Background: TSG-6 transfers heavy chains (HCs) from the inter-α-inhibitor to hyaluronan (HA), increasing its avidity for leukocytes.
Results: Recombinant TSG-6 increased leukocyte adhesion to HA and its accumulation in airway cells.
Conclusion: In addition to its ability to transfer HCs to HA, TSG-6 amplifies HA synthesis.
Significance: TSG-6 is a potent regulator of HA synthesis and is likely to be involved in a variety of inflammatory diseases.
Keywords: Enzymes, Extracellular Matrix, Glycobiology, Inflammation, Virus, Hyaluronan Synthesis, Inter-α-inhibitor, TSG-6, Airway Smooth Muscle, Poly(I:C)
Abstract
We tested the hypothesis that the artificial addition of heavy chains from inter-α-inhibitor to hyaluronan (HA), by adding recombinant TSG-6 (TNF-stimulated gene-6) to the culture medium of murine airway smooth muscle (MASM) cells, would enhance leukocyte binding to HA cables produced in response to poly(I:C). As predicted, the addition of heavy chains to HA cables enhanced leukocyte adhesion to these cables, but it also had several unexpected effects. (i) It produced thicker, more pronounced HA cables. (ii) It increased the accumulation of HA in the cell-associated matrix. (iii) It decreased the amount of HA in the conditioned medium. Importantly, these effects were observed only when TSG-6 was administered in the presence of poly(I:C), and TSG-6 did not exert any effect on its own. Increased HA synthesis occurred during active, poly(I:C)-induced HA synthesis and did not occur when TSG-6 was added after poly(I:C)-induced HA synthesis was complete. MASM cells derived from TSG-6−/−, HAS1/3−/−, and CD44−/− mice amplified HA synthesis in response to poly(I:C) + TSG-6 in a manner similar to WT MASM cells, demonstrating that they are expendable in this process. We conclude that TSG-6 increases the accumulation of HA in the cell-associated matrix, partially by preventing its dissolution from the cell-associated matrix into the conditioned medium, but primarily by inducing HA synthesis.
Introduction
Characterization of hyaluronan (HA)2 isolated from fibroblast cultures identified a unique, covalently bound protein described as a serum-derived HA-associated protein (called SHAP) (1), which was subsequently shown to be heavy chains (HCs) derived from inter-α-inhibitor (IαI) (2). IαI is a serum proteoglycan synthesized by hepatocytes (3). It is composed of three polypeptides: the trypsin inhibitor called bikunin and two HCs that are covalently attached to the single chondroitin sulfate chain of bikunin by an ester linkage between a HC aspartate and the 6-OH of a GalNAc in the chondroitin sulfate chain. The HA-HC complex has been found in the sera of patients with rheumatoid arthritis (4), osteoarthritis (4), ovarian cancer (5), cervical ripening (6), and chronic liver disease caused by hepatitis virus infection (7). It is also an important component of the cumulus cell-oocyte complex in the ovary, where it cross-links the HA matrix and is necessary for female fertility (8–10).
TSG-6 (TNF-stimulated gene-6) is a 35-kDa protein that is synthesized and secreted by many types of cells after treatment with TNFα and IL-1 (11). Through our investigations and others, TSG-6 was identified as the enzyme responsible for the covalent transfer of HCs from IαI to HA (9, 10, 12). Elevated levels of TSG-6 have been observed in asthmatic bronchoalveolar lavage fluid (13), in the airway epithelia and secretions of smokers (13), and in infarcted regions following stroke (14).
HA “cables” were first observed following viral infection or poly(I:C) treatment of human intestinal mucosal smooth muscle cells (15). These unique strand-like structures appear as multiple coalescing threads of HA that are responsible for adhesion of mononuclear leukocytes and their subsequent CD44-mediated mechanism to digest them into soluble HA fragments that are referred to as “danger signals” during inflammation (16).
In a previous study, we tested the hypothesis that leukocyte-adhesive HA cables produced by murine airway smooth muscle (MASM) cells are substituted with HCs from IαI (17). The rationale for this hypothesis was that HA-HC derived from the synovial fluid of arthritis patients promoted leukocyte binding more than HA alone (4). Furthermore, there was evidence that HA cables produced by human intestinal smooth muscle cells in response to poly(I:C) treatment promoted leukocyte adhesion in a HC-dependent manner (16). Surprisingly, we were unable to find HCs attached to leukocyte-adhesive HA cable structures produced by MASM cell cultures in response to poly(I:C) and concluded that if HCs were present, they were below the limit of detection (17). In the present study, we tested the hypothesis that the artificial addition of HCs to HA, by adding recombinant TSG-6 to the culture medium, would enhance leukocyte binding to HA cables. Our results show that TSG-6 not only promoted leukocyte binding to HA cables in a HC-dependent manner but also had a profound stimulatory effect on the synthesis of HA cables when MASM cultures were treated with TSG-6 in combination with poly(I:C), which has properties similar to a viral infection.
EXPERIMENTAL PROCEDURES
Primary Cell Culture
MASM cells were derived from mouse trachea outgrowths as described previously (18). The experiments were done on the first passage. Mice homozygous null for TSG-6 (10), HAS1 and HAS3 (19), and CD44 (20) have been described previously.
Experimental Culture
MASM cell cultures were either not treated or treated with poly(I:C) (10 μg/ml; P0913, Sigma-Aldrich), recombinant human TSG-6 (0.250 μg/ml; 2104-TS, R&D Systems), or a combination of poly(I:C) and TSG-6. Endotoxin levels in the recombinant TSG-6 preparation were <1.0 enzyme unit/1 μg of protein as determined by the Limulus amebocyte lysate method (as reported by the manufacturer). Unless stated otherwise, the cells were treated for 18 h, and TSG-6 was added at the same time as poly(I:C) for the full 18 h. Unless stated otherwise, these treatments were done in DMEM/F-12 medium with 5% FBS. The treatment volume was 0.25 ml/cm2 area of tissue culture well.
Leukocyte Adhesion Assay
U937 lymphoma cells were labeled with the lipophilic carbocyanine dye CM-DiI (5 μm; V-22888, Invitrogen) as described previously (21). U937 cells (2 million/ml, 0.5 million/cm2 of culture area) were applied to the MASM cells at 4 °C for 30 min and allowed to settle, by gravity, onto the surface of the MASM cells for 30 min, during which time they had the opportunity to come into contact with HA at the cell surface. Unbound cells were gently washed away with DMEM/F-12 medium at 4 °C. U937 cells bound to HA were then removed by the application of Streptomyces hyaluronidase (0.5 turbidity units/ml; 100740-1, Seikagaku America Inc., East Falmouth, MA) at 4 °C for 5 min. The U937 cells remaining bound to the MASM cells were lysed with 1% Triton X-100 in PBS for 5 min at room temperature. The U937 cells released by hyaluronidase were lysed by the addition of 10 μl Triton X-100 to each 1 ml extract and incubation at room temperature for 5 min (final concentration of 1%). Aliquots of the detergent and hyaluronidase extracts were transferred to a 96-well plate, and the cell number was quantified with a fluorometer (excitation and emission at 520 and 590 nm, respectively) by comparing the relative fluorescence intensities of extracts with a known number of CM-DiI-labeled U937 cells.
Fluorophore-assisted Carbohydrate Electrophoresis
HA contents in the cell layers and conditioned media of MASM cell cultures were measured as described previously (18). Briefly, after proteolytic digestion and ethanol precipitation purification steps, the HA in the samples was digested into disaccharides with hyaluronidase SD (2.5 milliunits/μl; 100741-1A, Seikagaku America Inc.) and labeled with 2-aminoacridone (A-6289, Invitrogen) at 6.25 mm in 42.5% Me2SO, 7.5% glacial acetic acid, and 0.625 m sodium cyanoborohydride (1.25 μl/cm2 of tissue culture surface area). The labeled HA disaccharides were electrophoresed using a Bio-Rad mini-PROTEAN Tetra system. The gel composition consisted of 20% acrylamide (37.5:1; Bio-Rad), 40 mm Tris acetate (pH 7.0), 2.5% glycerol, 10% ammonium persulfate, and 0.1% TEMED. After electrophoresis at 500 V (constant voltage) for 50 min at 4 °C, the gels were imaged in their plates on a UV transilluminator at 365 nm using a CCD camera. The HA disaccharide band was quantified using Gel-Pro Analyzer® version 3.0 (Media Cybernetics, Silver Spring, MD). All statistics (Student's t tests) were done using Excel (2011 for MAC).
Immunohistochemistry
MASM cells were fixed in 100% methanol at −20 °C for 10 min, air-dried for 1 h, and rehydrated in PBS for 20 min. After blocking for 30 min in PBS with 1% BSA, HA was labeled with a biotinylated HA-binding protein (HABP; 5 μg/ml; 385911, EMD Chemicals, Gibbstown, NJ) in the blocking solution, and streptavidin conjugated to Alexa Fluor® 488 (S-11223, Invitrogen) was applied at 1:500. In some slides, CD44 was stained with the simultaneous incubation of a mouse monoclonal antibody against CD44 (1:100; C7923, Sigma-Aldrich) and HABP using an Alexa Fluor® 594-conjugated donkey anti-mouse secondary antibody (1:250; A-21203, Invitrogen) for CD44. In some slides, antibodies against HC1 (1:50; sc-33944, Santa Cruz Biotechnology) and HC2 (1:50; sc-21978, Santa Cruz Biotechnology) were applied simultaneously with HABP using Alexa Fluor® 594-conjugated anti-goat IgG (1:250; A-11058, Invitrogen) as a secondary antibody for the anti-HC antibodies. Imaging was done by either confocal microscopy or standard fluorescence microscopy (as indicated in the figure legends).
Western Blotting
HCs were extracted from HA cables by the addition of Streptomyces hyaluronidase (10 turbidity units/ml; 40 μl/cm2 for 5 min on ice). These extracts were then transferred to 1.5-ml tubes and incubated at 37 °C for 1 h to further remove HA from the HA-bound HCs before electrophoresis. Samples were electrophoresed on 4–15% Mini-PROTEAN TGX gels (Bio-Rad) and blotted using a Bio-Rad nitrocellulose and Trans-Blot Turbo system. The blots were blocked for 1 h with blocking buffer (catalog no. 927-40000, LI-COR) and probed with the antibodies against HC1 and HC2 in blocking buffer with 0.1% Tween 20 for 1 h. The blots were washed five times with PBS with 0.1% Tween 20 and probed with IRDye 800CW donkey anti-goat IgG secondary antibody (catalog no. 926-32214, LI-COR) at 1:15,000 dilution in blocking buffer with 0.1% Tween 20 and 0.01% lauryl sulfate for 45 min. The blots were washed as described above and imaged on an Odyssey infrared imaging system (LI-COR).
Real-time PCR
We used the same mouse primer sets for double-labeled HAS2, and HAS3 as reported previously (22). We used the SYBR Green method (Invitrogen) for real-time PCR on a thermal cycler 7500 system (Applied Biosystems). Data were normalized to GAPDH for each treatment. Linear regression of efficiency was used to model PCR amplification as described previously (23, 24). This method uses a universal standard (λ genomic DNA) to calculate an optical calibration factor that allows for the calculation of absolute copy number of the HAS enzymes without the need for standard curves. This absolute copy number was expressed as the ratio of the absolute copy number of the HAS genes to the absolute copy number of GAPDH.
RESULTS
Artificial Addition of HCs to HA Cables via Recombinant TSG-6
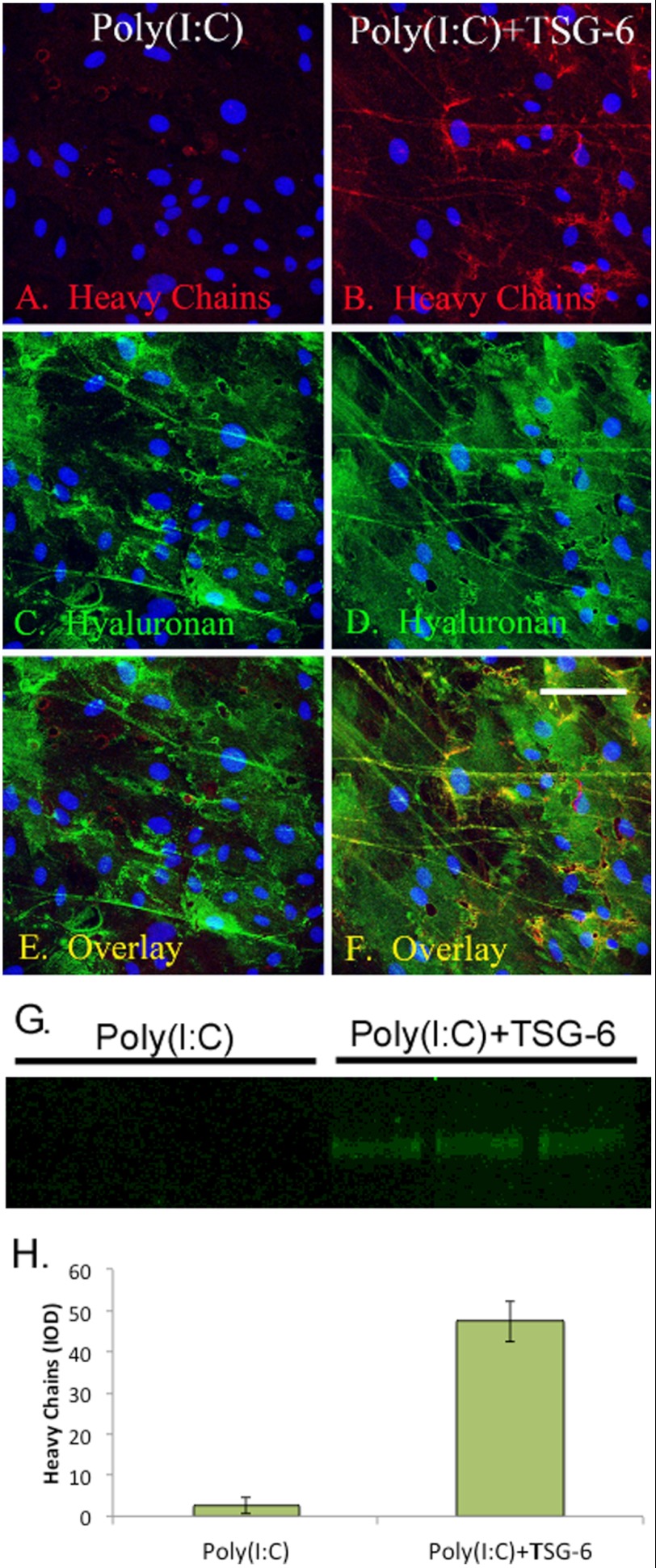
We tested the hypothesis that the artificial addition of HCs to HA, by adding recombinant TSG-6 to the culture medium, would enhance leukocyte binding to HA cables. Fig. 1 (A, C, and E) shows that MASM cells treated with poly(I:C) synthesized HA cables without detectable HCs. However, when TSG-6 was added to the culture medium at the same time as poly(I:C), HC staining colocalized with HA staining (Fig. 1 B, D, and F). In parallel cultures, Streptomyces hyaluronidase was applied in serum-free medium to digest HA cables and solubilize the HCs. Western blots of the digests probed with the same anti-HC antibodies used for the confocal microscopy analyses of Fig. 1 (A and B) showed a single band slightly over 75 kDa only when TSG-6 was present (Fig. 1G). The quantification of this gel is shown in Fig. 1H. In Fig. 2, we confirmed that the MASM cells did not secrete their own HC donor (IαI and/or pre-IαI) into the conditioned medium, demonstrating that the sole source of these HC donors is the FBS in the culture medium.
FIGURE 1.
Artificial addition of HCs to HA cables via recombinant TSG-6. MASM cells were cultured in the presence of 5% FBS, treated with poly(I:C) (A, C, and E) or poly(I:C) plus recombinant TSG-6 (B, D, and F) for 18 h, fixed in methanol, and double-labeled with HABP (green; C, and D) with the simultaneous incubation of antibodies against the HC1 and HC2 isotypes (red; A and B). Nuclei were stained with DAPI (blue). The samples were imaged by confocal microscopy at ×4. Scale bar = 100 μm. An overlay of the red/green channels is shown in E and F. In separate cultures, Streptomyces hyaluronidase was added in serum-free medium to release HCs bound to HA cables. Aliquots of these hyaluronidase extracts (n = 3) were analyzed by Western blotting (G), probing the blots with the same anti-HC antibodies probed in A and B, yielding a single band slightly over 75 kDa. Quantification of this band is shown in H. Error bars represent S.D. IOD, integrated optical density.
FIGURE 2.
MASM cells do not make their own HC donor. The Western blots of MASM cell-conditioned media show the absence of endogenous HC donors IαI and/or pre-IαI. Conditioned media from untreated MASM cells (lanes 2–4) and cells treated with poly(I:C) + TSG-6 (lanes 5–7) for 12 h were compared with unconditioned medium alone (M; lane 8) or an aliquot of mouse serum (lanes 9 and 10) as a positive control. Some samples were treated with chondroitinase ABC (c′ABC; lanes 3, 6, and 10) to release free HCs from IαI and/or pre-IαI (shown as a single 83-kDa band in lane 10). Other samples were incubated with Streptomyces hyaluronidase (HAase); lanes 4 and 7 to release free HCs from high molecular mass HA-HC that may have been in the conditioned media (which was not observed). The blot was probed with the simultaneous incubation of antibodies against the HC1 and HC2 isotypes. These antibodies are species-specific for only mouse HCs and do not cross-react with the HC epitopes of bovine origin (as supplied by FBS in the medium). Thus, IαI and/or pre-IαI from FBS is truly present in lanes 2–8, but the antibodies are unable to detect it. This is different from the situation in Fig. 1, where the culture medium was supplemented with mouse serum as the IαI source instead of FBS, which was used throughout the rest of this work.
TSG-6 Promotes HA Cable Formation
Interestingly, in addition to transferring HCs to HA cables (Fig. 1), TSG-6 also greatly enhanced the thickness and possibly the number of the cables when added with poly(I:C) but had no effect beyond that of the untreated control when added in the absence of poly(I:C) (Fig. 3). This also demonstrates the lack of possible proinflammatory cytotoxins in our TSG-6 preparation that could influence HA synthesis.
FIGURE 3.
TSG-6 promotes HA cable formation. MASM cells were either not treated (A) or treated with only recombinant TSG-6 (B), poly(I:C) (C), or the simultaneous incubation of poly(I:C) + TSG-6 (D) for 18 h. The samples were then fixed in methanol and probed with HABP (green). Nuclei were stained with DAPI (blue). The samples were imaged by confocal microscopy at ×40. Scale bar = 100 μm.
TSG-6 Promotes Leukocyte Adhesion to HA Cables
As expected, the addition of HCs to HA cables, by adding recombinant TSG-6 with poly(I:C) to the culture medium of MASM cells, significantly increased the number of leukocytes that bound to the HA cables (Fig. 4). Whereas poly(I:C) induced the formation of single HA cables that were leukocyte-adhesive (Fig. 4, D–F), the addition of TSG-6 promoted leukocyte adhesion to the extent that the appearance was more sheet-like than cable-like (Fig. 4, G–I). This was more likely because TSG-6 promoted a greater HA cable density in a given area rather than promoting the actual formation of HA sheet structures. TSG-6 enhanced leukocyte binding by 41% (p < 0.0001) compared with poly(I:C) alone (Fig. 5).
FIGURE 4.
TSG-6 promotes leukocyte adhesion to HA cables. MASM cells were either not treated (A–C) or treated with only poly(I:C) (D–F) or the simultaneous incubation of poly(I:C) + TSG-6 (G–I) for 18 h. U937 monocytic cells were then applied to the MASM cells for 30 min at 4 °C, and unbound cells were gently washed away in 4 °C medium. The MASM and bound U937 cells were fixed in methanol and probed with antibodies against CD44 (red; A, D, and G) or HA (green; B, E, and H). The samples were imaged by confocal microscopy at ×40. Scale bar = 100 μm. An overlay of the red/green channels is shown in C, F, and I.
FIGURE 5.

Quantification of leukocytes bound to HA cables formed in the presence of TSG-6. MASM cells were either not treated (NT) or treated with only recombinant TSG-6, poly(I:C), or the simultaneous incubation of poly(I:C) + TSG-6 for 18 h. U937 monocytic cells were labeled with CM-DiI and applied to the MASM cells for 30 min at 4 °C, gently washing away unbound cells in 4 °C medium. Streptomyces hyaluronidase was added to the cultures in 4 °C medium for 5 min to release U937 cells bound to HA cables. The number of U937 cells remaining bound to the MASM cells with or without hyaluronidase treatment was measured as described under “Experimental Procedures”( n = 16). Error bars represent S.D.
TSG-6 Amplifies HA Synthesis
Although a TSG-6-mediated increase in leukocyte adhesion to HA cables was expected, we were surprised to find that TSG-6 also stimulated HA synthesis by MASM cells (Fig. 6). Induction of HA synthesis by poly(I:C) was observable in the cell layer 4 h after its addition to the culture medium, peaking at 18 h (Fig. 6A). The addition of recombinant TSG-6 to the culture medium demonstrated similar kinetics and also a greatly increased HA synthesis over poly(I:C) treatment alone (3.58-fold increase at 18 h; p = 0.0013). By 24 h, the amount of HA in the cell layer was significantly reduced (65% for poly(I:C) alone and 58% for poly(I:C) + TSG-6; p = 0.018 and 0.0034, respectively), indicating significantly increased catabolism and decreased synthesis after 18 h. Cultures that were not treated or treated only with TSG-6 showed similar kinetics but at much lower levels.
FIGURE 6.
TSG-6 amplifies HA synthesis. MASM cells were either not treated (NT; blue) or treated with only TSG-6 (red), poly(I:C) (green), or the simultaneous incubation of poly(I:C) + TSG-6 (purple) for 2, 4, 6, 12, 18, and 24 h. HA attached to the cells (A) and secreted into the media (B) was quantified by FACE (n = 3 for each time point). Error bars represent S.D.
Although poly(I:C) induced HA synthesis in the cell layer, it did not induce the accumulation of HA in the conditioned medium beyond the levels for untreated cells and for cells treated with TSG-6 alone (Fig. 6B). In these cultures, the HA concentrations in the media steadily increased, peaking at 18 h, and diminished by 24 h, similar to HA kinetics in the cell layers. In contrast, when TSG-6 was added with poly(I:C), the amount of HA in the conditioned medium was significantly lower than in the other treatment groups and was not decreased at 24 h, possibly because the cells were preoccupied with removing the much larger amounts of HA present in the cell layer of these cultures compared with the amount of HA synthesis induced by the other treatments. Although this decrease in HA in the medium could contribute to the increase in the cell layer for the combined TSG-6 and poly(I:C) cultures, the amount in the medium at 18 h (0.01 μg) was very small compared with the amount in the cell layer (0.25 μg).
Regulation of HAS Enzymes by Poly(I:C) but Not TSG-6
Because TSG-6 increased HA synthesis in combination with poly(I:C), we tested the hypothesis that this induction was by increased transcription of the HAS enzymes (Fig. 7). Real-time PCR showed a respective 6.51- and 1.79-fold 4 h induction of HAS1 and HAS2 mRNA levels by poly(I:C) treatment (p < 0.0001 and 0.001, respectively) (Fig. 7, A and B), but the addition of TSG-6 did not have an additive effect. In contrast, HAS3 mRNA levels actually decreased by 58% upon treatment with TSG-6 alone and by 63% with poly(I:C) alone (Fig. 7C). This decrease in HAS3 mRNA levels was additive when TSG-6 was combined with poly(I:C), giving a further 57% decrease compared with poly(I:C) alone. Using the linear regression of efficiency method to model PCR amplification (23, 24), we found that, in untreated cells, HAS2 was 19-fold more abundant than HAS1 and 134-fold more abundant than HAS3, suggesting that HAS2 is the most important enzyme mediating this process. These data demonstrate that the amplification of poly(I:C)-induced HA synthesis by TSG-6 does not occur by increased transcription of the HAS genes and also demonstrate that HAS3 is not likely to be one of the enzymes mediating this process.
FIGURE 7.

TSG-6 does not amplify HA synthesis by increased transcription. MASM cells were either not treated (NT) treated with only TSG-6, poly(I:C), or the simultaneous incubation of poly(I:C) + TSG-6 for 2 h (white bars) and 4 h (gray bars). Transcription of mRNA for HAS1, HAS2, and HAS3 (A–C, respectively) was determined by real-time PCR (n = 6 for each treatment). Error bars represent S.D.
TSG-6 Promotes Accumulation of HA in the Cell-associated Matrix during Active HA Synthesis
In the data shown up to this point, TSG-6 was added at the same time as poly(I:C) for the entire treatment period. We next tested whether TSG-6 could amplify HA synthesis and promote leukocyte adhesion if it was applied for 6 h after an 18 h treatment with poly(I:C) alone (Fig. 8). Regardless of whether TSG-6 was added at the same time as poly(I:C) or 18 h later, leukocyte adhesion was induced by 48% compared with cells treated with poly(I:C) alone (Fig. 8A). Interestingly, an induction of HA synthesis in the cell layer with its concomitant decrease in the conditioned medium was observed only when TSG-6 was applied at the same time as poly(I:C) (Fig. 8B), i.e. the addition of TSG-6 at 18 h increased leukocyte binding at 24 h without inducing an additive effect on HA synthesis beyond poly(I:C) treatment alone. We also observed an enhancement in the thickness of the cables when TSG-6 was added after 18 h of treatment with poly(I:C) (Fig. 8F).
FIGURE 8.
TSG-6 promotes the accumulation of HA into the cell-associated matrix during active HA synthesis. MASM cells were either not treated (NT) or treated with only poly(I:C) or the simultaneous incubation of poly(I:C) + TSG-6 (TSG-6 During poly(I:C)) for 24 h. Parallel cultures were treated with poly(I:C) for the first 18 h, and then an aliquot of TSG-6 was added to the conditioned culture medium for an additional 6 h (TSG-6 After poly(I:C)) for a total of 24 h. The U937 leukocyte binding assay was performed on these cultures (A; n = 3), and HA levels were measured by FACE (B; n = 6). Error bars represent S.D. C–F portray images of the MASM cells probed with HABP (green) and either not treated (C) or treated with poly(I:C) alone for 24 h (D), treated with poly(I:C) + TSG-6 for 24 h (E), or treated with poly(I:C) for 18 h followed by treatment with TSG-6 for another 6 h (F).
Endogenous TSG-6 Is Not Required for Synthesis of Leukocyte-adhesive HA Cables or for Induction of HA Synthesis by Exogenous TSG-6
We next tested whether endogenous TSG-6 has any role during the formation of HA cables and if the lack of endogenous TSG-6 has any effect on the ability of recombinant TSG-6 to amplify HA synthesis. To accomplish this, MASM cell cultures were prepared from mice homozygous null for TSG-6 (10) and treated as indicated in Fig. 9. HA cables produced by TSG-6−/− MASM cells promoted leukocyte binding (Fig. 9A), essentially identical to HA cables produced by WT MASM cells (Fig. 5). Furthermore, amplification of HA synthesis in the cell layer by the addition of recombinant TSG-6 to poly(I:C)-treated TSG-6−/− cultures (Fig. 9B) was essentially identical to that in MASM cells derived from WT mice at 18 h (Fig. 6A). Additionally, depletion of HA from the conditioned medium of cultures treated with poly(I:C) + TSG-6 (Fig. 9C) was also similar to that of WT cultures (Fig. 6B). Thus, we conclude that endogenous TSG-6 does not have a role in the formation of HA cables induced by poly(I:C) alone, and it is not necessary for the exogenous TSG-6-mediated induction of HA synthesis when recombinant TSG-6 is added with poly(I:C).
FIGURE 9.

Endogenous TSG-6 is not required for the synthesis of leukocyte-adhesive HA cables or induction of HA synthesis by exogenous TSG-6. MASM cells derived from TSG-6−/− mice were either not treated (NT) or treated with only TSG-6, poly(I:C), or the simultaneous incubation of poly(I:C) + TSG-6. The U937 leukocyte binding assay was performed on these cultures (A; n = 4). HA attached to the cells (B; n = 6) and secreted into the media (C; n = 6) was quantified by FACE. Error bars represent S.D.
Neither HAS1 Nor HAS3 Is Required for Amplification of HA Synthesis by TSG-6
Because the fold increase in HAS1 expression by poly(I:C) was greater compared with HAS2 (Fig. 7), we determined whether exogenous TSG-6 could amplify HA synthesis with HAS2 alone. MASM cell cultures were prepared from mice that were homozygous null for both HAS1 and HAS3 (19, 25, 26) and treated as indicated in Fig. 10. With the exception of a 62% increase (p < 0.0001) in the amount of HA released into the conditioned medium of poly(I:C)-treated cells, the results were essentially identical to those for the TSG-6 null and WT MASM cells. Thus, we conclude that, although the amplification of HA synthesis by HAS1 may occur, HAS2 alone is sufficient for this induction.
FIGURE 10.

Neither HAS1 nor HAS3 is required for amplification of HA synthesis by TSG-6. MASM cells derived from mice homozygous null for both HAS1 and HAS3 were either not treated or treated with only TSG-6, poly(I:C), or the simultaneous incubation of poly(I:C) + TSG-6. The U937 leukocyte binding assay was performed on these cultures (A; n = 6). HA attached to the cells (B; n = 6) and secreted into the media (C; n = 6) was quantified by FACE. Error bars represent S.D.
HA Receptor CD44 Is Not Required for Amplification of HA Synthesis by TSG-6
Alone, TSG-6 has no effect on HA synthesis by MASM cells (as shown throughout this study). The amplification of HA synthesis was observed only when TSG-6 was combined with the viral mimic poly(I:C). We wanted to see if the HA receptor CD44 was involved in this process. MASM cells were isolated from CD44−/− mice and either not treated or treated with TSG-6 alone, poly(I:C) alone, or the simultaneous incubation of poly(I:C) with TSG-6 (Fig. 11). MASM cells from CD44−/− promoted U937 leukocyte binding (Fig. 11A) and HA synthesis in the cell layer (Fig. 11B) and conditioned media (Fig. 11C) in a manner very similar to MASM cells derived from WT mice with TSG-6 + poly(I:C), clearly increasing leukocyte adhesion and HA matrix accumulation. These data show that the amplification of HA synthesis by TSG-6 is independent of CD44.
FIGURE 11.

CD44 is not required for amplification of HA synthesis by TSG-6. MASM cells derived from mice homozygous null for CD44 were either not treated (NT) or treated with only TSG-6, poly(I:C), or the simultaneous incubation of poly(I:C) + TSG-6. The U937 leukocyte binding assay was performed on these cultures (A; n = 6). HA attached to the cells (B; n = 6) and secreted into the media (C; n = 6) was quantified by FACE. Error bars represent S.D.
DISCUSSION
Although we showed that HCs appear to be absent from HA cables produced by MASM cell cultures (17), they can be artificially added to the cables by supplementing the medium with recombinant TSG-6. The treatment of MASM cell cultures with TSG-6 in combination with poly(I:C) had the following effects: (i) greatly increased HA synthesis; (ii) improved HA cable formation compared with poly(I:C) alone, resulting in the formation of thicker and more pronounced cables; (iii) enhanced leukocyte adhesion to these cables; and (iv) diminished amounts of HA released into the medium. It is important to emphasize that these effects were observed only when TSG-6 was applied at the same time as poly(I:C) and that the application of TSG-6 alone had no effect. This suggests that TSG-6 may have a similar effect with other agents that induce HA synthesis, particularly in inflammation responses. It is also important to emphasize that when TSG-6 was added after poly(I:C)-induced HA synthesis was complete, TSG-6 did not increase HA synthesis, although it did improve the adhesion of leukocytes to the HA cables.
The mechanism whereby HC transfer to HA promotes leukocyte adhesion is not clear, although several hypotheses present themselves. For example, HA-HC may increase the avidity of leukocytes to HA matrices by functioning as a ligand that binds to a receptor(s) on the leukocyte surface, such as the HA receptor CD44. Indeed, binding of leukocytes to HA-HC matrices has been shown to involve CD44 (27). Alternatively, it is not clear to what extent the three-dimensional HA cable structure itself contributes to leukocyte binding. We observed that HC transfer to HA cables results in the formation of thicker and more pronounced cables. This could occur if HCs effectively cross-link HA cables into larger bundles that contain structural information that promotes the adhesion of leukocytes. Furthermore, we have not ruled out the possibility that TSG-6 itself might promote the assembly of HA cables because purified TSG-6 has been shown to have a cross-linking effect with purified HA (28).
Although it is clear that TSG-6 induces the accumulation of HA in the cell-associated matrix, the mechanism that mediates this process remains to be established. We have shown that the amount of HA released into the conditioned medium is significantly diminished in cultures treated with poly(I:C) + TSG-6 compared with poly(I:C) alone (Figs. 6, 9, and 10). This suggested the possibility that the enhanced accumulation of HA in the cell-associated matrix is simply by more efficient retention of HA in the cell layer, which could be accomplished by HC-mediated cross-linking of HA monomers. Although this contributes some additional HA to the matrix, it is clear that the very small amount of HA released into the conditioned medium compared with the amount in the matrix could not by itself account for the much larger accumulation of HA in the cell layer when MASM cell cultures were treated with the combination of TSG-6 and poly(I:C) compared with poly(I:C) alone.
It is also possible that decreased turnover/degradation of HA contributes to the increased accumulation of HA in the cell layer. Clearly, the MASM cells have a robust capacity for catabolism of HA, as demonstrated by the sharp decrease in HA levels from 18 to 24 h (Fig. 6). Our hypothesis is that this decrease is most likely an artifact of the cell culture model related to the depletion of nutrients (such as glucose) in the culture medium and that this would not occur with the relatively unlimited source of nutrients available in vivo. This catabolism of HA could be related to the turnover of membrane receptors for HA, such as has been previously shown for the rapid CD44-mediated turnover of HA in keratinocytes (29). Although possible, it is unlikely that the accumulation of HA in the cell layer for the first 18 h would be explained by decreased turnover alone. The increased expression of HAS1 and HAS2 upon treatment with poly(I:C) demonstrates that up-regulation of HA synthesis is likely to be the dominant contributor (Fig. 7). The most likely interpretation of these results is that TSG-6 actually increases the synthesis of HA in combination with another proinflammatory stimulus that induces HA synthesis (i.e. poly(I:C)). In these cases, TSG-6 may act as a cytokine to increase either the protein translation or activity of the HAS enzymes, although up-regulation of HAS mRNA transcription is not the mechanism for the TSG-6-mediated induction of HA synthesis (Fig. 7).
Alone, TSG-6 has no effect on HA synthesis, but did affect HA synthesis when used in combination with the viral mimic poly(I:C). This mechanism most likely involves increasing HAS translation, activity, and/or trafficking to the cell surface beyond that mediated by poly(I:C) alone. Although this mechanism clearly does not involve CD44, we have not ruled out the possibility that other HA receptors, such as RHAMM, HARE, LYVE-1, and LAYLIN, could be involved. Similarly, Toll-like receptors, such as TLR2 and TLR4, have been shown to bind HA (30) and may play a role in this process, especially because one of the pathways whereby poly(I:C) is recognized by cells is TLR3.
These data predict that TSG-6 would regulate HA synthesis in a variety of inflammatory diseases in which increased HA synthesis is involved. In our accompanying article (31), we showed that mice homozygous null for TSG-6 developed a milder form of asthma, with less leukocyte accumulation and, importantly, less HA accumulation in the lung tissue. In summary, our results indicate that TSG-6 synergizes with the viral stimulant poly(I:C) and extends its catalytic function in the formation of HA-HC to the actual induction of the synthesis of HA matrices.
Acknowledgment
We thank Diane Leigh (Department of Biomedical Engineering, Cleveland Clinic) for generous help with the linear regression of efficiency method to model PCR amplification.
This work was supported, in whole or in part, by National Institutes of Health Grants P11 HL081064 and P01 HL107147 from NHLBI.
- HA
- hyaluronan
- HC
- heavy chain
- IαI
- inter-α-inhibitor
- MASM
- murine airway smooth muscle
- HAS
- HA synthase
- FACE
- fluorophore-assisted carbohydrate electrophoresis
- TEMED
- N,N,N′,N′-tetramethylethylenediamine
- HABP
- HA-binding protein.
REFERENCES
- 1. Huang L., Yoneda M., Kimata K. (1993) A serum-derived hyaluronan-associated protein (SHAP) is the heavy chain of the inter α-trypsin inhibitor. J. Biol. Chem. 268, 26725–26730 [PubMed] [Google Scholar]
- 2. Zhao M., Yoneda M., Ohashi Y., Kurono S., Iwata H., Ohnuki Y., Kimata K. (1995) Evidence for the covalent binding of SHAP, heavy chains of inter-α-trypsin inhibitor, to hyaluronan. J. Biol. Chem. 270, 26657–26663 [DOI] [PubMed] [Google Scholar]
- 3. Fries E., Kaczmarczyk A. (2003) Inter-α-inhibitor, hyaluronan and inflammation. Acta Biochim. Pol. 50, 735–742 [PubMed] [Google Scholar]
- 4. Kida D., Yoneda M., Miyaura S., Ishimaru T., Yoshida Y., Ito T., Ishiguro N., Iwata H., Kimata K. (1999) The SHAP-HA complex in sera from patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 26, 1230–1238 [PubMed] [Google Scholar]
- 5. Obayashi Y., Yabushita H., Kanyama K., Noguchi M., Zhuo L., Kimata K., Wakatsuki A. (2008) Role of serum-derived hyaluronan-associated protein-hyaluronan complex in ovarian cancer. Oncol. Rep. 19, 1245–1251 [PubMed] [Google Scholar]
- 6. Kishida T., Yabushita H., Wakatsuki A., Zhuo L., Kimata K. (2008) Hyaluronan (HA) and serum-derived hyaluronan-associated protein (SHAP)-HA complex as predictive markers of cervical ripening in premature labor. Connect. Tissue Res. 49, 105–108 [DOI] [PubMed] [Google Scholar]
- 7. Shen L., Zhuo L., Okumura A., Ishikawa T., Miyachi M., Owa Y., Ishizawa T., Sugiura N., Nagata Y., Nonami T., Kakumu S., Kimata K. (2006) The SHAP-hyaluronan complex in serum from patients with chronic liver diseases caused by hepatitis virus infection. Hepatol. Res. 34, 178–186 [DOI] [PubMed] [Google Scholar]
- 8. Mukhopadhyay D., Hascall V. C., Day A. J., Salustri A., Fülöp C. (2001) Two distinct populations of tumor necrosis factor-stimulated gene-6 protein in the extracellular matrix of expanded mouse cumulus cell-oocyte complexes. Arch Biochem. Biophys. 394, 173–181 [DOI] [PubMed] [Google Scholar]
- 9. Mukhopadhyay D., Asari A., Rugg M. S., Day A. J., Fülöp C. (2004) Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J. Biol. Chem. 279, 11119–11128 [DOI] [PubMed] [Google Scholar]
- 10. Fülöp C., Szántó S., Mukhopadhyay D., Bárdos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6-deficient mice. Development 130, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 11. Wisniewski H.-G., Vilcek J. (2004) Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 15, 129–146 [DOI] [PubMed] [Google Scholar]
- 12. Jessen T. E., Ødum L. (2003) Role of tumour necrosis factor-stimulated gene 6 (TSG-6) in the coupling of inter-α-trypsin inhibitor to hyaluronan in human follicular fluid. Reproduction 125, 27–31 [DOI] [PubMed] [Google Scholar]
- 13. Forteza R., Casalino-Matsuda S. M., Monzon M. E., Fries E., Rugg M. S., Milner C. M., Day A. J. (2007) TSG-6 potentiates the antitissue kallikrein activity of inter-α-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 36, 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al'Qteishat A., Gaffney J., Krupinski J., Rubio F., West D., Kumar S., Kumar P., Mitsios N., Slevin M. (2006) Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain 129, 2158–2176 [DOI] [PubMed] [Google Scholar]
- 15. de La Motte C. A., Hascall V. C., Calabro A., Yen-Lieberman B., Strong S. A. (1999) Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I·C). J. Biol. Chem. 274, 30747–30755 [DOI] [PubMed] [Google Scholar]
- 16. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., Strong S. A. (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 163, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauer M. E., Fulop C., Mukhopadhyay D., Comhair S., Erzurum S. C., Hascall V. C. (2009) Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-α-inhibitor heavy chain attachment. J. Biol. Chem. 284, 5313–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lauer M. E., Mukhopadhyay D., Fulop C., de la Motte C. A., Majors A. K., Hascall V. C. (2009) Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J. Biol. Chem. 284, 5299–5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mack J. A., Feldman R. J., Itano N., Kimata K., Lauer M., Hascall V. C., Maytin E. V. (2012) Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J. Invest. Dermatol. 132, 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoop R., Kotani H., McNeish J. D., Otterness I. G., Mikecz K. (2001) Increased resistance to collagen-induced arthritis in CD44-deficient DBA/1 mice. Arthritis Rheum. 44, 2922–2931 [DOI] [PubMed] [Google Scholar]
- 21. Lauer M. E., Erzurum S. C., Mukhopadhyay D., Vasanji A., Drazba J., Wang A., Fulop C., Hascall V. C. (2008) Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J. Biol. Chem. 283, 26283–26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng G., Swaidani S., Sharma M., Lauer M. E., Hascall V. C., Aronica M. A. (2011) Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 30, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leigh D. R., Mesiha M., Baker A. R., Walker E., Derwin K. A. (2012) Host response to xenograft ECM implantation is not different between the shoulder and body wall sites in the rat model. J. Orthop. Res. 30, 1725–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rutledge R. G., Stewart D. (2008) A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnol. 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi N., Miyoshi S., Mikami T., Koyama H., Kitazawa M., Takeoka M., Sano K., Amano J., Isogai Z., Niida S., Oguri K., Okayama M., McDonald J. A., Kimata K., Taniguchi S., Itano N. (2010) Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 70, 7073–7083 [DOI] [PubMed] [Google Scholar]
- 26. Bai K.-J., Spicer A. P., Mascarenhas M. M., Yu L., Ochoa C. D., Garg H. G., Quinn D. A. (2005) The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 172, 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhuo L., Kanamori A., Kannagi R., Itano N., Wu J., Hamaguchi M., Ishiguro N., Kimata K. (2006) SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 281, 20303–20314 [DOI] [PubMed] [Google Scholar]
- 28. Baranova N. S., Nilebäck E., Haller F. M., Briggs D. C., Svedhem S., Day A. J., Richter R. P. (2011) The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J. Biol. Chem. 286, 25675–25686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tammi R., Rilla K., Pienimaki J. P., MacCallum D. K., Hogg M., Luukkonen M., Hascall V. C., Tammi M. (2001) Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 276, 35111–35122 [DOI] [PubMed] [Google Scholar]
- 30. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 31. Swaidani S., Cheng G., Lauer M. E., Sharma M., Mikecz K., Hascall V. C., Aronica M. A. (2013) TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J. Biol. Chem. 288, 412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]