Background: TSG-6 is important in the organization of hyaluronan (HA).
Results: Lack of TSG-6 results in diminished HA accumulation, inflammation, and airway hyperresponsiveness.
Conclusion: TSG-6 is essential for the pathological manifestations in a murine model of asthma.
Significance: TSG-6 is likely to contribute to the pathogenesis of asthma.
Keywords: Asthma, Extracellular Matrix, Extracellular Matrix Proteins, Hyaluronate, Lung, Heavy Chains, TSG-6, Airway Hyperresponsiveness
Abstract
Hyaluronan (HA) deposition is often correlated with mucosal inflammatory responses, where HA mediates both protective and pathological responses. By modifying the HA matrix, Tnfip6 (TNF-α-induced protein-6; also known as TSG-6 (TNF-stimulated gene-6)) is thought to potentiate anti-inflammatory and anti-plasmin effects that are inhibitory to leukocyte extravasation. In this study, we examined the role of endogenous TSG-6 in the pathophysiological responses associated with acute allergic pulmonary inflammation. Compared with wild-type littermate controls, TSG-6−/− mice exhibited attenuated inflammation marked by a significant decrease in pulmonary HA concentrations measured in the bronchoalveolar lavage and lung tissue. Interestingly, despite the equivalent induction of both humoral and cellular Th2 immunity and the comparable levels of cytokines and chemokines typically associated with eosinophilic pulmonary inflammation, airway eosinophilia was significantly decreased in TSG-6−/− mice. Most importantly, contrary to their counterpart wild-type littermates, TSG-6−/− mice were resistant to the induction of airway hyperresponsiveness and manifested improved lung mechanics in response to methacholine challenge. Our study demonstrates that endogenous TSG-6 is dispensable for the induction of Th2 immunity but is essential for the robust increase in pulmonary HA deposition, propagation of acute eosinophilic pulmonary inflammation, and development of airway hyperresponsiveness. Thus, TSG-6 is implicated in the experimental murine model of allergic pulmonary inflammation and is likely to contribute to the pathogenesis of asthma.
Introduction
Eosinophilic pulmonary inflammation is often associated with allergic asthma, where it triggers airway hyperresponsiveness (AHR)3 that is clinically manifested as recurrent episodes of dyspnea, wheezing, and coughing. Central to allergic pathology are cellular Th2 (T helper type 2) responses that are hallmarked by infiltration of CD4+ Th2 cells, natural killer T cells, neutrophils, eosinophils, and mast cells, as well as morphological changes due to goblet cell hyperplasia, smooth muscle hypertrophy/hyperplasia, subepithelial fibrosis, angiogenesis, and extracellular matrix (ECM) deposition (1–4). ECM modifications during inflammatory responses have been shown to influence the distribution, activation, survival, and adhesion of inflammatory cells, and the ECM can act as a reservoir for inflammatory mediators and growth factors (5, 6). This is of particular relevance in the asthmatic airways, in which the profile of the ECM is altered (7, 8).
One of the major components of the ECM is hyaluronan (HA). HA is a nonsulfated glycosaminoglycan (GAG) that has been implicated in both physiological and pathophysiological tissue responses, including ovulation and fertilization, embryonic development, tissue fluid homeostasis, inflammation, tissue repair and remodeling, and tumor progression and metastasis (9–16). The mechanisms by which HA mediates many of these biological responses is complex and context-dependent and involves HA deposition and degradation, as well as HA modifications (9, 17, 18). This was reported in the context of pulmonary biology, where macromolecular HA mediates protective pulmonary responses, whereas HA degradation products lead to pathological consequences (9, 14, 19). Although clinical studies have long suggested a direct correlation between HA content of bronchoalveolar lavage (BAL) and severity of asthmatic responses (20–22), the molecular mechanism by which HA impacts pulmonary biology and pathology remains to be further defined.
Tnfip6 (TNF-α-induced protein-6; also known as TSG-6 (TNF-stimulated gene-6)) is a member of the HA-binding protein or hyaladherin family. TSG-6 is a 35-kDa secreted protein that is involved in both physiological and pathophysiological tissue responses. In addition to HA (23–28), TSG-6 has been shown to interact with a wide range of ECM components such as the GAGs (29), chondroitin 4-sulfate (25), and heparan sulfate and heparin (30). TSG-6 hyaladherin properties are endowed by virtue of the link module (24) and have been shown to be important for mediating HA interaction with CD44, which is also a link module-containing hyaladherin (29, 31). In addition, TSG-6 interacts with the inter-α-inhibitor (IαI), a proteoglycan composed of the trypsin inhibitor bikunin with heavy chain 1 (HC1) and heavy chain 2 (HC2) covalently bound by GalNAc ester linkages on the chondroitin 4-sulfate GAG chain. TSG-6 catalyzes the transfer of HC1 and HC2 from the chondroitin 4-sulfate chain to GlcNAc residues on HA in a HC2-dependent manner (32). The lack of this transesterification of HCs destabilizes the HA matrix of the cumulus cell-oocyte complexes and contributes to the infertility of female TSG-6−/− mice (33–35). In asthmatics, both TSG-6 mRNA expression and TSG-6-HC complexes have been shown to increase after allergen challenge (36, 37), suggesting that TSG-6 may play an important role in the asthmatic airway. Nevertheless, how TSG-6 contributes to the pathogenesis of inflammatory responses in asthma remains to be defined. In this study, we examined the role of endogenous TSG-6 during the induction and propagation of pathophysiological responses associated with a murine ovalbumin (OVA) model of allergic pulmonary inflammation and AHR in TSG-6−/− mice and wild-type littermates.
EXPERIMENTAL PROCEDURES
Mice
All mice were maintained in the Biological Resource Unit of the Cleveland Clinic Lerner Research Institute in a temperature-controlled facility with an automatic 12-h light-dark cycle and were given free access to food and water. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. TSG-6−/− mice (35) were a generous gift from Dr. Tibor T. Glant (Rush University Medical Center). 6–8-Week-old female TSG-6−/− mice and wild-type littermate controls on a BALB/c background were used for OVA sensitization and challenge.
OVA Sensitization
Standard induction of allergic airway disease was done as described previously (38, 39). In brief, BALB/c mice were immunized with an intraperitoneal injection with 10 μg of chicken OVA (grade V, Sigma-Aldrich) adsorbed to 20 mg of Al(OH)3. 2 weeks later, mice were exposed to an aerosol of 1% OVA in PBS for 45 min using an Ultrasonic 2000 nebulizer (NOUVAG) for a total of 6 days. Control TSG-6−/− and age-matched wild-type littermates were immunized with 20 mg of aluminum hydroxide. Analysis was performed 24 h after the last OVA aerosol.
Collection of BAL
Mice were killed with an intraperitoneal injection of sodium pentobarbital. BAL was obtained by cannulating the trachea with a 24-gauge feeding needle and lavaging the lungs with 700 μl of PBS. The typical BAL fluid return was 400–600 μl. White blood cells were counted on a hemocytometer using ethidium bromide/acridine orange staining of nucleated cells. Differential counts were based on counts of 100 cells after staining with Hema 3 (Fisher Scientific) using standard morphologic criteria to classify the cells as eosinophils, lymphocytes, neutrophils, and macrophages. The remainder of each BAL sample was centrifuged at 1000 rpm for 5 min at 4 °C. Supernatants were transferred to a new tube for additional analysis.
AHR and Lung Mechanics
AHR and lung mechanics were measured in mice in response to increasing doses of inhaled methacholine as described (39). Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (40–50 mg/kg). Following the intubation and placement of a 19-gauge cannula through a tracheotomy incision, the cannula was affixed with a silk suture ligation. Mice were connected to a computer-controlled piston ventilator (flexiVent, SCIREQ, Montreal, Canada) run at a rate of 150 breaths/min, a tidal volume of 10 ml/kg, and a positive end-expiratory pressure of 3 cm of H2O. Mice were then administered a muscle relaxant (pancuronium bromide, 0.8 mg/kg), and the lungs were expanded twice to total lung capacity at an amplitude pressure of 30 cm of H2O. Each mouse was aerosolized with methacholine in saline at doses of 0, 2.5, 5, 10, and 25 mg/ml delivered over 10 s through an in-line nebulizer. flexiVent 5.2 software (SCIREQ) was utilized to obtain resistance measurements through multiple linear regression fitting of measured pressure and volume from each mouse to the model of linear motion of the lung. Coefficients of determination of <0.90 were excluded. Lung resistance and lung compliance were obtained through the forced oscillation technique, whereas tissue elastance (H) and dampening were obtained by a constant phase model.
Enzyme-linked Immunosorbent Spot Analyses
Single-cell suspensions were prepared from spleens from immunized mice and cultured using HL-1 medium (BioWhittaker, Walkersville, MD) supplemented with 1 mm l-glutamine and penicillin/streptomycin (Invitrogen). Purification was done by negative selection using EasySep mouse CD4+ T cell reagents (STEMCELL Technologies). CD4+ T cells (100 × 103) were cultured with 200 × 103 mitomycin C-treated stimulator cells (panned splenocytes from naïve mice) and incubated with 10 μg/ml OVA for 4 h at 37 °C and 5% CO2, followed by three washes with HL-1 medium in 96-well ImmunoSpot M200 plates (Cellular Technology Ltd., Shaker Heights, OH). The plates were previously coated with capture antibodies for IL-5 or IL-13 (4 μg/ml antibody in PBS overnight at 4 °C; eBioscience), blocked with 1% BSA (Sigma-Aldrich) in PBS, and washed three times with PBS and 0.5% Tween 20. After 48 h of incubation at 37 °C and 5% CO2, the plates were washed three times with PBS and 0.5% Tween 20, and biotinylated detection antibodies for IL-5 or IL-13 (eBioscience) in 1% BSA were added to the wells for 2 h, followed by washing and incubation for 2 h with alkaline phosphatase-conjugated streptavidin (R&D Systems). Spots were developed with 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt and nitro blue tetrazolium chloride in organic solvent (R&D Systems). The plates were analyzed with an ImmunoSpot Series 2 analyzer (Cellular Technology Ltd.).
OVA-specific Immunoglobulin ELISA
OVA-specific antibodies were determined using an ELISA. Briefly, 96-well flat-bottom protein absorbent polystyrene plates were coated with chicken OVA (grade V) at 10 μg/well. Plates were then blocked with 2% FBS in PBS and washed with PBS. Serum samples and standards (OVA-specific IgG1 and IgE) were incubated overnight at 4 °C. Biotinylated anti-mouse IgG1 or IgE was used, followed by horseradish peroxidase-conjugated streptavidin. Plates were developed, stained with 2,2′-azino-di(3-ethylbenzthiazoline 6-sulfonate), and quantified using a Molecular Devices plate reader. Data were analyzed using SoftMax Pro v.5 software.
ELISA-like Assay for Quantification of HA
HA was quantified using a HA test kit (Corgenix, Broomfield, CO) according to the manufacturer's instructions. The HA test kit uses a capture molecule known as “hyaluronan-binding protein” (HABP). Diluted BAL samples were incubated in HABP-coated microwells, which allows the HA present in samples to react with the immobilized HABP. After removal of unbound BAL molecules by washing with PBS, HABP conjugated with horseradish peroxidase solution was added to the microwells to form complexes with the immobilized HA. After washing with PBS, a chromogenic substrate of tetramethylbenzidine and hydrogen peroxide were added to develop color reaction. The intensity of the color was measured in absorbance units with a spectrophotometer at 450 nm. HA levels in BAL and control samples were determined against a reference curve prepared from the reagent blank and the HA reference solution provided with the kit.
Fluorophore-assisted Carbohydrate Electrophoresis
The fluorophore-assisted carbohydrate electrophoresis method for the quantification of HA has been described (40). Briefly, the lower lobe of the left lung was digested with proteinase K (Invitrogen) at 1 mg/ml in 100 mm ammonium acetate (pH 7.0) with 0.01% lauryl sulfate at 60 °C for 4 h, and HA was recovered by ethanol precipitation. The HA was digested to disaccharides at 37 °C overnight with hyaluronidase SD (Seikagaku America Inc., East Falmouth, MA) at 2.5 milliunits/μl and labeled with 2-aminoacridone (Invitrogen). The samples were electrophoresed on polyacrylamide gels, and the gel plates were washed with distilled water. After electrophoresis, the gels (in their glass plates) were placed on a UV transilluminator (Ultra-Lum, Inc., Claremont, CA) and illuminated at 365 nm. Images were captured on a Quantix CCD camera (Photometrics, Tucson, AZ), and the HA disaccharide band was quantified using Gel-Pro Analyzer® version 3.0 (Media Cybernetics, Silver Spring, MD). The lower lobes of the right and left lungs from six mice were also collected and lyophilized to obtain the dry weight of the lung tissue. The total amount (micrograms) of HA from each lower lobe of the right and left lungs was calculated per dry weight.
Multiplex Cytokine Assay
Cell culture supernatants or BAL fluids were collected, centrifuged at 13,000 rpm for 10 min at 4 °C, and stored at −80 °C until further analysis. Multiplex assays were done with an Invitrogen 20-Plex panel kit following the manufacturer's guidelines. Supernatants were diluted with culture sample diluent (Invitrogen). 50 μl of each bead mixture was aliquoted into a well of a 96-well filter plate, which was washed twice with 100 μl of PBS buffer/wash buffer. The culture supernatant or standard (50 μl of each) was added to each well and incubated for 1 h at room temperature in the dark. Plates were then vacuum-washed three times with wash buffer. The detection reagent was prepared by mixing 2.5 μl of 10× detection antibody mixture with detection antibody diluent buffer, and 25 μl was added to each well, followed by a 30-min incubation at room temperature in the dark. Streptavidin-phycoerythrin reagent (50 μl) was added to each well, followed by a 30-min incubation at room temperature in the dark. Plates were then vacuum-washed three times with wash buffer, and 125 μl of assay buffer was added to each well. Plates were then analyzed using Bio-Plex Manager 4.1 software using a Bio-Plex system reader.
Lung Collection and Histology
Lungs were collected for histology, fixed overnight in 10% formalin, and then embedded in paraffin. For immunohistochemistry, lungs were inflated with optimal cutting temperature compound (Sakura Finetek USA Inc., Torrance, CA), and embedded in this compound, and stored at −80 °C (fresh frozen lung tissue). Paraffin-embedded lung tissues were stained with H&E.
Immunohistochemistry
Fresh frozen sections were fixed in 50:50 cold acetone/methanol for 10 min. Sections were then blocked with 3% BSA in PBS for all antibodies used. In addition, sections stained with HABP, double-stained with HABP, or stained with HC1 and HC2 were also blocked with avidin, biotin, and streptavidin (Vector Laboratories, Burlingame, CA). Sections were incubated with 5 μg/ml biotinylated HABP (Seikagaku America Inc.) or with the primary antibody for 1 h at room temperature or overnight at 4 °C. The primary antibody was a goat polyclonal antibody against eosinophil major basic protein (EMBP; 4 μg/ml; sc-18241, Santa Cruz Biotechnology, Santa Cruz, CA) and a goat polyclonal antibody against IαI, HC1 (4 μg/ml; sc-33944, Santa Cruz Biotechnology), and HC2 (4 μg/ml; sc-21978, Santa Cruz Biotechnology). After washing, secondary reagents were added and incubated for 1 h at room temperature in the dark. Alexa Fluor 488-conjugated (for HABP) or Alexa Fluor 594-conjugated (for EMBP) donkey anti-goat streptavidin was used as the secondary reagent. Each staining included a control of only the secondary reagent to demonstrate the specificity of staining. Sections were examined with a Leica fluorescence microscope, and images were captured with a Retiga 2000R camera and QCapture Pro 6 software and quantitated by Image-Pro Plus 7.0 software.
Hyaluronidase Extraction of HCs from HA-HC Complexes
The lung tissues of mice were collected, cut with a scalpel, and transferred to preweighed 1.5-ml tubes that were prechilled on dry ice. The weights of the lungs were measured, and prechilled PBS was added to the tubes such that 100 μl of cold PBS was added for every 30 mg of tissue. The tissue was minced on ice in the PBS for ∼20 s using a small motorized pestle. A 50-μl aliquot of the minced tissue suspension was transferred to two new prechilled 1.5-ml tubes. Streptomyces hyaluronidase (10 μl of a 0.5 turbidity units/ml stock; product 100740-1, Seikagaku America Inc.) was added to one of these tubes, and PBS (10 μl) was added to the other. The tubes were incubated on ice for 30 min and then centrifuged at 13,200 rpm for 5 min at 4 °C. The supernatants were transferred to new prechilled 1.5-ml tubes and incubated for another 30 min at 37 °C. Then, 25 μl of the digests was added per lane on 4–15% Mini-PROTEAN TGX gels (Bio-Rad) and blotted using a Bio-Rad nitrocellulose and Trans-Blot Turbo system. The blots were blocked for 1 h with blocking buffer (catalog no. 927-40000, LI-COR, Lincoln, NE) and probed with a rabbit polyclonal antibody against IαI (1:8000 dilution; A0301, Dako North America, Inc., Carpinteria, CA). The secondary antibody was IRDye 800CW anti-rabbit IgG (1:15,000 dilution; catalog no. 926-32211, LI-COR). The blots were washed and imaged using an Odyssey infrared imaging system (LI-COR).
Statistical Analysis
Data are presented as means ± S.E.; n is indicated in the figure legends in representative experiments. The significance of differences between two groups was determined by Student's t test (two-tailed) using KaleidaGraph v3.6 software (Synergy Software, Reading, PA). Statistical significance was reported if p < 0.05 was achieved. Data analysis and figures were generated using Prism 5.0a (GraphPad Software, Inc.).
RESULTS
TSG-6 Deficiency Results in Reduced Eosinophilic Airway Inflammation
Atopic asthma is often associated with the induction of recurrent episodic flares of eosinophilic airway inflammation as a consequence of inhaled allergen triggers. The OVA murine model of allergic pulmonary inflammation has been extensively utilized to examine the in vivo development of eosinophilic inflammation and the associated increase in AHR, in which T cell-mediated Th2 immunity is heavily implicated (41–43). To examine the biological role of TSG-6 in the development of allergic pulmonary inflammation, we used mice in which the TSG-6-coding gene was interrupted (35). Following OVA sensitization and aerosol challenge, we assessed the lungs for inflammatory infiltrates by analyzing cells retrieved by BAL. A significant reduction in total leukocyte recovery (Fig. 1A) was observed in TSG-6−/− mice compared with their wild-type littermates (mean ± S.E. of 42.8 ± 9.3 versus 153.1 ± 35.4, p < 0.05). Furthermore, following differential counts of BAL Cytospin preparations, the reduction in total leukocytes was largely attributed to the lower number of airway eosinophils (mean ± S.E. of 29.3 ± 6.0 versus 113.2 ± 28.8, p < 0.05) (Fig. 1B) and macrophages (mean ± S.E. of 11.1 ± 3.4 versus 33.7 ± 8.5, p < 0.05) (Fig. 1D). Neutrophils and lymphocytes also appeared to be less in the OVA/alum BAL, but the smaller numbers were not sufficient to reach statistical significance (Fig. 1, C and E). Thus, TSG-6 deficiency resulted in reduced airway inflammation after aerosol antigen exposure, indicating that endogenous TSG-6 has an essential role in the development of eosinophilic pulmonary inflammation.
FIGURE 1.
Effects of TSG-6 deficiency on BAL leukocyte distributions during acute antigen-induced pulmonary inflammation. BAL from wild-type (white bars) and TSG-6−/− (black bars) mice subjected to OVA-induced pulmonary inflammation was analyzed for leukocytes as described under “Experimental Procedures.” A, total BAL leukocytes. B–E, differential count analyses of BAL Cytospin preparations stained with Wright-Giemsa. Data represent means ± S.E. for 104 cells/ml (BAL; n = 6). The experiment was repeated at least three independent times. *, p < 0.05. KO, knock-out; I.P., intraperitoneal.
TSG-6 Deficiency Results in Reduced Induction of AHR
To investigate the pathophysiological development of AHR, which is typically associated with antigen-induced pulmonary eosinophilia in the mouse, we analyzed lung mechanics in response to increasing doses of methacholine 24 h after the last aerosol challenge. The parameters in the lung mechanics assessment (44) included dynamic lung resistance and lung compliance. In addition, we compared tissue elastance, which correlates with the elastic rigidity of the lungs, and dampening, which correlates with tissue resistance and reflects energy dissipation in the lung tissues.
The induction of lung resistance was markedly attenuated in TSG-6−/− mice compared with the methacholine dose-dependent increase in lung resistance typically observed in the OVA/alum-immunized and OVA-challenged wild-type mice (Fig. 2A). Interestingly, TSG-6−/− mice did not exhibit any protection against the base line or in methacholine-induced reduction of lung compliance following antigen exposure (Fig. 2B), even though both tissue elastance and dampening responses in TSG-6−/− mice were significantly attenuated (Fig. 2, C and D). It is also worth noting that TSG-6 deficiency did not affect lung mechanics at the base line or in unsensitized controls. Thus, although endogenous TSG-6 is expendable for the antigen-induced decrease in lung compliance, it is required for the development of AHR and the increases in lung resistance, tissue elastance, and tissue dampening that occur in wild-type lungs in response to methacholine challenge.
FIGURE 2.
Effects of TSG-6 deficiency on AHR. TSG-6 deficiency resulted in reduced induction of AHR following acute antigen-induced pulmonary inflammation. Wild-type (▵ and ○) and TSG-6−/− (▴ and ●) mice were immunized with alum alone (▵ and ▴) or with OVA/alum (OVA/OVA; ○ and ●) as indicated. After OVA aerosol challenge, AHR measurements were done as described under “Experimental Procedures.” A, dynamic lung resistance (RL). B, lung compliance (C). C, tissue elastance (H). D, tissue dampening (G). *, p < 0.05 (significant differences between wild-type and TSG-6−/− mice at the indicated methacholine doses; n = 6). KO, knock-out.
TSG-6 Is Dispensable for the Induction of Th2 Immunity
Changes in the airways and lung parenchyma that are associated with eosinophilic pulmonary inflammation are mediated in part by Th2 responses that engage innate and adaptive immune compartments. The significant reduction in airway eosinophilia in TSG-6−/− mice prompted us to examine the levels of induction of cellular and humoral Th2 responses that are known to drive systemic as well as pulmonary eosinophilia (43).
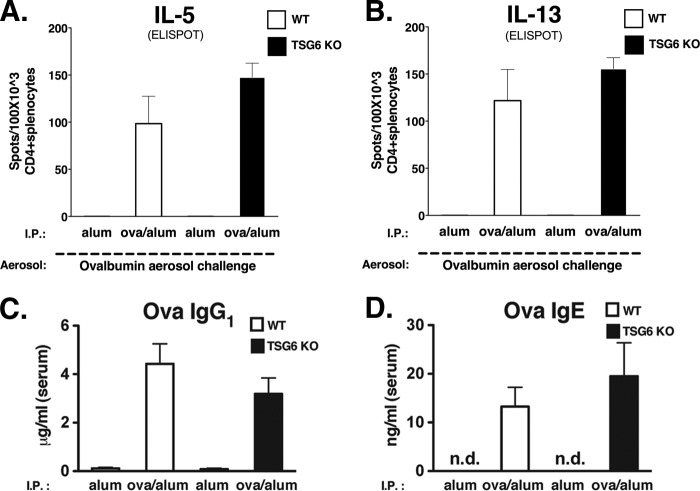
To evaluate the induction of OVA-specific Th2 immunity in wild-type and TSG-6−/− mice, we first examined the effects of OVA/alum immunization in both strains of mice before the OVA aerosol challenge. This was accomplished by the ex vivo assessment of Th2 cytokine production from CD4+ splenocytes with enzyme-linked immunosorbent spot analysis. Comparing wild-type and TSG-6−/− mice, we observed no significant changes in the levels of antigen-specific IL-5- or IL-13-producing CD4+ splenocytes (Fig. 3, A and B). Furthermore, upon analysis of serum samples obtained from these immunized mice, we were unable to detect any significant variations in the levels of OVA-specific IgG1 or IgE (Fig. 3, C and D). Thus, endogenous TSG-6 is not necessary for the induction of antigen-specific cellular and humoral responses following OVA/alum sensitization.
FIGURE 3.
Lack of effects of TSG-6 deficiency on TH2 immunity. Wild-type (white bars) and TSG-6−/− (black bars) mice were immunized with alum alone or with OVA/alum as indicated. A and B, enzyme-linked immunosorbent spot analyses were done using enriched CD4+ splenocytes incubated with OVA: IL-5-producing (A) and IL-13-producing (B) spots. Data represent means ± S.E. for the number of spots out of 100 × 103 cells (n = 4). C and D, serum levels of OVA-specific antibodies IgG1 and IgE, respectively. Data represent means ± S.E. (n = 4). n.d., none detected; KO, knock-out; I.P., intraperitoneal.
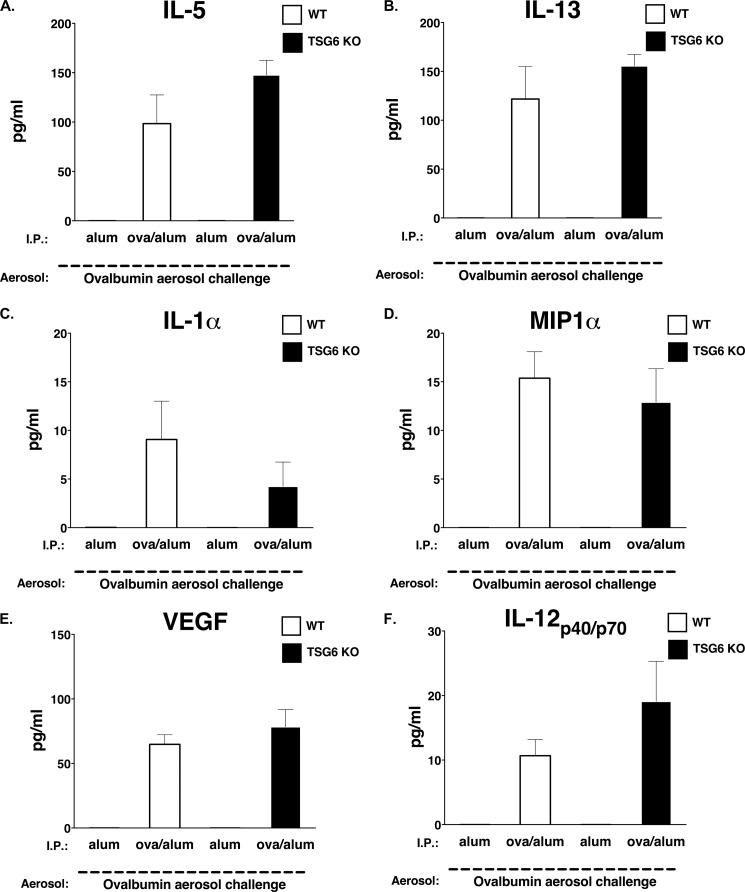
Because we were unable to measure any ex vivo variation in Th2 immunity, we next examined the in vivo levels of other proinflammatory cytokines, chemokines, and growth factors that have been implicated in the murine asthma model. No substantial differences were detected in the protein levels of IL-5, IL-13, IL-1α, macrophage inflammatory protein-1α, VEGF, or IL-12 p40/p70 (Fig. 4). Thus, our data suggest that endogenous TSG-6 is not necessary for the induction of antigen-specific cellular and humoral responses following antigen/alum sensitization and is not important for the induction of secondary antigen responses during aerosol antigen challenge.
FIGURE 4.
Lack of effects of TSG-6 on pulmonary Th2 immunity. Wild-type (white bars) and TSG-6−/− (black bars) mice were subjected to allergen-mediated pulmonary inflammation as described under “Experimental Procedures” and as indicated. A–F, multiplex cytokine analyses of BAL fluid. Data represent means ± S.E. (n = 6). KO, knock-out; I.P., intraperitoneal; MIP1α, macrophage inflammatory protein-1α.
TSG-6 Deficiency Results in Reduced Pulmonary HA Deposition
We have recently described the temporal association between pulmonary HA deposition and eosinophilia during the induction and propagation of acute and chronic antigen-induced pulmonary inflammation and found that the maximum HA lung deposition and eosinophilia occur at around day 6 (45). Additionally, in our accompanying article (59), we show that TSG-6 enhanced HA accumulation and leukocyte binding in an in vitro model of HA synthesis. Because TSG-6 is a hyaladherin that is involved in HA modification during many biological responses, we next examined the correlation between the antigen-induced inflammatory responses and the HA deposition in wild-type and TSG-6−/− mice (Fig. 5). H&E staining revealed a muted perivascular and peribronchial accumulation of leukocytes in TSG-6−/− mice compared with wild-type mice (Fig. 5A). Furthermore, immunohistochemical staining with HABP revealed a reduction in parenchymal HA deposition in TSG-6−/− mice compared with wild-type littermates (Fig. 5, B and D). The HA and eosinophils appeared to correlate in the parenchymal and alveolar interstitial compartments, as well as in the perivascular and peribronchial regions (Fig. 5, B and C).
FIGURE 5.
Localization of HA and eosinophils in lung tissue. Wild-type (left panels) and TSG-6−/− (right panels) mice were immunized with OVA/alum and OVA aerosol challenged as described under “Experimental Procedures.” A, H&E staining of lung tissue. B–D, immunofluorescence staining of frozen lung sections using anti-mouse EMBP goat antibody (red) and HABP (green). B, EMBP and HABP. C, EMBP only. D, HABP only.
Given the immunohistochemical evidence of reduced HA deposition in TSG-6−/− mice, we next quantified the levels of HA in the mucosal and lung tissue compartments. Following antigen challenge, BAL HA levels in OVA/alum-immunized wild-type mice were significantly increased compared with the control mice immunized with alum alone (47.6 ± 3.7 versus 7.6 ± 0.4 ng/ml, p < 0.05) (Fig. 6A). Although this same trend was also observed in TSG-6−/− mice (34.3 ± 5.9 versus 8.1 ± 0.8 ng/ml, p < 0.05) (Fig. 6A), the level of HA in TSG-6−/− mice was reduced by ∼30% from that in the counterpart wild-type littermates (p < 0.05).
FIGURE 6.
Effect of TSG-6 deficiency on HA in BAL and lung tissue. BAL and lung tissue samples were obtained from wild-type (white bars) and TSG-6−/− (black bars) mice. Mice were immunized with alum alone or with OVA/alum as indicated and subjected to OVA aerosol challenge. A, HA quantification in BAL using the HABP ELISA-like assay. B, HA quantification in lung tissue by the fluorophore-assisted carbohydrate electrophoresis assay normalized to dry weight. Data represent means ± S.E. for the amount of HA in the BAL (ng/ml) and the amount of HA in the lung (μg/g, dry weight (d.w)) (n ≥ 8). *, p < 0.05. KO, knock-out; I.P., intraperitoneal.
Furthermore, fluorophore-assisted carbohydrate electrophoresis assays were used to estimate the level of parenchymal HA and revealed a similar pattern of HA induction (Fig. 6B). Thus, TSG-6 deficiency results in reduced pulmonary HA deposition following OVA aerosol challenge in OVA-immunized mice during induction of allergic pulmonary inflammation.
TSG-6 Deficiency Is Associated with the Absence of Pulmonary HA-HC Complexes
We developed a procedure to evaluate the presence of the HA-HC complexes in lung tissue to determine the presence of HA-HC complexes at the site of the ongoing inflammation. Furthermore, because TSG-6 catalyzes the transesterification reactions that form HA-HC complexes (34, 35, 37), we speculated that the absence of endogenous TSG-6 would affect the formation of these HA-HC complexes. We used Streptomyces hyaluronidase to degrade HA and to dissociate HA-associated molecules, including HCs from pulmonary tissue obtained from WT and TSG-6−/− animals undergoing OVA challenge. Western blot analysis of these samples for IαI and HC protein content revealed the presence of 240-kDa IαI and 120-kDa pre-α-inhibitor in all samples (Fig. 7). Nevertheless, the HC band (75 kDa) was present only in hyaluronidase-treated samples in OVA-sensitized/challenged WT lungs and was not detectable in TSG-6−/−-derived lung tissue.
FIGURE 7.
Effects of TSG-6 deficiency on formation of HA-HC in asthmatic lungs of mice. Mice were immunized with alum alone and challenged with OVA aerosol (PBS/OVA) or immunized with OVA/alum and challenged with OVA aerosol (OVA/OVA), as in Fig. 2. Minced lung tissue was treated with Streptomyces hyaluronidase (Hyase; +) or left untreated (−) to release HCs from HA-HC. The supernatants of this extraction were analyzed by Western blotting and probed with antibodies against IαI. HCs (green) were released by hyaluronidase in the lungs of OVA/OVA WT mice (75 kDa; arrow) but not in the lungs of PBS/OVA-treated WT mice. In contrast, no HCs were released by hyaluronidase in the minced lung tissue of lungs derived from TSG-6−/− mice after either OVA/alum or PBS/OVA treatment. The bands were identified as 240-kDa IαI, 120-kDa pre-α-inhibitor, and 75-kDa HCs. Molecular mass standards are shown in red on the left in kilodaltons. Identical results were obtained from three replicates (data not shown).
Furthermore, to address this marked difference in the presence of HA-HC complexes in TSG-6−/− mice compared with their WT littermates and to better characterize the localization of these complexes, we performed double immunostaining of HA, HC1, and HC2 on lung sections (Fig. 8). We observed a rather profound increase in both HA and HC deposition in WT lung tissue after the OVA challenge in immunized animals (Fig. 8B) but not in non-immunized animals (data not shown). Interestingly, an absence of HC deposition in similarly treated TSG-6−/− mice was observed (Fig. 8F).
FIGURE 8.
Distribution of HA and HCs in lung sections of WT and TSG-6−/− mice. Lung sections in WT or TSG-6−/− OVA/OVA treated mice were stained with HABP (green) for detection of HA and with anti-HC antibodies (red) for detection of HCs. There was no staining for the HCs on the TSG-6−/− OVA/alum mouse lung tissue with reduced HA staining.
Thus, our data suggest a non-redundant role for TSG-6 in the formation of HA-HC complexes during pulmonary inflammation. HA-HC complexes were absent in the TSG-6−/− mice following OVA aerosol challenge in the OVA-immunized mice.
DISCUSSION
GAG components of the pulmonary ECM are integral components of the structural scaffold required for pulmonary development, mechanics, and many processes that contribute to maintaining lung homeostasis (8). Increasing evidence now suggests that pulmonary GAGs are also involved in pulmonary inflammation, repair, and pathology (8, 9). In this study, we examined the role of TSG-6 during the induction and propagation of antigen-induced pulmonary eosinophilia and AHR. TSG-6 has been shown to interact with GAGs that are present in the pulmonary compartment, including chondroitin 4-sulfate, heparan sulfate, heparin, and HA. TSG-6 has also been shown to mediate HA interaction with cell surface CD44 molecules, where TSG-6-HA complexes had higher avidity for CD44 (31). Following segmental allergen challenge of asthmatic individuals, TSG-6 (in addition to the Th2 cytokines IL-3, IL-4, and IL-5) is one of 24 genes that are induced by 4-fold or more (36). Additionally, TSG-6 and TSG-6-HC complexes are also found in the BAL fluid of asthmatics after segmental allergen challenge (37). Nevertheless, how TSG-6 contributes to the pathogenesis of inflammatory responses in asthma has not been well defined.
Utilizing a well described model of allergic pulmonary inflammation, we evaluated the role of TSG-6 during the induction and propagation of OVA-induced innate and adaptive immune responses in the lung. We have shown that TSG-6 deficiency leads to decreased airway eosinophilia. In contrast, other investigators have shown that TSG-6 deficiency has an enhancing effect on neutrophil migration into peripheral joints of mice undergoing a murine model of proteoglycan-induced arthritis (46). Reciprocally, exogenous TSG-6 inhibits neutrophil extravasation in a murine model of acute inflammation (47). The observations from TSG-6−/− mice undergoing proteoglycan-induced arthritis are consistent with the in vitro and in vivo studies demonstrating the link module-dependent inhibitory effects of TSG-6 on neutrophil migrations (29, 48, 49). It is not clear how TSG-6 can have two opposite effects on granulocyte extravasation in murine models of experimental arthritis and in a model of allergic pulmonary inflammation. Although these opposing observations could be model-dependent (given the predominant Th1 profile related to proteoglycan-induced arthritis and the predominant Th2 profile of asthma) as well as tissue response-dependent, additional work is needed to clarify this dichotomy.
The unique and somewhat surprising finding in our in vivo model is the significant reduction in HA deposition in TSG-6−/− mice despite equivalent base-line levels. In our accompanying article (59), we show that TSG-6 can induce HA synthesis in murine airway smooth muscle cells during active HA synthesis. Although this provides a plausible explanation for our findings, given TSG-6 pluripotent activities, it is likely not the only contributing mechanism.
TSG-6 has also been suggested to potentiate both inflammatory and anti-inflammatory responses by up-regulating the expression of the rate-limiting enzyme COX-2 (cyclooxygenase isoenzyme-2) and the generation of its downstream product, prostaglandin D2 (PGD2) (50). Interestingly, PGD2 and its high affinity receptors DP1 and CRTH2 (chemoattractant-homologous receptor expressed on Th2 cells) have been shown to be crucial for the promotion and maintenance of allergic responses (51–55). Importantly, although PGD2 promotes inflammatory responses through its CRTH2 receptor, which is selectively expressed on eosinophils, Th2 T cells, and basophils (54, 55), PGD2 also has anti-inflammatory and inhibitory effects on neutrophils (56, 57). Therefore, it is possible that TSG-6 contributes to the induction of robust allergic inflammation and AHR through promoting the production of PGD2. In contrast, the TSG-6-induced production of PGD2 may potentiate anti-inflammatory effects on neutrophils. Further investigation is needed to examine and address the complex role of TSG-6 in these important pathways.
Also, in addition to the reduced eosinophilic inflammation, TSG-6−/− mice were resistant to the induction of AHR and manifested improved lung mechanics in response to methacholine challenge. Although the role of TSG-6 in lung mechanics has not been previously studied, HA was shown to mediate AHR in mice following ozone exposure in a TLR4-dependent fashion (58). It is plausible that the requirement of TSG-6 for the development of AHR is also contingent on HA-TLR4-mediated mechanisms, which remains to be determined. It is important to note that, in our study, the reduced allergic responses observed in TSG-6−/− mice occurred despite the equivalent induction of both humoral and cellular Th2 responses. This suggests that the lack of eosinophilia and AHR is independent of any defect in Th2 immune responses, which are known to be required for the induction of these allergic processes.
This study provides the first description of the absence of HA-HC complexes in TSG-6−/− mice after OVA/OVA treatment. This might be one of the possible mechanisms for reduced HA, eosinophilia and AHR in TSG-6−/− mice.
In summary, we have demonstrated that endogenous TSG-6 is dispensable for the induction of Th2 immunity but is essential for the robust increase in pulmonary HA deposition, propagation of acute eosinophilic pulmonary inflammation, and development of AHR. Thus, TSG-6 is likely to contribute to the pathogenesis of asthma by enhancing HA accumulation and deposition, pulmonary eosinophilic inflammation, and AHR. TSG-6 is required for the formation of HA-HC in the asthmatic lungs of mice in the OVA/OVA asthma model.
This work was supported, in whole or in part, by National Institutes of Health Grant AI067816 from NIAID (to M. A. A.) and Grant HL081064 from NHLBI (to V. C. H. and M. A. A.).
- AHR
- airway hyperresponsiveness
- ECM
- extracellular matrix
- HA
- hyaluronan
- GAG
- glycosaminoglycan
- BAL
- bronchoalveolar lavage
- IαI
- inter-α-trypsin inhibitor
- HC
- heavy chain
- OVA
- ovalbumin
- HABP
- HA-binding protein
- EMBP
- eosinophil major basic protein
- PGD2
- prostaglandin D2.
REFERENCES
- 1. Elias J. A., Lee C. G., Zheng T., Ma B., Homer R. J., Zhu Z. (2003) New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laitinen A., Laitinen L. A. (1994) Pathology of asthma. Allergy Proc. 15, 323–328 [DOI] [PubMed] [Google Scholar]
- 3. Holgate S. T., Davies D. E. (2009) Rethinking the pathogenesis of asthma. Immunity 31, 362–367 [DOI] [PubMed] [Google Scholar]
- 4. Wills-Karp M. (1999) Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17, 255–281 [DOI] [PubMed] [Google Scholar]
- 5. Raines E. W. (2000) The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 81, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaday G. G., Lider O. (2000) Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 67, 149–159 [DOI] [PubMed] [Google Scholar]
- 7. Burgess J. K. (2009) The role of the extracellular matrix and specific growth factors in the regulation of inflammation and remodelling in asthma. Pharmacol. Ther. 122, 19–29 [DOI] [PubMed] [Google Scholar]
- 8. Papakonstantinou E., Karakiulakis G. (2009) The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Br. J. Pharmacol. 157, 1111–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang D., Liang J., Noble P. W. (2007) Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 23, 435–461 [DOI] [PubMed] [Google Scholar]
- 10. Culley F. J., Fadlon E. J., Kirchem A., Williams T. J., Jose P. J., Pease J. E. (2003) Proteoglycans are potent modulators of the biological responses of eosinophils to chemokines. Eur. J. Immunol. 33, 1302–1310 [DOI] [PubMed] [Google Scholar]
- 11. Cantor J. O., Cerreta J. M., Armand G., Osman M., Turino G. M. (1999) The pulmonary matrix, glycosaminoglycans and pulmonary emphysema. Connect. Tissue Res. 40, 97–104 [DOI] [PubMed] [Google Scholar]
- 12. Negrini D., Passi A., Moriondo A. (2008) The role of proteoglycans in pulmonary edema development. Intensive Care Med. 34, 610–618 [DOI] [PubMed] [Google Scholar]
- 13. Jiang D., Liang J., Noble P. W. (2010) Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat. Rec. 293, 982–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noble P. W., Jiang D. (2006) Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc. Am. Thorac. Soc. 3, 401–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wisniewski H. G., Vilcek J. (2004) Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 15, 129–146 [DOI] [PubMed] [Google Scholar]
- 16. Blundell C. D., Mahoney D. J., Almond A., DeAngelis P. L., Kahmann J. D., Teriete P., Pickford A. R., Campbell I. D., Day A. J. (2003) The link module from ovulation- and inflammation-associated protein TSG-6 changes conformation on hyaluronan binding. J. Biol. Chem. 278, 49261–49270 [DOI] [PubMed] [Google Scholar]
- 17. Stern R. (2004) Hyaluronan catabolism: a new metabolic pathway. Eur. J. Cell Biol. 83, 317–325 [DOI] [PubMed] [Google Scholar]
- 18. Day A. J., de la Motte C. A. (2005) Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 26, 637–643 [DOI] [PubMed] [Google Scholar]
- 19. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 20. Monzon M. E., Casalino-Matsuda S. M., Forteza R. M. (2006) Identification of glycosaminoglycans in human airway secretions. Am. J. Respir. Cell Mol. Biol. 34, 135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sahu S., Lynn W. S. (1978) Hyaluronic acid in the pulmonary secretions of patients with asthma. Biochem. J. 173, 565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bousquet J., Chanez P., Lacoste J. Y., Enander I., Venge P., Peterson C., Ahlstedt S., Michel F. B., Godard P. (1991) Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J. Allergy Clin. Immunol. 88, 649–660 [DOI] [PubMed] [Google Scholar]
- 23. Parkar A. A., Kahmann J. D., Howat S. L., Bayliss M. T., Day A. J. (1998) TSG-6 interacts with hyaluronan and aggrecan in a pH-dependent manner via a common functional element: implications for its regulation in inflamed cartilage. FEBS Lett. 428, 171–176 [DOI] [PubMed] [Google Scholar]
- 24. Kohda D., Morton C. J., Parkar A. A., Hatanaka H., Inagaki F. M., Campbell I. D., Day A. J. (1996) Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 86, 767–775 [DOI] [PubMed] [Google Scholar]
- 25. Parkar A. A., Day A. J. (1997) Overlapping sites on the link module of human TSG-6 mediate binding to hyaluronan and chrondroitin 4-sulfate. FEBS Lett. 410, 413–417 [DOI] [PubMed] [Google Scholar]
- 26. Blundell C. D., Almond A., Mahoney D. J., DeAngelis P. L., Campbell I. D., Day A. J. (2005) Towards a structure for a TSG-6·hyaluronan complex by modeling and NMR spectroscopy: insights into other members of the link module superfamily. J. Biol. Chem. 280, 18189–18201 [DOI] [PubMed] [Google Scholar]
- 27. Carrette O., Nemade R. V., Day A. J., Brickner A., Larsen W. J. (2001) TSG-6 is concentrated in the extracellular matrix of mouse cumulus oocyte complexes through hyaluronan and inter-α-inhibitor binding. Biol. Reprod. 65, 301–308 [DOI] [PubMed] [Google Scholar]
- 28. Kahmann J. D., O'Brien R., Werner J. M., Heinegârd D., Ladbury J. E., Campbell I. D., Day A. J. (2000) Localization and characterization of the hyaluronan-binding site on the link module from human TSG-6. Structure 8, 763–774 [DOI] [PubMed] [Google Scholar]
- 29. Milner C. M., Higman V. A., Day A. J. (2006) TSG-6: a pluripotent inflammatory mediator? Biochem. Soc. Trans. 34, 446–450 [DOI] [PubMed] [Google Scholar]
- 30. Mahoney D. J., Mulloy B., Forster M. J., Blundell C. D., Fries E., Milner C. M., Day A. J. (2005) Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparan: implications for the inhibition of plasmin in extracellular matrix microenvironments. J. Biol. Chem. 280, 27044–27055 [DOI] [PubMed] [Google Scholar]
- 31. Lesley J., Gál I., Mahoney D. J., Cordell M. R., Rugg M. S., Hyman R., Day A. J., Mikecz K. (2004) TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J. Biol. Chem. 279, 25745–25754 [DOI] [PubMed] [Google Scholar]
- 32. Sanggaard K. W., Sonne-Schmidt C. S., Krogager T. P., Lorentzen K. A., Wisniewski H. G., Thøgersen I. B., Enghild J. J. (2008) The transfer of heavy chains from bikunin proteins to hyaluronan requires both TSG-6 and HC2. J. Biol. Chem. 283, 18530–18537 [DOI] [PubMed] [Google Scholar]
- 33. Mukhopadhyay D., Hascall V. C., Day A. J., Salustri A., Fülöp C. (2001) Two distinct populations of tumor necrosis factor-stimulated gene-6 protein in the extracellular matrix of expanded mouse cumulus cell-oocyte complexes. Arch. Biochem. Biophys. 394, 173–181 [DOI] [PubMed] [Google Scholar]
- 34. Mukhopadhyay D., Asari A., Rugg M. S., Day A. J., Fülöp C. (2004) Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J. Biol. Chem. 279, 11119–11128 [DOI] [PubMed] [Google Scholar]
- 35. Fülöp C., Szántó S., Mukhopadhyay D., Bárdos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6-deficient mice. Development 130, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 36. Lilly C. M., Tateno H., Oguma T., Israel E., Sonna L. A. (2005) Effects of allergen challenge on airway epithelial cell gene expression. Am. J. Respir. Crit. Care Med. 171, 579–586 [DOI] [PubMed] [Google Scholar]
- 37. Forteza R., Casalino-Matsuda S. M., Monzon M. E., Fries E., Rugg M. S., Milner C. M., Day A. J. (2007) TSG-6 potentiates the antitissue kallikrein activity of inter-α-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 36, 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aronica M. A., Mora A. L., Mitchell D. B., Finn P. W., Johnson J. E., Sheller J. R., Boothby M. R. (1999) Preferential role for NF-κB/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J. Immunol. 163, 5116–5124 [PubMed] [Google Scholar]
- 39. Swaidani S., Bulek K., Kang Z., Liu C., Lu Y., Yin W., Aronica M., Li X. (2009) The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J. Immunol. 182, 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lauer M. E., Fulop C., Mukhopadhyay D., Comhair S., Erzurum S. C., Hascall V. C. (2009) Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-α-inhibitor heavy chain attachment. J. Biol. Chem. 284, 5313–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar R. K., Herbert C., Foster P. S. (2008) The “classical” ovalbumin challenge model of asthma in mice. Curr. Drug Targets 9, 485–494 [DOI] [PubMed] [Google Scholar]
- 42. Fuchs B., Braun A. (2008) Improved mouse models of allergy and allergic asthma–chances beyond ovalbumin. Curr. Drug Targets 9, 495–502 [DOI] [PubMed] [Google Scholar]
- 43. Finkelman F. D., Hogan S. P., Hershey G. K., Rothenberg M. E., Wills-Karp M. (2010) Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 184, 1663–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Irvin C. G., Bates J. H. (2003) Measuring the lung function in the mouse: the challenge of size. Respir. Res. 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng G., Swaidani S., Sharma M., Lauer M. E., Hascall V. C., Aronica M. A. (2011) Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 30, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szántó S., Bárdos T., Gál I., Glant T. T., Mikecz K. (2004) Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 50, 3012–3022 [DOI] [PubMed] [Google Scholar]
- 47. Wisniewski H. G., Hua J. C., Poppers D. M., Naime D., Vilcek J., Cronstein B. N. (1996) TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-α-inhibitor and exerts a strong anti-inflammatory effect in vivo. J. Immunol. 156, 1609–1615 [PubMed] [Google Scholar]
- 48. Cao T. V., La M., Getting S. J., Day A. J., Perretti M. (2004) Inhibitory effects of TSG-6 link module on leukocyte-endothelial cell interactions in vitro and in vivo. Microcirculation 11, 615–624 [DOI] [PubMed] [Google Scholar]
- 49. Getting S. J., Mahoney D. J., Cao T., Rugg M. S., Fries E., Milner C. M., Perretti M., Day A. J. (2002) The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-α-inhibitor-independent manner. J. Biol. Chem. 277, 51068–51076 [DOI] [PubMed] [Google Scholar]
- 50. Mindrescu C., Le J., Wisniewski H. G., Vilcek J. (2005) Up-regulation of cyclooxygenase-2 expression by TSG-6 protein in macrophage cell line. Biochem. Biophys. Res. Commun. 330, 737–745 [DOI] [PubMed] [Google Scholar]
- 51. Pettipher R. (2008) The roles of the prostaglandin D2 receptors DP1 and CRTH2 in promoting allergic responses. Br. J. Pharmacol. 153, S191–S199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herrerias A., Torres R., Serra M., Marco A., Pujols L., Picado C., de Mora F. (2009) Activity of the cyclooxygenase 2-prostaglandin E prostanoid receptor pathway in mice exposed to house dust mite aeroallergens, and impact of exogenous prostaglandin E2. J. Inflamm. 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spik I., Brénuchon C., Angéli V., Staumont D., Fleury S., Capron M., Trottein F., Dombrowicz D. (2005) Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J. Immunol. 174, 3703–3708 [DOI] [PubMed] [Google Scholar]
- 54. Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K. (2001) Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Honda K., Arima M., Cheng G., Taki S., Hirata H., Eda F., Fukushima F., Yamaguchi B., Hatano M., Tokuhisa T., Fukuda T. (2003) Prostaglandin D2 reinforces Th2-type inflammatory responses of airways to low-dose antigen through bronchial expression of macrophage-derived chemokine. J. Exp. Med. 198, 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ney P., Schrör K. (1991) PGD2 and its mimetic ZK 110.841 are potent inhibitors of receptor-mediated activation of human neutrophils. Eicosanoids 4, 21–28 [PubMed] [Google Scholar]
- 57. Darius H., Michael-Hepp J., Thierauch K. H., Fisch A. (1994) Inhibition of human platelets and polymorphonuclear neutrophils by the potent and metabolically stable prostaglandin D2 analog ZK 118.182. Eur. J. Pharmacol. 258, 207–213 [DOI] [PubMed] [Google Scholar]
- 58. Garantziotis S., Li Z., Potts E. N., Lindsey J. Y., Stober V. P., Polosukhin V. V., Blackwell T. S., Schwartz D. A., Foster W. M., Hollingsworth J. W. (2010) TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am. J. Respir. Crit. Care Med. 181, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 59. Lauer M. E., Cheng G., Swaidani S., Aronica M. A., Weigel P. H., Hascall V. C. (2013) Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J. Biol. Chem. 288, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]