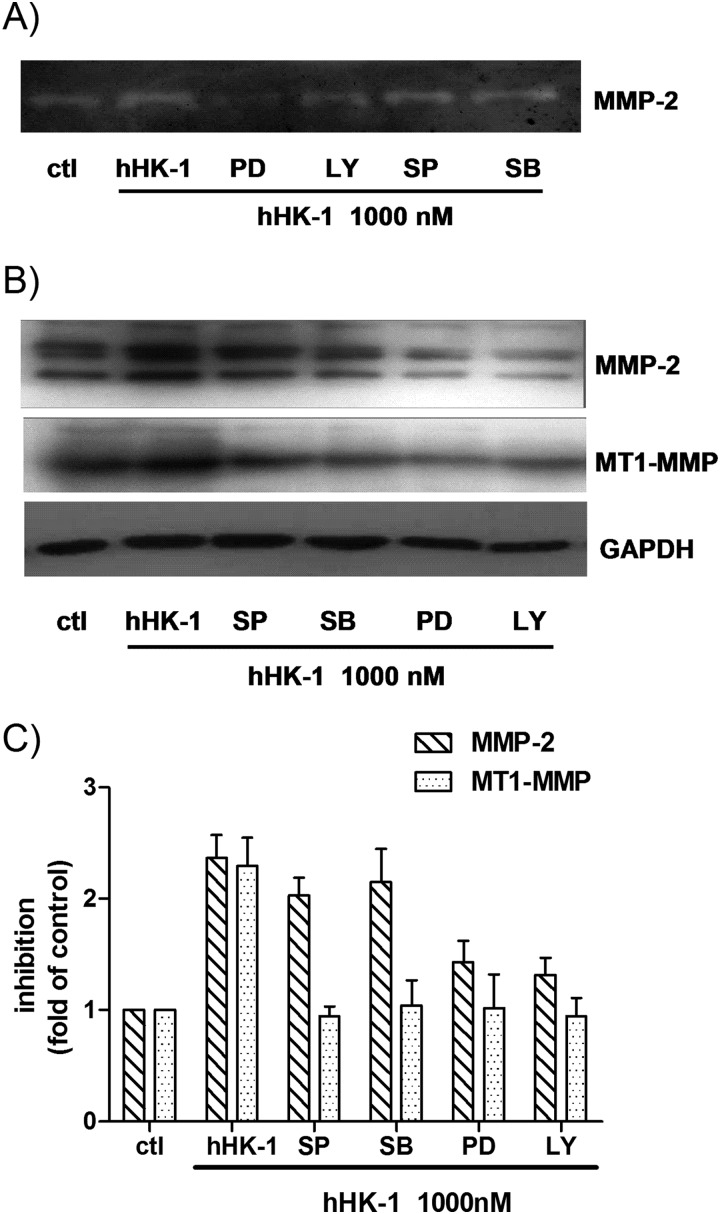
To further determine the involvement of kinase phosphorylation in the up-regulation of MMP-2 and MT1-MMP, the cells were treated with hHK-1 in the presence or absence of the specific inhibitors of ERK (PD98059), JNK (SP600125), p38 (SB203580), and phosphatidylinositol-3 kinase (PI3K, LY294002). These chemicals inhibited the kinase activity dose-dependently (Fig. 6, D–F). Gelatin zymography showed that PD98059 and LY294002 strongly inhibited the activity of MMP-2 induced by hHK-1, whereas SP600125 and SB203580 had a slightly inhibitory effect (Fig. 7A). Western blot analysis result showed similar inhibitory effects on MMP-2 expression level. However, all the inhibitors significantly blocked the increase of MT1-MMP by hHK-1 (Fig. 7, B and C). The above data suggested the following: 1) NK1R, activated by hHK-1, induced multiple signaling pathways, including ERK, JNK, and Akt phosphorylation; 2) these kinases were involved in regulating the level of MMP-2 and MT1-MMP. Although hHK-1 had no effect on p38 phosphorylation, the inhibition of basal p38 activity in U-251 did show substantial blocking effect on MT1-MMP expression.
FIGURE 7.
hHK-1-elicited up-regulation of MMP-2, and MT1-MMP was regulated by the phosphorylation of ERK, JNK, p38, and Akt. U-251 cells were pretreated with different kinase inhibitors for 30 min before stimulation with 1000 nm hHK-1. The following abbreviations are used: SP600125 (SP, 10 μm), SB203580 (SB, 10 μm), PD98059 (PD, 100 μm), and LY294002 (LY, 100 μm); control (ctl). 24 h later, the conditioned media were collected, and the cells were lysed. A, gelatin zymography analysis of conditioned medium from U-251 cells to detect the secretion of MMP-2. B, Western blotting analysis of whole cell lysates of U-251 MG to detect the protein level of MMP-2 and MT1-MMP. C, densitometric analysis of MMP-2 and MT1-MMP protein expression relative to GAPDH. Results are means ± S.E. for three independent experiments.
Involvement of NF-κB and AP-1 Activity in hHK-1 Induced MMP Up-regulation
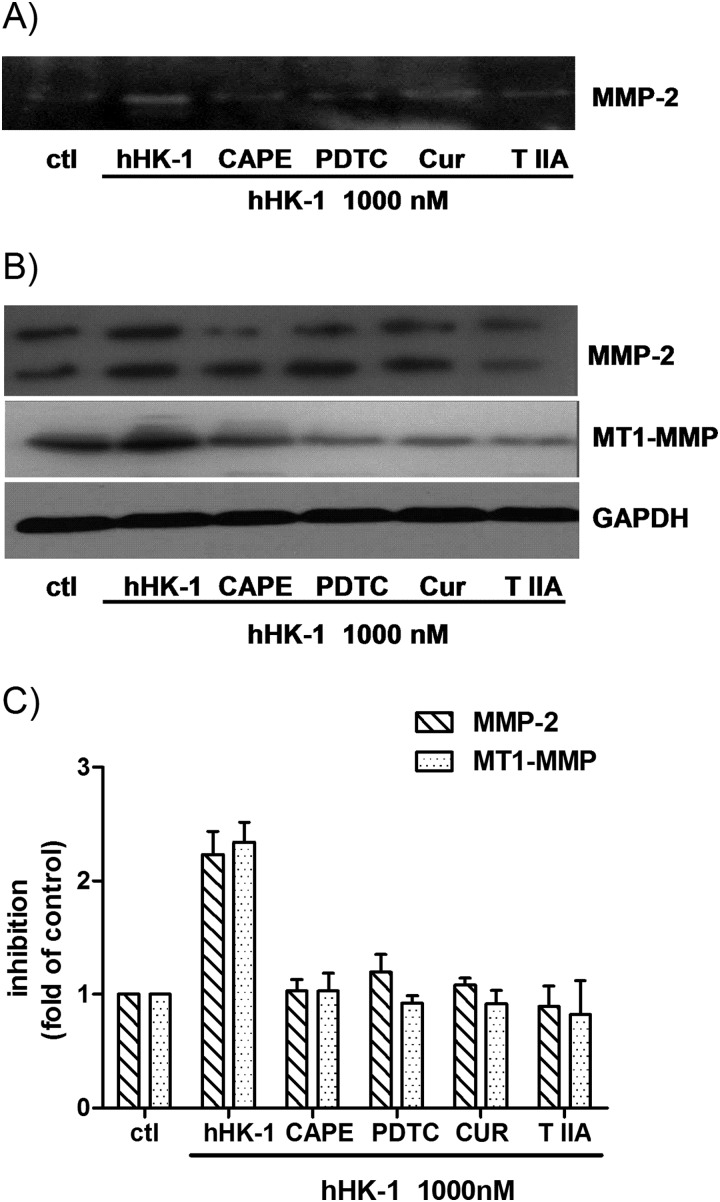
It has been reported that several transcriptional factors, including NF-κB and AP-1, were correlated with the expression of MMP-2 and MT1-MMP (18, 19). To determine whether the transcriptional factors were involved in hHK-1-induced MMP expression, we tested the effect of different inhibitors on the event, including PDTC and CAPE, the inhibitors of NF-κB, curcumin, and tanshinone IIA, the inhibitors of AP-1. As shown in Fig. 8A, gelatin zymography results indicated that pretreatment of the cells with PDTC, CAPE, curcumin, or tanshinone IIA significantly reduced the secretion of MMP-2 by hHK-1. In Western blotting analysis, these inhibitors subdued the protein level of both MMP-2 and MT1-MMP by hHK-1 (Fig. 8, B and C). These results suggested that the induction of MMP-2 and MT1-MMP expression by hHK-1 was regulated by both NF-κB and AP-1 transcriptional activities.
FIGURE 8.
Transcription factors AP-1 and NF-κB were involved in the induction of MMP-2 and MT1-MMP caused by hHK-1. U-251 cells were pretreated with different inhibitors for 30 min before stimulation with 1000 nm hHK-1 as follows: CAPE (25 μm), PDTC (100 μm), curcumin (Cur, 25 μm), and tanshinone IIA (T IIA, 25 μm). 24 h later, the conditioned media were collected, and the cells were lysed. A, gelatin zymography analysis of conditioned medium from U-251 cells to detect the secretion of MMP-2. B, Western blotting analysis of whole cell lysates of U-251 MG to detect the protein level of MMP-2 and MT1-MMP. C, densitometric analysis of MMP-2 and MT1-MMP protein expression relative to GAPDH. Results are means ± S.E. for three independent experiments.
We next examined the effect of hHK-1 on p65 and c-JUN phosphorylation in U-251 cells, showing that hHK-1 induced both p65 and c-JUN phosphorylation time-dependently (Fig. 9A). To quantitatively determine the activation of NF-κB and AP-1, a reporter gene assay was used. Because the up-regulation of MMP-2 and MT1-MMP induced by hHK-1 was blocked by the inhibitors of ERK1/2, JNK, and Akt, respectively (Fig. 7, A–C), we also examined the effect of the kinase inhibitors on the NF-κB and AP-1 activity. hHK-1 significantly enhanced the NF-κB-driven luciferase gene expression. PD98059 and LY294002 strongly blocked the NF-κB-driven luciferase activity induced by hHK-1 in a dose-dependent manner (Fig. 9, B and D). SP600125 also had significant inhibitory effect at a higher concentration (100 μm) in the transfected U-251 cells (Fig. 9C). However, only PD98059 strongly reduced the AP-1-driven luciferase activity induced by hHK-1 (Fig. 9E). SP600125 (1–100 μm) modestly reduced the AP-1-driven luciferase activity (Fig. 9F), whereas LY294002 (10–100 μm) had no effect on the AP-1 activity even at a concentration of 100 μm (Fig. 9G). These results showed the following: 1) hHK-1 induced both NF-κB and AP-1 transcriptional activities in U-251 cells; 2) activities of NF-κB and AP-1 were differentially regulated by the phosphorylation of upstream ERK, JNK, and Akt.
FIGURE 9.
Activation of ERK, JNK, and Akt regulated hHK-1-elicited activity of NF-κB. A, hHK-1 induced time-dependent phosphorylation of p65 and c-JUN determined by Western blotting. B–D, U-251 cells transfected by p-NF-κB-luciferase were pretreated with or without different kinases inhibitors for 30 min before stimulating with 1000 nm hHK-1. The activity of NF-κB-driven luciferase was detected in U-251 cells pretreated with PD98059 (10–100 μm) (B), SP600125 (5–100 μm) (C), or LY294002 (10–100 μm) (D). E–G, U-251 cells transfected by p-AP1-luciferase were pretreated with or without different kinase inhibitors for 30 min before stimulated with 1000 nm hHK-1. The activity of AP-1-driven luciferase was detected in U-251 cells pretreated with PD98059 (10–100 μm) (E), SP600125 (5–100 μm) (F), and LY294002 (10–100 μm) (G). ***, p < 0.001 versus untreated controls; ###, p < 0.001 versus hHK-1-treated cells.
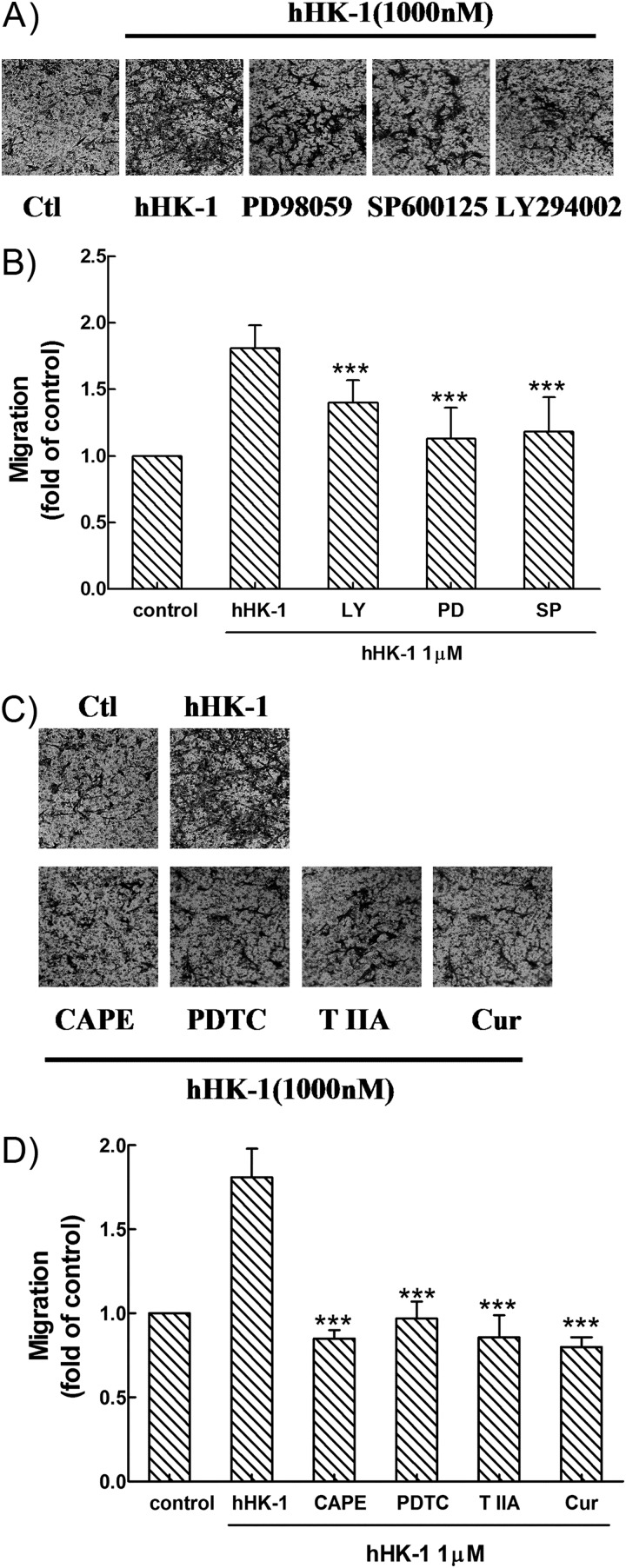
Involvement of hHK-1-induced Multiple Signaling Pathways in U-251 Cell Migration
As shown above, because multiple signaling pathways were involved in the induction of MMP-2 and MT1-MMP expression in U-251 MG cells by hHK-1, we next examined whether the inhibition of these pathways impaired the migration activity of the cells. In transwell assays, the treatment of PD98059, SP600125, and LY294002 suppressed the hHK-1-mediated migration of U-251 cells to a different extent (Fig. 10, A and B), suggesting that the activity of ERK, JNK, and Akt is required for the enhancement of cell migration. When U-251 cells were pretreated with PDTC, CAPE, curcumin, and tanshinone IIA, the migration activity of hHK-1 was strongly inhibited (Fig. 10, C and D), suggesting that the activation of both NF-κB and AP-1 played a key role in this event. These inhibitory effects on U-251 cell migration were corrected by the cytotoxicity data from MTT assays. The results indicated that hHK-1-induced U-251 cell migration was mediated by multiple signaling pathways, linking the activation of ERK, JNK, Akt, NF-κB, and AP-1.
FIGURE 10.
Effect of specific inhibitors for various kinases and transcription factors on U-251 cell migration induced by hHK-1 (1000 nm). Cells were pretreated for 30 min with PD98059 (100 μm) (A), SP600125 (10 μm) and LY294002 (100 μm) (B), and CAPE (25 μm), PDTC (100 μm), curcumin (25 μm), and tanshinone IIA (25 μm) in the presence or absence of hHK-1 (1000 nm) (C). B and D, extraction of stained cells was measured by a microplate reader at 600 nm. Using untreated cells as control, data were presented as the means ± S.E. for five independent experiments. ***, p < 0.001 versus hHK-1 controls. ctl, control.
DISCUSSION
NK1R has attracted great attention as a novel anti-tumor target because of its role in modulating cell proliferation and cytokine secretion (6–8). In addition, the receptor was suggested to be an important regulator of the motility of different cell types. The studies had been performed in immune cells and endothelial cells such as natural killer cells, peripheral blood leukocytes, human monocytes, and human umbilical vein endothelial cells (24–29). These features were important for the host-tumor interaction and the nourishment supply in tumor growth. However, whether NK1R activation directly influences the migration of tumor cells themselves has not yet been investigated. In this study, we sought to determine the involvement of NK1R in the migration of human glioma cells. Here, we first reported that the activation of NK1R by hHK-1 significantly increased the migration of human glioma cells U-251 and U-87 MG; the up-regulation of MMP-2 and MT1-MMP in U-251 cells induced by hHK-1 treatment was responsible for the enhancement of cell migration.
First of all, we determined the migration of U-251 and U-87 cells with a transwell assay, finding that hHK-1 induced dose-dependent cell migration in both cell lines (Fig. 1, A and B). hHK-1 was a functional agonist for the NK1, NK2, and NK3 receptor, showing higher affinity for NK1R. Our data support that the migration of U-251 and U-87 cells induced by hHK-1 was the result of NK1R activation because the selective antagonist of the NK1 receptor L-732138 inhibited both U-251 and U-87 cell migration, although SR48968 (NK2 antagonist) and SB22200 (NK3 antagonist) had no influence (Fig. 2, A and B). L-732138 also blocked the calcium mobilization (Fig. 4C) and downstream phosphorylation of ERK, JNK, and Akt induced by hHK-1 in U-251 cells (Fig. 6C). The evidence is consistent with the previous reports that functional NK1R was expressed in human glioma U-251 cells (also named U-373) (12, 13). They can be used as an appropriate model to study the biological functions of NK1R in human malignant gliomas.
The mechanisms of NK1R-mediated cell motility were poorly understood (24–29). To explore the possible machinery, we started with the analysis of MMP activities in the supernatants of U-251 cells. When the cells were treated with hHK-1, significant enhancement of MMP-2 activity was detected in the supernatants of U-251 culture by gelatin zymography (Fig. 3A); however, the activity of MMP-9 was barely visible. Aberrant activation of MMP-2 was potentially correlated with both glioma cell invasion and tumor grade. The activity of MMP-2 was delicately regulated at several levels, including gene expression, the activation of pro-enzyme, and the inhibition of active enzymes by its specific inhibitor (TIMP-2) (34–36). It has been reported that MT1-MMP acted as cell surface activator of pro-MMP2 (36, 37). Therefore, we examined the expression of both MMP-2 and MT1-MMP in U-251 cells. Compared with the untreated control, the parallel enhancement of MMP-2 and MT1-MMP expression was observed in hHK-1-treated U-251 MG cells at both the RNA and protein level (Fig. 3, B–D). To identify the role of MT1-MMP in MMP-2 release, we used anti-MT1-MMP antibody to block the effect of MT1-MMP, finding that the activity of MMP-2 was reduced I the U-251 cell culture supernatant treated by hHK-1 (Fig. 3E). The elevated MMP-2 and MT1-MMP productions were responsible for the cell migration induced by hHK-1, because antibody neutralizing MMP-2 or MT1-MMP was able to inhibit the U-251 cell migration (Fig. 3F). Taken together, our results indicated that the up-regulation of MMP-2 and MT1-MMP played a crucial role in U-251 cell migration mediated by NK1R.
We attempted to elucidate the intracellular signaling pathways that were involved in the up-regulation of the MMPs and the migration of U-251 MG cells initiated by the NK1-hHK-1 interaction. NK1R couples to different G proteins dependent on the cell types (38–40). Previously, we reported that hHK-1 differentially activated Gs and Gq signaling pathways in CHO cells expressing NK1 receptors (38). Here, we monitored the activation of both Gq and Gs protein by hHK-1 in U-251 cells. The peptide ligand triggered fast and significant intracellular calcium release mediated by the Gq protein (EC50 = 5.8 nm, see Fig. 4A), which was completely blocked by the PLC inhibitor U-73122 (Fig. 5A). However, there was no detectable change in the cAMP level induced by Gs activation (Fig. 4B). Then we examined the effect of U-73122 on U-251 cell migration and MMP up-regulation induced by hHK-1. The results indicated that U-73122 dose-dependently inhibited the up-regulation of MMP-2 and MT1-MMP and the migration of U-251 cells (Fig. 5, B–D). These data suggested that in U-251 cells the interaction of NK1-hHK-1 mainly activated the Gq-PLC pathway, which was responsible for the up-regulation of MMP-2 and MT1-MMP and the cell migration induced by hHK-1.
The events occurring after the stimulation of NK1R by hHK-1 are largely unresolved. Except for the intracellular calcium release, little is known about hHK-1 signaling, especially in glioma cells (12, 51, 52). Recently hHK-1, by binding NK1R, was demonstrated to rescue bone marrow-derived dendritic cells from apoptosis and promote dendritic cell survival via PI3K/Akt signaling (41). Wang et al. (42) showed that hHK-1 acted as a co-stimulatory factor for B cell activation possibly through synergistic activation of the MAPK pathway and induction of transcription factors critical for plasmacytic differentiation. In line with these studies, we examined the MAPK and Akt pathways induced by hHK-1 in U-251 cells. As shown in Fig. 6, A and B, hHK-1 potentially stimulated ERK and Akt phosphorylation in a manner of time dependence; hHK-1 also increased the phosphorylation of JNK, but the level was relatively low; however, hHK-1 had no influence on p38 phosphorylation. U-73122 markedly blocked the intracellular calcium release caused by hHK-1, indicating that PLC was effectively inactivated (Fig. 5A). The phosphorylation of ERK, JNK, and Akt was significantly reduced by U-73122, suggesting that these effects were mostly dependent on the classical Gq-PLC pathway (Fig. 5C).
The involvement of these pathways in hHK-1-induced U-251 cell migration was further investigated by the specific inhibitors of kinases. We found that PD98059 and LY294002, the specific inhibitors of ERK and Akt, respectively, strongly inhibited the up-regulation of both MMP-2 and MT1-MMP induced by hHK-1, although the specific inhibitor of JNK, SP600125, slightly inhibited the secretion and expression of MMP-2 but strongly inhibited the expression of MT1-MMP. It was noted that although hHK-1 had no significant effect on p38 phosphorylation in U-251 cells, its specific inhibitor SB203580 did show a faintly inhibitory effect on MMP-2 secretion and expression level and a significant inhibitory effect on MT1-MMP expression (Fig. 7, A–C). It was possibly related to the high basal level of phosphorylated p38 in U-251 cells (Fig. 6A). In the transwell migration assay, it was shown that the blockade of ERK, JNK, and Akt pathways exhibited significant suppression of U-251 cell migration mediated by hHK-1 (Fig. 10, A and B). Actually these kinase signalings had been correlated with the increased invasiveness of glioblastoma cells (49, 50). For example, the alteration of the IP3K/Akt pathway was reported to regulate the migration and invasion of human glioma cells LN229, T89G, and U-373 through the reduction of MMP-2 and MT1-MMP (43). NK1R activation was verified to induce MAPK and Akt activation, mediating cell proliferation and the anti-apoptosis effect in glioma cells and other tumor cells (15, 16, 21). In this study, we proved the following in U-251 cells: 1) that NK1R activation by hHK-1 stimulated intracellular calcium release and the phosphorylation of ERK, JNK, and Akt through the Gq-PLC pathway; 2) that activation of these kinases was related to the up-regulation of MMP-2 and MT1-MMP expression and the promotion of cell migration induced by hHK-1.
MMP-2 and MT1-MMP gene expressions are regulated at the transcription level, involving the activation of several transcription factors, including AP-1, Sp1, and NF-κB (36, 43–45). It has been evidenced that NK1R activation induced both NF-κB-dependent and AP-1-dependent cytokine and chemokine expression (18–20). Previously, we reported that hHK-1 stimulated significant NF-κB activity in CHO cells expressing the human NK1 receptor (38). Here, our results showed that in U-251 cells hHK-1 induced both p65 and c-JUN phosphorylation time-dependently (Fig. 9A). In U-251 cells transfected with the AP-1 or NF-κB reporter gene, hHK-1 significantly enhanced the luciferase gene expression driven by AP-1 or NF-κB (fold: ∼1.4 and ∼1.6, respectively, see Fig. 9, B and E). Using specific NF-κB inhibitors PDTC and CAPE, we found that the up-regulation of MMP-2 and MT1-MMP by hHK-1 was inhibited, and the inhibitory effect seemed more evident on MT1-MMP expression. Curcumin and tanshinone IIA, which are known as the inhibitors of AP-1, also showed a similar inhibitory pattern on hHK-1-induced MMP up-regulation (Fig. 8, A–C). All these inhibitors exhibited a strong effect of suppression on U-251 cell migration promoted by hHK-1 (Fig. 10, C and D). These results suggested that the activity of AP-1 and NF-κB induced by NK1R activation was important for the regulation of MMP-2 and MT1-MMP expression. They also reinforced that the MMP up-regulation was crucial in hHK-1-induced U-251 cell migration.
Next, we examined whether and how MAPKs and Akt signaling pathways were linked with the activation of AP-1 and NF-κB. The results showed that AP-1 and NF-κB were differentially activated by these kinases. In a dose-dependent manner, the NF-κB-driven luciferase activity was dramatically blocked by PD98059 and LY294002; SP600125 also had a significant but relatively lower inhibitory effect at higher concentrations (Fig. 9, B–D). However, the AP-1-driven luciferase activity was robustly reduced by only PD98059; SP600125 modestly reduced the AP-1-driven luciferase activity, and LY294002 had no effect on the AP-1 activity even at the concentration of 100 μm (Fig. 9, E–G). LY294002 only affected the activity of NF-κB; however, it also exhibited a significant inhibitory effect on hHK-1-induced up-regulation of MMP-2 and MT1-MMP (Fig. 6). This meant that there were possibly other transcription factors that were regulated by Akt activity and were involved in the control of the MMP expression induced by hHK-1. Further work is needed to elucidate the mechanism of NK1R-mediated MMPs expression.
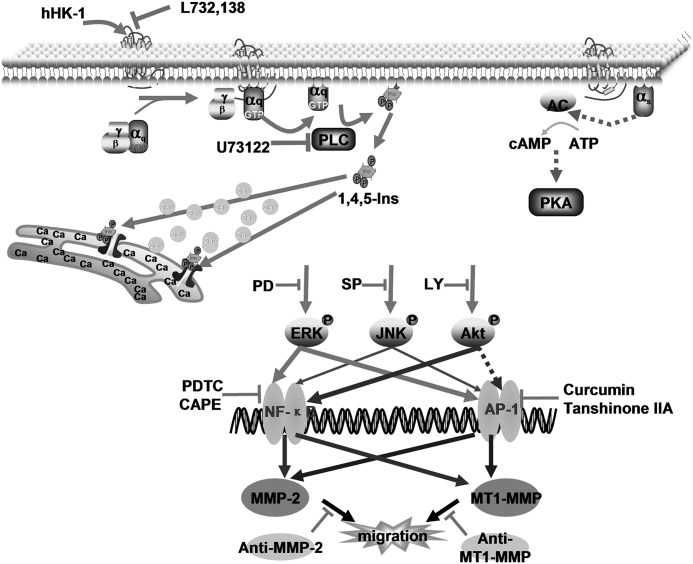
In summary, these results suggested that NK1R, activated by hHK-1, promoted the cell migration of human glioblastoma U-251 and U-87. The up-regulation of MMP-2 and MT1-MMP was responsible for the enhancement of U-251 cell migration. The molecular mechanism underlying hHK-1-induced MMP up-regulation and cell migration is complicated; by binding to NK1R, hHK-1 activated the Gq-PLC pathway, which in turn initiated intracellular calcium release, triggering a large array of signaling molecules, including ERK, JNK, and Akt, followed by AP-1 and NF-κB activation (Fig. 11). NK1R has been considered as a potential target for a new generation of antitumor drugs due to its role in tumor development, including cell proliferation, anti-apoptosis, and cytokine secretion (reviewed in Refs. 6, 7, 46). Our results provided evidence that NK1R was an important regulator of glioma cell migration. This afforded a new perspective for understanding the regulatory mechanisms of NK1R in tumor pathology.
FIGURE 11.
Schematic diagram of the signaling pathways involved in NK1R-mediated MMPs up-regulation and cell migration in U-251 MG cells. hHK-1 binds to NK1R inducing Gq-mediated intracellular calcium release but has no effect on cAMP level. Phosphorylation of ERK, JNK, and Akt enhances AP-1 and/or NF-κB activity, resulting in the up-regulation of MMP-2 and MT1-MMP and glioma cell migration, as demonstrated by specific inhibitors, respectively.
This study was supported by National Nature Science Foundation of China Grants 91213302, 20932003, and 21272102, Key National S & T Major Project and Major New Drugs Development of China Grant 2012ZX09504-001-003, and Fundamental Research Funds for the Central Universities Grant lzujbky-2012-117.
- NK1R
- neurokinin-1 receptor
- MMP
- matrix metalloproteinase
- PLC
- phospholipase C
- hHK-1
- human hemokinin-1
- PDTC
- pyrrolidinedithiocarbamic acid
- CAPE
- caffeic acid phenethyl ester
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Davis F. G., Freels S., Grutsch J., Barlas S., Brem S. (1998) Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type. An analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J. Neurosurg. 88, 1–10 [DOI] [PubMed] [Google Scholar]
- 2. Nakada M., Nakada S., Demuth T., Tran N. L., Hoelzinger D. B., Berens M. E. (2007) Molecular targets of glioma invasion. Cell. Mol. Life Sci. 64, 458–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stylli S. S., Kaye A. H., Lock P. (2008) Invadopodia. At the cutting edge of tumour invasion. J. Clin. Neurosci. 15, 725–737 [DOI] [PubMed] [Google Scholar]
- 4. Stupp R., Mason W. P., van den Bent M. J., Weller M., Fisher B., Taphoorn M. J., Belanger K., Brandes A. A., Marosi C., Bogdahn U., Curschmann J., Janzer R. C., Ludwin S. K., Gorlia T., Allgeier A., Lacombe D., Cairncross J. G., Eisenhauer E., Mirimanoff R. O., European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, and National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 [DOI] [PubMed] [Google Scholar]
- 5. Koul D., Shen R., Bergh S., Sheng X., Shishodia S., Lafortune T. A. (2006) Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol. Cancer Ther. 5, 637–644 [DOI] [PubMed] [Google Scholar]
- 6. Palma C. (2006) Tachykinins and their receptors in human malignancies. Curr. Drug Targets 7, 1043–1052 [DOI] [PubMed] [Google Scholar]
- 7. Muñoz M., Rosso M., Coveñas R. (2011) The NK-1 receptor. A new target in cancer therapy. Curr. Drug Targets 12, 909–921 [DOI] [PubMed] [Google Scholar]
- 8. Reubi J. C., Mäcke H. R., Krenning E. P. (2005) Candidates for peptide receptor radiotherapy. Today and in the future. J. Nucl. Med. 46, 67–75 [PubMed] [Google Scholar]
- 9. Page N. M. (2005) New challenges in the study of the mammalian tachykinins. Peptides 26, 1356–1368 [DOI] [PubMed] [Google Scholar]
- 10. Hennig I. M., Laissue J. A., Horisberger U., Reubi J. C. (1995) Substance-P receptors in human primary neoplasms: tumoral and vascular localization. Int. J. Cancer 61, 786–792 [DOI] [PubMed] [Google Scholar]
- 11. Łazarczyk M., Matyja E., Lipkowski A. (2007) Substance P and its receptors–a potential target for novel medicines in malignant brain tumour therapies (mini-review). Folia Neuropathol. 45, 99–107 [PubMed] [Google Scholar]
- 12. Berger A., Paige C. J. (2005) Hemokinin-1 has substance P-like function in U-251 MG astrocytoma cells. A pharmacological and functional study. J. Neuroimmunol. 164, 48–56 [DOI] [PubMed] [Google Scholar]
- 13. Palma C., Nardelli F., Manzini S., Maggi C. A. (1999) Substance P activates responses correlated with tumor growth in human glioma cell lines bearing tachykinin NK1 receptors. Br. J. Cancer 79, 236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogo H., Kuroyanagi N., Inoue A., Nishio H., Hirai Y., Akiyama M., DiMaggio D. A., Krause J. E., Nakata Y. (1996) Human astrocytoma cells (U-87 MG) exhibit a specific substance P-binding site with the characteristics of an NK-1 receptor. J. Neurochem. 67, 1813–1820 [DOI] [PubMed] [Google Scholar]
- 15. Luo W., Sharif T. R., Sharif M. (1996) Substance P-induced mitogenesis in human astrocytoma cells correlates with activation of the mitogen-activated protein kinase signaling pathway. Cancer Res. 56, 4983–4991 [PubMed] [Google Scholar]
- 16. Akazawa T., Kwatra S. G., Goldsmith L. E., Richardson M. D., Cox E. A., Sampson J. H., Kwatra M. M. (2009) A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J. Neurochem. 109, 1079–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitsuhashi M., Ohashi Y., Shichijo S., Christian C., Sudduth-Klinger J., Harrowe G., Payan D. G. (1992) Multiple intracellular signaling pathways of the neuropeptide substance P receptor. J. Neurosci. Res. 32, 437–443 [DOI] [PubMed] [Google Scholar]
- 18. Lieb K., Fiebich B. L., Berger M., Bauer J., Schulze-Osthoff K. (1997) The neuropeptide substance P activates transcription factor NF-κB and κB-dependent gene expression in human astrocytoma cells. J. Immunol. 159, 4952–4958 [PubMed] [Google Scholar]
- 19. Christian C., Gilbert M., Payan D. G. (1994) Stimulation of transcriptional regulatory activity by substance P. Neuroimmunomodulation 1, 159–164 [DOI] [PubMed] [Google Scholar]
- 20. Eistetter H. R., Mills A., Brewster R., Alouani S., Rambosson C., Kawashima E. (1992) Functional characterization of neurokinin-1 receptors on human U373MG astrocytoma cells. Glia 6, 89–95 [DOI] [PubMed] [Google Scholar]
- 21. Koon H. W., Zhao D., Zhan Y., Moyer M. P., Pothoulakis C. (2007) Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc. Natl. Acad. Sci. U.S.A. 104, 2013–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guha A., Mukherjee J. (2004) Advances in the biology of astrocytomas. Curr. Opin. Neurol. 17, 655–662 [DOI] [PubMed] [Google Scholar]
- 23. Furnari F. B., Fenton T., Bachoo R. M., Mukasa A., Stommel J. M., Stegh A., Hahn W. C., Ligon K. L., Louis D. N., Brennan C. (2007) Malignant astrocytic glioma. Genetics, biology, and paths to treatment. Gene Dev. 21, 2683–2710 [DOI] [PubMed] [Google Scholar]
- 24. Ziche M., Morbidelli L., Pacini M., Geppetti P., Alessandri G., Maggi C. A. (1990) Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 40, 264–278 [DOI] [PubMed] [Google Scholar]
- 25. Guha S., Eibl G., Kisfalvi K., Fan R. S., Burdick M., Reber H., Hines O. J., Strieter R., Rozengurt E. (2005) Broad-spectrum G protein-coupled receptor antagonist, [d-Arg1,d-Trp5,7,9,Leu11]SP. A dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 65, 2738–2745 [DOI] [PubMed] [Google Scholar]
- 26. Song H., Yin W., Zeng Q., Jia H., Lin L., Liu X., Mou L., Wang R. (2012) Hemokinins modulate endothelium function and promote angiogenesis through neurokinin-1 receptor. Int. J. Biochem. Cell B. 44, 1410–1421 [DOI] [PubMed] [Google Scholar]
- 27. Feistritzer C., Clausen J., Sturn D. H., Djanani A., Gunsilius E., Wiedermann C. J., Kähler C. M. (2003) Natural killer cell functions mediated by the neuropeptide substance P. Regul. Pept. 116, 119–126 [DOI] [PubMed] [Google Scholar]
- 28. Schratzberger P., Reinisch N., Prodinger W. M., Kähler C. M., Sitte B. A., Bellmann R., Fischer-Colbrie R., Winkler H., Wiedermann C. J. (1997) Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J. Immunol. 158, 3895–3901 [PubMed] [Google Scholar]
- 29. Ruff M. R., Wahl S. M., Pert C. B. (1985) Substance P receptor-mediated chemotaxis of human monocytes. Peptides 6, 107–111 [DOI] [PubMed] [Google Scholar]
- 30. Meshki J., Douglas S. D., Lai J.-P., Schwartz L., Kilpatrick L. E., Tuluc F. (2009) Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells through a Rho/Rho-associated coiled-coil kinase-dependent mechanism. J. Biol. Chem. 284, 9280–9289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meshki J., Douglas S. D., Hu M., Leeman S. E., Tuluc F. P. (2011) Substance P induces rapid and transient membrane blebbing in U373MG cells in a p21-activated kinase-dependent manner. PloS ONE 6, e25332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dzwonek J., Rylski M., Kaczmarek L. (2004) Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 567, 129–135 [DOI] [PubMed] [Google Scholar]
- 33. Yong V. W., Power C., Forsyth P., Edwards D. R. (2001) Metalloproteinases in biology and pathology of nervous system. Neuroscience 2, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao J. S. (2003) Molecular mechanisms of glioma invasiveness. The role of proteases. Nat. Rev. Cancer 3, 489–501 [DOI] [PubMed] [Google Scholar]
- 35. Vanmeter T. E., Rooprai H. K., Kibble M. M., Fillmore H. L., Broaddus W. C., Pilkington G. J. (2001) The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J. Neuro-oncol. 53, 213–235 [DOI] [PubMed] [Google Scholar]
- 36. Clark I. M., Swingler T. E., Sampieri C. L., Edwards D. R. (2008) The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 40, 1362–1378 [DOI] [PubMed] [Google Scholar]
- 37. Nishida Y., Miyamori H., Thompson E. W., Takino T., Endo Y., Sato H. (2008) Activation of matrix metalloproteinase-2 (MMP-2) by membrane type 1 matrix metalloproteinase through an artificial receptor for proMMP-2 generates active MMP-2. Cancer Res. 68, 9096–9104 [DOI] [PubMed] [Google Scholar]
- 38. Mou L., Xing Y., Kong Z., Zhou Y., Chen Z., Wang R. (2011) The N-terminal domain of human hemokinin-1 influences functional selectivity property for tachykinin receptor neurokinin-1. Biochem. Pharmacol. 81, 661–668 [DOI] [PubMed] [Google Scholar]
- 39. Nakajima Y., Tsuchida K., Negishi M., Ito S., Nakanishi S. (1992) Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J. Biol. Chem. 267, 2437–2442 [PubMed] [Google Scholar]
- 40. Sagan S., Chassaing G., Pradier L., Lavielle S. (1996) Tachykinin peptides affect differently the second messenger pathways after binding to CHO-expressed human NK-1 receptors. J. Pharmacol. Exp. Ther. 276, 1039–1048 [PubMed] [Google Scholar]
- 41. Janelsins B. M., Mathers A. R., Tkacheva O. A., Erdos G., Shufesky W. J., Morelli A. E., Larregina A. T. (2009) Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood 113, 3017–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang W., Li Q., Zhang J., Wu H., Yin Y., Ge Q., Zhang Y. (2010) Hemokinin-1 activates the MAPK pathway and enhances B cell proliferation and antibody production. J. Immunol. 184, 3590–3597 [DOI] [PubMed] [Google Scholar]
- 43. Kwiatkowska A., Kijewska M., Lipko M., Hibner U., Kaminska B. (2011) Down-regulation of Akt and FAK phosphorylation reduces invasion of glioblastoma cells by impairment of MT1-MMP shuttling to lamellipodia and down-regulates MMPs expression. Biochim. Biophys. Acta 1813, 665–667 [DOI] [PubMed] [Google Scholar]
- 44. Yan C., Boyd D. D. (2007) Regulation of matrix metalloproteinase gene expression. J. Cell Physiol. 211, 19–26 [DOI] [PubMed] [Google Scholar]
- 45. Lohi J., Lehti K., Valtanen H., Parks W. C., Keski-Oja J. (2000) Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene 242, 75–86 [DOI] [PubMed] [Google Scholar]
- 46. Reddy B. Y., Trzaska K. A., Murthy R. G., Navarro P., Rameshwar P. (2008) Neurokinin receptors as potential targets in breast cancer treatment. Curr. Drug Discov. Technol. 5, 15–19 [DOI] [PubMed] [Google Scholar]
- 47. Kurtz M. M., Wang R., Clements M. K., Cascieri M. A., Austin C. P., Cunningham B. R., Chicchi G. G., Liu Q. (2002) Identification, localization, and receptor characterization of novel mammalian substance P-like peptides. Gene 296, 205–212 [DOI] [PubMed] [Google Scholar]
- 48. Page N. M., Bell N. J., Gardiner S. M., Manyonda I. T., Brayley K. J., Strange P. G., Lowry P. J. (2003) Characterization of the endokinins. Human tachykinins with cardiovascular activity. Proc. Natl. Acad. Sci. U.S.A. 100, 6245–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Molina J. R., Hayashi Y., Stephens C., Georgescu M. M. (2010) Invasive glioblastoma cells acquire stemness and increased Akt activation 12. Neoplasia 12, 453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu D. Y., Leung Y. M., Cheung C. W., Chen Y. R., Wong K. L. (2010) Glial cell line-derived neurotrophic factor induces cell migration and matrix metalloproteinase-13 expression in glioma cells. Biochem. Pharmacol. 80, 1201–1209 [DOI] [PubMed] [Google Scholar]
- 51. Sakai A., Takasu K., Sawada M., Suzuki H. (2012) Hemokinin-1 gene expression is up-regulated in microglia activated by lipopolysaccharide through NF-κB and p38 MAPK signaling pathways. PLoS ONE 7, e32268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsumura T., Sakai A., Nagano M., Sawada M., Suzuki H., Umino M., Suzuki H. (2008) Increase in hemokinin-1 mRNA in the spinal cord during the early phase of a neuropathic pain state. Br. J. Pharmacol. 155, 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]