Background: AlkB dioxygenase removes alkyl and exocyclic lesions via an oxidative mechanism, restoring the native DNA bases.
Results: AlkB repair efficiency is pH- and Fe(II) concentration-dependent, which correlates with the substrate pKa.
Conclusion: AlkB recognizes and repairs protonated substrates.
Significance: This study provides experimental evidence for the molecular mechanism of action of AlkB.
Keywords: DNA Enzymes, DNA Repair, Docking, Enzyme Mechanisms, Ligand-binding Protein, Molecular Modeling, AlkB Dioxygenase, Etheno Adducts to DNA Bases, Exocyclic Adducts to DNA Bases
Abstract
Efficient repair by Escherichia coli AlkB dioxygenase of exocyclic DNA adducts 3,N4-ethenocytosine, 1,N6-ethenoadenine, 3,N4-α-hydroxyethanocytosine, and reported here for the first time 3,N4-α-hydroxypropanocytosine requires higher Fe(II) concentration than the reference 3-methylcytosine. The pH optimum for the repair follows the order of pKa values for protonation of the adduct, suggesting that positively charged substrates favorably interact with the negatively charged carboxylic group of Asp-135 side chain in the enzyme active center. This interaction is supported by molecular modeling, indicating that 1,N6-ethenoadenine and 3,N4-ethenocytosine are bound to AlkB more favorably in their protonated cationic forms. An analysis of the pattern of intermolecular interactions that stabilize the location of the ligand points to a role of Asp-135 in recognition of the adduct in its protonated form. Moreover, ab initio calculations also underline the role of substrate protonation in lowering the free energy barrier of the transition state of epoxidation of the etheno adducts studied. The observed time courses of repair of mixtures of stereoisomers of 3,N4-α-hydroxyethanocytosine or 3,N4-α-hydroxypropanocytosine are unequivocally two-exponential curves, indicating that the respective isomers are repaired by AlkB with different efficiencies. Molecular modeling of these adducts bound by AlkB allowed evaluation of the participation of their possible conformational states in the enzymatic reaction.
Introduction
Exocyclic DNA base adducts are produced by a variety of bifunctional electrophilic agents of exogenous and endogenous origin. These agents include products of endogenous lipid peroxidation occurring as a consequence of oxidative stress, α,β-unsaturated aldehydes (enals) such as malondialdehyde, acrolein (ACR),2 croton aldehyde, and trans-4-hydroxy-2-nonenal (1). Other sources of such compounds are environmental pollutants (tobacco smoke and automobile exhaust), dietary toxins (formed for example in overfried food), and metabolites of the industrial carcinogen vinyl chloride, chloroethylene epoxide and chloroacetaldehyde.
Structurally, exocyclic adducts are analogous but can differ in ring size, saturation, angularity, and nature or location of substituents. Some of them occur as stereoisomers (2). The rings, usually five- or six-membered, are formed via bridging of an exocyclic NH2 group with an endocyclic nitrogen atom of a purine or pyrimidine base. The only DNA base lacking an exocyclic NH2 group and thus resistant to this kind of modification is thymine (3). Reaction of DNA bases with chloroacetaldehyde proceeds via hydroxyethano intermediates, which are spontaneously dehydrated to an etheno (ϵ) form (4). We have shown that the most stable among them, 3,N4-α-hydroxyethanocytosine (HEC) with a t½ at 37 °C of about 24 h (5), plays an independent role in mutagenesis (6, 7).
Exocyclic ring modifications are highly mutagenic and cytotoxic DNA lesions, leading to miscoding or replication blocks and generating various degenerative disorders and several types of cancer. Different mechanisms appear to have evolved for the removal of specific exocyclic adducts. These include base excision repair, nucleotide excision repair, mismatch repair, and apurinic/apyrimidinic endonuclease-mediated repair (for reviews, see Refs. 2 and 8). Recently, yet another single protein AlkB-directed repair system protecting cellular DNA and RNA against alkylating agents has been discovered.
Escherichia coli AlkB is the best known member of the superfamily of α-ketoglutarate (αKG)- and iron-dependent dioxygenases (9) that remove alkyl lesions from DNA bases via an oxidative mechanism, restoring the native bases in the DNA. Its primary substrates are 1-methyladenine (m1A) and 3-methylcytosine (m3C) (10, 11). AlkB can also revert bulkier adducts such as ethyl, propyl, and hydroxyalkyl groups (12, 13) as well as exocyclic ϵ and ethano adducts (14–16). It has been reported that AlkB also removes methyl groups from 1-methylguanine (m1G) and 3-methylthymine (m3T) although much less efficiently (17–19). Further studies have revealed that AlkB homologs are present in almost all organisms including human. Besides methylated single-stranded DNA, dsDNA and RNA also can be substrates for AlkB and its homologs (20–22). As do other members of this superfamily, AlkB requires oxygen (O2) and non-heme Fe(II) as cofactors and αKG as cosubstrate to initiate oxidative demethylation of DNA bases, resulting in the formation of succinate and CO2. The methyl and ethyl groups are hydroxylated and spontaneously released as formaldehyde and acetaldehyde, respectively (10–12). In the case of an etheno bridge, the double bond is epoxidized, then spontaneously hydroxylated to glycol, and released as glyoxal, leading to regeneration of the natural base (16, 23). Recently, we have found that AlkB repairs not only 3,N4-ethenocytosine (ϵC) but also its precursor HEC, and we also formulated a hypothesis that AlkB more readily recognizes and repairs substrates if they exist in a protonated form (7). This was also previously suggested by Koivisto et al. (18) and in a crystallographic study by Yi et al. (23) both on the basis of differences of repair efficiency between the best AlkB substrates m1A and m3C and very poorly repaired non-protonated m3T.
Here, we provide experimental evidence for the molecular mechanism of action confirming that E. coli AlkB dioxygenase preferentially repairs substrates in cationic form. Our study focuses on the specificity of AlkB-directed repair of various exocyclic DNA adducts, which differ in their protonation ability. These include two unsaturated, five-membered etheno adducts, 1,N6-ethenoadenine (ϵA) and ϵC, which are both known AlkB substrates (14, 16, 24), and two saturated adducts, the five-membered HEC and a six-membered acrolein adduct, 3,N4-α-hydroxypropanocytosine (HPC) (25, 26). Additionally, as a positive control, m3C was used (Fig. 1). HEC described by us recently (7) and HPC reported here for the first time form a new, chemically distinct group of AlkB substrates. Using HPLC technique and single-stranded pentamer oligodeoxynucleotides containing the modifications studied, we established optimal pH and Fe(II) and αKG concentrations for the AlkB-directed repair reaction. In parallel, using the known crystal structure of AlkB in complex with Fe(II), αKG, and methylated trinucleotide T(m1A)T (Protein Data Bank code 3I2O) (27), we performed molecular modeling of putative complexes with the modified TXT trinucleotides (where X represents a modified base). The consensus achieved between the simulated annealing procedure coupled with molecular dynamics (MD) studies and screening for the best location for X done with the aid of a restricted docking algorithm allowed building of reliable structures for all of the analyzed trinucleotides in complex with AlkB.
FIGURE 1.
Structures of studied adducts to DNA bases. Shown are methylated (m3C) and lipid peroxidation products, exocyclic unsaturated (ϵC and ϵA) and exocyclic saturated (HEC and HPC). Note that Cα of HEC and HPC is chiral.
EXPERIMENTAL PROCEDURES
Detailed descriptions of all the methods are presented in supplemental Methods.
Preparation of AlkB Substrates
We incubated pentamer deoxynucleotides (TTXTT where X is cytosine (C) or adenine (A)) with chloroacetaldehyde, ACR, or methyl methanesulfonate and obtained derivatives containing modified cytosines HEC, ϵC, m3C, and HPC, respectively, or ϵA. After HPLC purification, the modified pentamers were used as AlkB substrates.
AlkB Assay
The reaction mixtures in a volume of 20 μl contained appropriate 50 mm buffer, 1 mm dithiothreitol, Fe(NH4)2(SO4)2 and αKG at varying concentrations, and 500 pmol of the substrate (see Supplemental Table S1 for details). Reactions were started by addition of purified AlkB protein or the component studied, allowed to proceed at 37 °C, stopped by adding 230 μl of ice-cold water, frozen at −20 °C to deactivate AlkB, and then analyzed by HPLC. Enzyme concentrations and reaction times (5–30 min) were screened in preliminary experiments to produce results enabling estimation of adduct repair efficiency. We began with determination of optimal pH for a given substrate and then at that particular pH we established optimal Fe(II) and finally optimal αKG concentrations. The time course reactions for rate determinations were carried out under conditions optimal for each adduct and in so called “physiological” conditions (50 mm HEPES/NaOH buffer, pH 7.5, 100 μm Fe(NH4)2(SO4)2, and 50 μm αKG).
Ab Initio Calculations
The initial structures of modified bases were adopted from the structure of corresponding native nucleoside where the sugar moiety was mimicked by a methyl group. This was followed by ab initio analysis with the aid of the Firefly version 7.1 program (28). The density functional theory calculations were performed with the B3LYP functional (29) using the 6-31G(d,p) basis set (30) previously found applicable in the analysis of similar systems (31, 32).
Protein-Ligand Interaction: Simulated Annealing Procedure
Initial coordinates of the complexes were adopted from the structure of T(m1A)T bound to AlkB (Protein Data Bank code 3I2O) (27). Structural calculations were performed with the aid of the simulated annealing protocol implemented in X-PLOR (33, 34).
Molecular Dynamics in Explicit Aqueous Solvent
MD analysis was done with the aid of YASARA Structure package using the standard YASARA2 force field (35) extended for the non-standard ligands by adding their ab initio derived topologies and charge distributions. The starting structures of the complexes were taken for the various ligands directly from the output of the simulated annealing procedure. The protein was then solvated by explicit water molecules to give an average solvent density of 1.004 g/ml. Because the structural alignments of all the accessible structures of AlkB (including those with ligands) are identical within the root of the mean squared deviation (r.m.s.d.) threshold of 0.3 Å (calculated for Cα atoms of residues located in secondary structure elements with Gln48–Met57 and Gly68–Ser79 the only exceptions), coordinates of the protein residues that were separated by more than 6 Å from the modified base were fixed during MD simulations. Similarly, all distant water molecules (threshold, 8 Å) were also frozen. For each of the base modification analyzed, a 10-ps MD simulation was performed to allow the protein to tune its structure to accommodate the ligand, and the last 3 ps of the trajectory were then subjected to detailed analysis. The stability of the four complexes of the two possible stereoisomers of HEC and HPC, all in anti conformation on the glycoside angle, were additionally tested in terms of 15-ns molecular dynamics. Those simulations were done with the use of the YASARA package in an explicit water box of size 60/63/60 Å using the YASARA2 force field (35). The simulations were done in isothermal-isobaric (NTP) ensemble at 298 K, keeping the solvent density of 0.997 g·cm−3. So the system closely mimicked that analyzed experimentally, all the modified nucleosides were flanked at both sides by deoxythymidine residues, the locations of which were adopted directly from the crystal structure of T(m3C)T trinucleotide bound to AlkB (Protein Data Bank code 3I49). The protein conformations and locations of modified residues were taken directly from the preliminary 10-ps simulations. During the first 1 ns of MD trace, the coordinates of the whole protein and those of aromatic bases were constrained. In the following steps, all the side chains of the protein were iteratively unrestricted within the center at the Fe(II) sphere with the radius growing in four 1-ns steps. After that, the modified base, αKG, iron cation, protein backbone, and flanking deoxythymidine residues were freed in 1-ns steps. The last 5 ns of MD simulation were performed for the fully solvated system with the only constraints fixing the stacking orientation of thymine bases.
Docking Procedure: Estimation of Free Energy of Binding
This was done by de novo docking performed for the MD-derived structures with the aid of the Autodock 4.2 program (36) implemented in the YASARA Structure package. Interactions between ligands and the protein were scored by van der Waals, electrostatic, hydrogen bonding, and desolvation energy terms adopted from the AMBER03 force field (37).
Numerical Analysis of the Rates of the Repair Process
Time-dependent changes in the concentrations of modified (substrate) and unmodified (repaired product) nucleotides were monitored in several independent experiments performed for various rationally selected mixtures of reagents. For each substrate, all the experimental data collected in a series of independent experiments (1 … n) were analyzed together, assuming the common model of a second order reaction controlled by a single transition state described by Equation 1
where [AlkB]i is the enzyme concentration in the ith experiment, Si(t) is the time evolution of substrate concentration in that experiment, and k is the universal rate of repair related directly to a given type of substrate. The product k·[AlkB]i represents the actual pseudo first order rate of the repair in the ith experiment, ki, that depends on the enzyme concentration, leading to a simplified pseudo first order equation.
The formal integration of Equation 1 leads to the formula
in which Si0 and [AlkB]i are the initial concentrations of substrate and AlkB, respectively; k is the second order repair rate; Δ is a delay related to the setup of the experimental procedure; and Ri is the upper limit of substrate that can be repaired in the ith experiment. For each substrate, all the kinetic data were analyzed together in terms of a model (2) consisting of two global parameters (k and Δ) and n experiment-specific Ri values, together giving a multibranch function (see Fig. 5 under Results and supplemental Figs. S1 and S2). These parameters were then optimized to obtain the best agreement with experimental data in which every branch of the curve represents an individual experiment. In the case of HEC and HPC, the model (Equation 3) was additionally extended to describe the repair of the mixture (1:α) of individual stereoisomers with two isomer-specific repair rates (k1 and k2),
 |
where α stands for relative ratio of two isomers. All the calculations were done with the aid of the R package.
FIGURE 5.
Kinetics of HPC stereoisomer mixture repair by various amounts of AlkB. Marks of a given type represent sets of experimental data, and solid lines follow the single substrate model (A) and two-substrate model (B) fitted to the same data. The visibly better agreement was obtained for the two-substrate model (see Table 1 for details), pointing to the substantial difference in repair rates of both stereoisomers (see Fig. 8 for molecular basis of that effect). Error bars represent S.D. estimated for those experiments that were repeated three times. The figure shows the initial 30 min of reactions. See supplemental Fig. S2 for the full course of the reactions.
RESULTS
Optimal Reaction Conditions for AlkB-directed Repair
Pentamers containing modified bases, m3C, HPC, HEC, ϵC, and ϵA, were studied as potential substrates for AlkB protein. Using HPLC to separate the modified and unmodified (repaired) oligomers (see Fig. 2 as an example), we found that all the modifications were repaired by E. coli AlkB dioxygenase. The appearance of the repaired oligomers was also confirmed by mass spectrometry (MS) (supplemental Table S2).
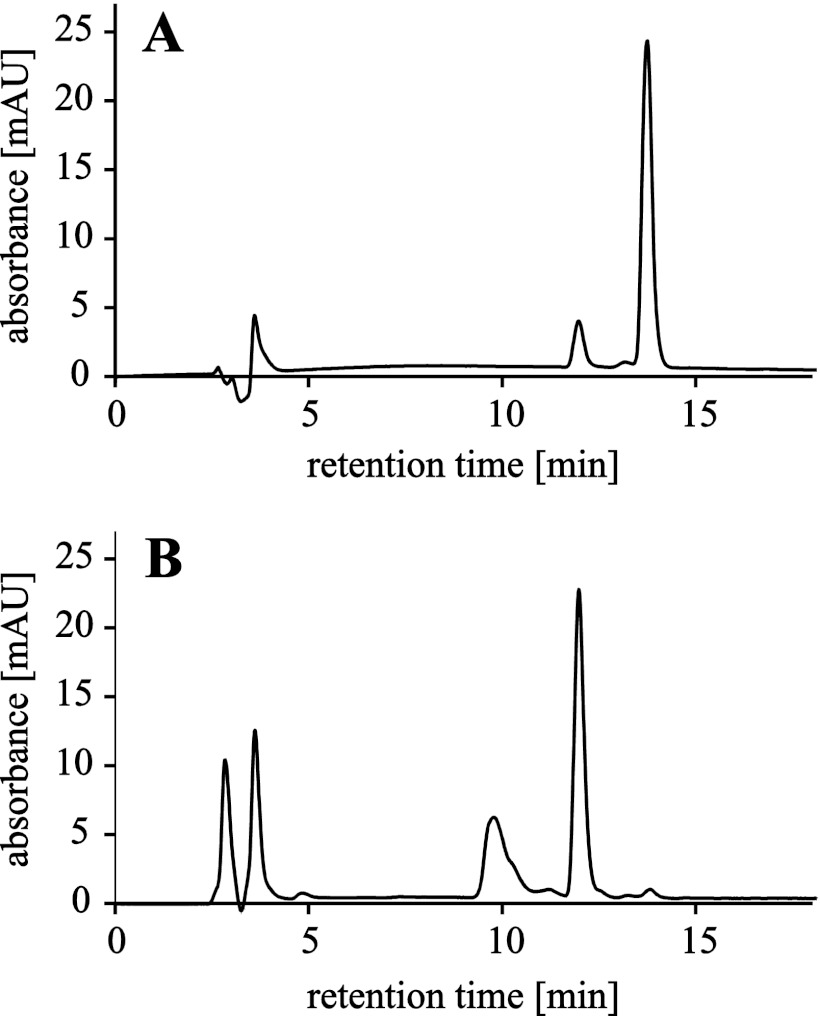
FIGURE 2.
HPLC profiles of HPC repair by AlkB protein. A, substrate TT(HPC)TT pentamer (retention time, 13.7 min) containing 12% unmodified TTCTT (retention time,11.9 min). B, 4-min reaction, 98% repair. AlkB reaction conditions were as follows: 50 mm HEPES buffer, pH of 7.5, 1 mm dithiothreitol, 100 μm Fe(NH4)2(SO4)2, 50 μm αKG, 500 pmol of TT(HPC)TT substrate, and 50 pmol of AlkB protein. The HPLC peak occurring at 9.6 min represents dithiothreitol. mAU, arbitrary units.
pH Dependence
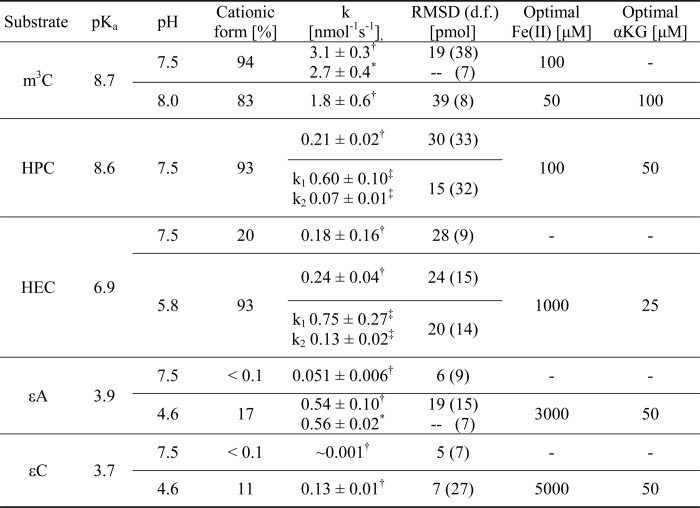
The optimal pH for m3C and HPC repair was found to be 7.5, and for HEC, the optimal pH was 5.8, whereas both ϵA and ϵC were most efficiently repaired at pH 4.6. The experimentally determined values of optimal pH for repair reactions strictly followed the order of pKa of the lesions (see Table 1). The optimal pH for the repair of adducts that display a pKa for protonation higher than 5.8 was generally 1 pH unit below the pKa. This clearly indicates much more efficient repair for the substrate in its cationic form. The comparison of repair kinetics of ϵA determined at neutral pH and optimal pH is shown in supplemental Fig. S1.
TABLE 1.
Kinetic parameters of AlkB repair of m3C and exocyclic DNA adducts at physiological pH of 7.5 and pH optimal for repair of each substrate
RMSD, r.m.s.d. of the fitted function; d.f., number of degrees of freedom; dashes, not applicable.
† Estimated according to single substrate model (Equation 3).
‡ Estimated according to two-substrate model (Equation 4).
* Estimated for a restricted set of data according to pseudo first order model (Equation 2).
Fe(II) Concentration Dependence
Optimal iron concentration ranged between 50 μm for m3C repair at pH 8.0 and 5 mm for ϵC repair at an optimal pH of 4.6 (far away from the physiological condition). These values are anticorrelative with pKa and optimal pH for the repair of a given adduct (see Fig. 3). Corresponding values are collected in Table 1.
FIGURE 3.
Interdependence between optimal concentrations of H+ and Fe(II) cations for repair of various adducts by AlkB protein.
α-Ketoglutarate Concentration Dependence
For αKG, we did not observe as strong a tendency as for Fe(II). The optimal αKG concentrations varied in the range of 25–100 μm, which are definitely below the commonly used 500 μm. Although we applied the lower, optimal αKG concentrations in further experiments, this was not the focus of our study. The experimentally observed dependences of the repair rates on pH, iron, and αKG concentrations are presented in supplemental Fig. S3.
Pseudo First Order Kinetics of AlkB Repair (Equation 2)
The kinetics of the repair processes for ϵA and HPC monitored for a long time using relatively high AlkB concentrations are shown in Fig. 4. The linearity observed for ϵA repair in Fig. 4A identifies, in accordance to Equation 2, a pseudo first order reaction. In the case of HPC, two different asymptotes clearly identify two species visibly differing in their repair rates; however, both processes can be individually considered as pseudo first order (see Fig. 4B).
FIGURE 4.
Pseudo first order kinetics of AlkB repair. The kinetics of the repair processes for ϵA (A) and HPC (B) are shown. The linearity observed for ϵA repair identifies, in accordance with Equation 2, a pseudo first order reaction. In the case of HPC, two evident asymptotes clearly identify two species visibly differing in their repair rates. Errors bars represent S.D.
Second Order Kinetics of AlkB Repair (Equation 1)
The initial rates of the repair of m3C and ϵA were estimated for nine individual experiments performed for three rationally selected AlkB concentrations. These short time experiments (shown in supplemental Fig. S4 for ϵA as an example) clearly demonstrated the pseudo first order of the repair kinetics (Equation 2). Moreover, the pseudo first order rates, ki, are proportional to the AlkB concentration, confirming a simple second order rate of the repair process (Equation 1; see Table 1 for rates estimated for ϵA and m3C).
Time Course
The kinetics of the repair process measured enabled us to determine the relative efficiency of this process normalized for enzyme concentration. This is expressed further as k1 (and additionally k2 for HEC and HPC), representing the rate of repair by 1 nmol of enzyme/s (Table 1). To improve the reliability of the rates obtained for a given adduct, a set of independent experiments was analyzed in parallel as described under “Experimental Procedures.” Results of most of the kinetic experiments performed at both physiological and “optimal” pH conditions were in agreement with the branches of the hypothetical single exponent concentration-adjusted repair curve (see Table 1 for the rates). The quality of the fit could be judged in terms of the root of the mean squared deviation (r.m.s.d.) normalized per degree of freedom (df). Those values fall in the range of 10–20 pmol of substrate (i.e. 2–5%), strictly corresponding to the experimental errors estimated in selected experiments (see error bars in Fig. 5 and supplemental Fig. S2 as an example). The exceptions were HEC and HPC for which two stereoisomers on the hydroxylated exocyclic carbon atoms were generated upon the chemical reaction. For these two adducts, the repair process is visibly better described by two-exponential curves with r.m.s.d. reduced from 25 to 20 and from 30 to 15 pmol for HEC and HPC, respectively (see Table 1 for details), clearly indicating that stereoisomers of these two pairs differ significantly in their susceptibility to AlkB-directed repair. In fact, reliable estimates of the rates of repair of individual HEC stereoisomers could only be at the optimal pH of 5.8. Kinetic curves of the repair of HPC by various concentrations of AlkB are presented in Fig. 5 and supplemental Fig. S2. In general, we found that the repair efficiency of the adducts studied follows the order m3C > HEC(fast) > HPC(fast) > ϵA > ϵC > HEC(slow) and HPC(slow) (for more details, see “Discussion”). In Fig. 2, we have shown the HPLC profile of HPC repair. Almost complete disappearance of the peak corresponding to HPC confirms that at high AlkB concentration both components of the stereoisomeric mixture are completely repaired.
Ab Initio Studies of Adducts: Protomeric and Dissociation Equilibria
To estimate the influence of substrate protonation on the efficiency of AlkB-directed repair, various tautomeric/protomeric forms of the adducts studied were tested. A comparison of the calculated free energies of protonation, ΔGprot, with the experimentally established values of pKa was used for verification of the applicability of the level of theory used. The good correlation obtained validated the method used (see supplemental Fig. S5). Consequently, for each molecule, its lowest energy protomeric state was used in the following molecular mechanics simulations. Analysis of the electron density maps clearly evidenced delocalization of the charge through large parts of the respective aromatic electron systems (supplemental Fig. S6). Additionally, according to the postulated mechanism of the first step of ϵA and ϵC oxidation (16, 23), their appropriate epoxides were also analyzed (see “Discussion” for details).
Structures of Enzyme-Substrate Complexes
To predict the mode of accommodation of the substrates in the active site of the enzyme, preliminary theoretical models of AlkB complexes with modified trinucleotides were prepared using the simulated annealing protocol followed by 10-ps MD of the fully solvated system with the “far” residues fixed. For verification of the quality of the structural models, see supplemental Fig. S7. The resulting trajectories confirmed that AlkB easily accommodates all the adducts studied. A detailed inspection of the resulting structures points to a common pattern of protein-ligand interaction involving Thr-51, Tyr-55, Met-57, Ser-58, Trp-69, Tyr-76, Lys-127, Leu-128, Ser-129, His-131, Asp-133, Asp-135, Gln-132, and Arg-161 among which Trp-69, His-131, and Asp-135 display the largest contact areas with the modified base.
The detailed analysis of location in the active site of AlkB of both stereoisomers of T(HEC)T and T(HPC)T trinucleotides clearly demonstrated that all the interactions crucial for AlkB activity are strictly preserved. Thus, in accordance with the results obtained for AlkB, the orientation of αKG molecule is stabilized by the interactions with polar side chains of residues Arg-204, Arg-210, and Asn-120. Among the interactions of the latter, the hydrogen bond between the Asn side-chain amide and α carbonyl group of αKG could be observed during ∼80% of a simulation time, whereas the two Arg residues are almost strictly present. During the majority of the simulation time, the N4 amino group of modified bases interacts with the side chain of Asp-135. In all four complexes, the Fe(II) is within a 6-Å distance of the alkyl adducts.
Further analysis concerns the stability of the bound forms, which was estimated by a free energy scoring function implemented in Autodock (36). This was done for the last 30 snapshots uniformly sampled from the registered MD trace. The obtained structures of the neutral and charged forms of the adducts bound to AlkB indicate that ϵA and ϵC are bound more favorably (approximately 1 kcal/mol) in their cationic forms (see Fig. 6 for structures). An analysis of the pattern of intermolecular interactions that stabilize the location of the ligand points to the role of Asp-135 in recognition of the N-alkylated base in its protonated form, whereas Trp-69 and His-131 sandwich the modified base, orienting it in relation to the iron redox center (see Fig. 7, A and C). In general, the preference of AlkB for cationic substrates could be clearly related to the distribution of electrostatic potential within the binding pocket area (see colored regions inside the pocket accommodating ϵA in Fig. 7, B and D). The stereoisomeric pairs of HEC and HPC also differ in their free energy of binding, but it should be pointed out that the observed differences in their repair efficiency should be attributed to the differences in the accessibility of Cβ of HEC and Cγ of HPC (Fig. 1) for the redox attack by Fe(II) rather than to their different binding energies (Fig. 8).
FIGURE 6.
Proposed structures of AlkB complexed with ϵA and ϵC. The coordinates for neutral and charged substrates were obtained after 20 rounds of the simulated annealing procedure started from the accessible structure of m1A bound to AlkB (Protein Data Bank code 3I2O) (27). Asp-135, postulated to be crucial for recognition of substrates in their cationic forms, is shown in stick representation, and Fe(II) is marked by a pink sphere of the appropriate radius.
FIGURE 7.
ϵA location in AlkB active center. A and C, ribbon models. B and D, protein surface-colored according to electrostatic potential distribution from red (−250 kJ/mol) to blue (+250 kJ/mol).
FIGURE 8.
Stereoview of proposed structures of AlkB complexed with S (A and C) and R (B and D) stereoisomers of charged HEC (A and B) and HPC (C and D) substrates in anti conformation. Results were obtained from 15-ns molecular dynamics simulations. See supplemental Fig. S8 for proposed structures of complexes with those ligands in syn conformation.
DISCUSSION
The aim of this work was to assign the role of E. coli AlkB dioxygenase in the repair of exocyclic DNA adducts and to establish crucial aspects of its mechanism of action. It is known that the lesions studied here can be repaired via the base excision repair pathway. The exocyclic adducts ϵC, HEC, HPC (26, 38, 39), and to some extent ϵA (24, 40) are excised from DNA by bacterial Mug glycosylase. It has been shown in in vitro experiments that ϵA is removed from DNA by AlkA glycosylase (41). However, we found no evidence of this mode of repair in vivo (24). Here, we have shown that all the above mentioned modifications are repaired by AlkB. Bearing in mind that HPC was less efficiently removed from DNA by Mug than were ϵC and HEC (26) and that ϵA is a very poor substrate for E. coli glycosylases, it seems that AlkB plays a crucial role in the repair of these lesions. Our previous in vivo observations (7) strongly argue in favor of AlkB as a principal repair enzyme also for the HEC and ϵC lesions. For all of the above adducts, Mug may act only as a backup enzyme for AlkB. Nevertheless, it cannot be excluded that the enzymes complement each other because AlkB preferentially removes modifications from single-stranded substrates, whereas Mug acts on dsDNA. The fact that two independent systems, AlkB direct repair and base excision repair, are involved in the repair of exocyclic adducts strongly emphasizes how dangerous these lesions are for genome integrity.
Repair of Methylated and Exocyclic Adducts by AlkB Protein in Vitro
The best AlkB substrates, m3C and m1A, bear a positive charge at physiological pH, whereas neutral m3T and m1G are repaired very poorly (17–19). As we have shown in this study, efficient repair of the exocyclic adducts studied also occurs at a pH corresponding to their protonated, positively charged forms. These results suggest that in the active center of the AlkB enzyme the substrate is bound favorably in a cationic form. Table 1 shows absolute repair rates (i.e. k1 and when applicable k2) estimated for the repair of m3C and the exocyclic adducts at the physiological pH of 7.5 and at the optimal pH determined for each substrate. In the case of HPC, pH 7.5 was also found to be optimal. Not surprisingly, it appeared that m3C is better repaired at pH 7.5 where the residue is almost fully protonated, whereas at the commonly used pH of 8.0, 17% of m3C remains neutral.
It should be stressed that for ϵC and ϵA, whose pKa values are 3.7 and 3.9, respectively, the optimal pH of 4.6 is substantially higher than their pKa values. However, the latter value corresponds exactly to the pKa for the second carboxyl group of αKG (pKa1 = 2.47; pKa2 = 4.68; Ref. 42), whereas the pKa of the aspartic acid side-chain carboxyl group is usually close to 3.9. Taking into consideration that in similar systems the binding of Fe(II) cation has been shown to be a metal-to-ligand charge transfer process (43) requiring an electron from the coordination center, the existence of a lower pH limit for the repair reaction confirms that the doubly dissociated anionic form of αKG is the only form of the co-substrate involved in the catalytic reaction of ϵC and ϵA repair at pH 4.6. In the case of adducts displaying lower pKa values, the optimal pH for their repair is a compromise between the two factors determining the efficiency of the repair. A decrease of pH may also induce AlkB unfolding. The Fe(II) cofactor binding has also been confirmed to be pH-dependent, but this could be partially compensated for by variation in its concentration. The pH dependence of the optimal iron concentration has to be judged in terms of protonation of the imidazole ring of histidines that are involved in Fe(II) coordination in the AlkB catalytic center. Thus, the protonation of His-131 and His-187 at a lower pH needs to be compensated for by an increase of Fe(II) concentration, and consequently, an exact (anti) correlation is observed between the optimal values of pH and the logarithm of iron concentration (see Fig. 3).
On the other hand, a strong lowering of the pH of the reaction medium is accompanied by an increase of the population of the favored cationic form of the adduct. As a consequence, for high pKa adducts in the optimal pH state (1 unit below pKa), the AlkB state is identical with that at the neutral pH, whereas the adducts are almost fully protonated. In the case of ϵC and ϵA, at a pH required for their efficient protonation (i.e. ∼2.7), AlkB would be inactive, and the αKG co-factor would only be partially dissociated. The pH optimum of the reaction at ∼1 unit above the pKa of the substrate thus allows the enzyme to retain activity and αKG to be doubly dissociated while still producing approximately 10% of the substrate in the protonated form.
Conformational Changes of AlkB in Complex with Charged Adducts
Inspection of the MD trajectories clearly indicated that conformational preferences of Asp-135 are coupled with the ionic state of the ligand: the cationic form favors the Asp-135 side-chain rotamer with the side-chain carboxyl group located proximal to the protonated base (see Figs. 6 and 8). According to the 15-ns MD trajectories obtained, this interaction is responsible for the preferences of AlkB toward protonated substrates.
Ab Initio Analysis of Free Energy of Activation for the Oxidation of ϵA and ϵC
Oxidation of the double (etheno) bond is the first step of repair of etheno adducts by AlkB, resulting in the formation of a transient epoxide form of the modified base. The following steps, leading to regeneration of the unmodified base, occur spontaneously (16, 23).
Although ϵA and ϵC have similar pKa values and are best repaired at the same pH, we observed a 5-fold difference in their repair rates despite both adducts being (partially) protonated (see Table 1 for details). Therefore, we analyzed the thermodynamics of the repair process, calculating with ab initio methods the free energy difference between ϵA and ϵC and their epoxy derivatives. We found that for the neutral molecule in aqueous solution the putative barrier is higher by ∼0.4 and 0.7 kcal for ϵA and ϵC, respectively, in comparison with the same molecule in the monocationic state. This, in agreement with the experimentally confirmed tendency, indicates that the epoxidation step is 2–3 times faster for the charged substrates than for the neutral substrates. The relative epoxidation barrier was estimated to be 2.3 (for the protonated forms) and 2.5 kcal/mol (for the neutral forms) higher for ϵC than that for ϵA, indicating that ϵA is expected to be repaired ∼100-fold faster than ϵC (neglecting the differences in their binding by the enzyme). Both in vitro and in silico data confirm that AlkB more readily repairs etheno adducts in their cationic form, which at physiological conditions results in a substantial decrease of their repair rates. On the other hand, the results of our (7, 24) and other groups (16, 44) indicate that in E. coli cells AlkB does repair ϵ-adducts quite efficiently. One can thus speculate that in vivo some other, yet unknown factors may be involved in AlkB-mediated direct reversal of ϵ-adducts.
Kinetics of the Repair
We have experimentally proven that at high concentration of the enzyme the repair reaction is clearly single exponential (Fig. 4A) with the evident exception of HPC (Fig. 4B) and putatively HEC (results not as clear as for HPC possibly due to spontaneous HEC dehydration during the time of the reaction (4)). Moreover, using short time experiments with different AlkB concentrations (supplemental Fig. S4A), we have demonstrated that the series of pseudo first order kinetics of the repair (Equation 2) are in agreement with the global model (Equation 1) of the reaction (supplemental Fig. S4B). This validated the applicability of the more general Equation 1 for all AlkB concentrations used in long time kinetic studies. As expected, the repair rates estimated for ϵA and m3C with the aid of both approaches are virtually the same (Table 1). However, it should be emphasized that for low picomolar AlkB concentration only the partial repair of subnanomolar substrate was observed (see Fig. 5 and supplemental Figs. S1 and S2).
The proposed method is equivalent to the commonly used approach (45). However, the replacement of two-step analysis (i.e. time evolution of reagents in individual experiments followed by regression of the estimated rates against AlkB concentration) by one-step analysis (Equation 3) reduces the number of required parameters from 3n + 1 (i.e. ki, Ri, and Δi for every individual experiment and slope ki versus [AlkB] in the last step) down to n + 2 (Ri for each experiment and global values of k and Δ).
In the case of HPC and HEC, which are mixtures of stereoisomers, the proposed approach was required for data analysis because the standard approach is based on the initial step of substrate repair, and thus the slower process cannot be analyzed. We have previously demonstrated that global multibranch analysis of a set of individual experiments was valuable in the analysis of circular dichroism, calorimetric, and NMR data (46–48).
Mixtures of HPC and HEC Stereoisomers
Two-exponential repair kinetics of HPC (Figs. 4B and 5) and HEC indicates that both stereoisomers are repaired, although the efficiency of the repair differs by approximately 1 order of magnitude for HPC and about 6-fold in the case of HEC (Table 1).
In the reaction of dCMP with acrolein, an equimolar mixture of product diastereomers was observed (49). As a substrate for AlkB, we have used pentadeoxynucleotide with the modified base surrounded by thymine residues. In Fig. 4B, the extrapolated asymptote representing slower HPC isomer repair would cross the vertical axis below 100 pmol. This suggests that the HPC isomers in the pentamer are not equimolar. Indeed, in preliminary NMR rotating frame Overhauser effect spectroscopy and total correlation spectroscopy spectra recorded for T(HPC)T trideoxynucleotide, we have evidenced two forms of HPC in the ratio 3:1.3 This demonstrates that, in contrast to free nucleotide (49), a pattern of steric interactions with dT flanking dC creates in the oligomer a chiral environment that favors one of two possible HPC stereoisomers upon dC modification by acrolein.
Inspection of the proposed structures of the R and S isomers of both compounds showed that for both S isomers the exocyclic hydroxyl group points toward the iron redox center (Fig. 8). This should prevent an efficient oxidation of the exocyclic adduct. However, the unfavorable orientation of the substrate could be compensated for by the anti to syn transition around the glycosydic bond. As modeled (supplemental Fig. S8), the relative orientation of the S-syn forms resembles that obtained for the R-anti forms with the oxygen atom directed away from the iron atom. A similar orientation of the oxygen atom was recently determined for the ethenoadenine glycol adduct (23), which was identified as a transition state for the repair of ϵA (16, 23).
It is worth mentioning (see Fig. 8 for details) the substantial differences in topology of patterns of intermolecular interactions caused by the hydroxyl groups of the HEC (Fig. 8, A and B) and HPC (Fig. 8, C and D) lesions. Thus, hydroxyl groups in R stereoisomers, which point toward the iron atom, make hydrogen bonds with either Asp-135 or Glu-136 (Fig. 8, B and D) that interfere with the conserved interaction between Arg-210 and Glu-136 observed for S stereoisomers (Fig. 8, A and C) and in all crystal structures of AlkB.
Although we confirmed that both stereoisomers of HEC and HPC are repaired (see Fig. 2 and also k1 and k2 rates in Table 1), at the moment, we have no experimental basis to confirm whether the S forms are preferably repaired in the syn or the anti conformation. We also have no experimental evidence regarding which of the HEC and HPC stereoisomers is repaired more efficiently; however, molecular modeling suggests that S stereoisomers may be preferably repaired (see discussion above and also supplemental Fig. S8).
Conclusions and Perspectives
Using experimental and in silico approaches, we have confirmed our (7) and others' (18, 23) suggestions that E. coli AlkB preferentially recognizes and repairs protonated substrates. The best AlkB substrates, m1A and m3C, exist in a cationic form at physiological pH. Here, we have experimentally demonstrated that other AlkB substrates also are more readily repaired at a pH corresponding to their cationic form. Using computational methods, we have established that a crucial role in recognition of the positively charged substrate is played by negatively charged Asp-135 located in the proximity of the bound modified base. More generally, the negative electrostatic potential distributed over the whole area of the binding site is responsible for the substrate preferences of AlkB (Figs. 6 and 7).
Human AlkB dioxygenase homologs hABH1–3 (12, 18, 21, 50, 51) differ in their specificity to recognize single-stranded and dsDNA and the ability to repair RNA modifications. However, they share the same fold, including active center organization with a non-heme Fe(II) cation, and as one can suppose on the basis of their known substrate specificity, may share with the bacterial enzyme the ability to recognize and repair protonated substrates much more readily.
Exocyclic DNA adducts are produced directly by environmental chemical compounds or occur as end products of cellular oxidative stress. Ample data indicate that they play important roles in the development of several pathological states including inflammation, chronic infection, metal imbalance, and cancer (52). The ever expanding number of such adducts among AlkB substrates emphasizes the likely role of human AlkB homologs in the etiology and prevention of those pathologies.
Acknowledgments
We thank Jacek Olędzki, M.Sc., of the Laboratory of Mass Spectrometry, Institute of Biochemistry and Biophysics, Polish Academy of Sciences for the MS analyses and Katarzyna Ruszczyńska, Ph.D., of the Laboratory of Biological NMR, Institute of Biochemistry and Biophysics, Polish Academy of Sciences for assistance in setting NMR experiments.
This work was supported by Polish-Norwegian Research Fund Grant PNRF-143-AI-1/07 and partially by National Science Centre Grant UMO-2012/04/M/NZ1/00068. Molecular modeling performed by J. P. was partially supported by International Centre for Genetic Engineering and Biotechnology, Trieste, Italy Grant CRP/08/011.

This article contains supplemental Methods, Tables S1 and S2, and Figs. S1–S8.
A.M. Maciejewska, K. Ruszczyńska, and J. Poznański, unpublished data.
- ACR
- acrolein
- αKG
- α-ketoglutarate
- ϵ
- etheno (see Fig. 1 for structures of adducts)
- MD
- molecular dynamics
- HEC
- 3,N4-α-hydroxyethanocytosine
- m1A
- 1-methyladenine
- m3C
- 3-methylcytosine
- m1G
- 1-methylguanine
- m3T
- 3-methylthymine
- ϵC
- 3,N4-ethenocytosine
- ϵA
- 1,N6-ethenoadenine
- HPC
- 3,N4-α-hydroxypropanocytosine
- r.m.s.d.
- root of the mean squared deviation.
REFERENCES
- 1. Bartsch H. (1999) Keynote address: exocyclic adducts as new risk markers for DNA damage in man. IARC Sci. Publ. 1–16 [PubMed] [Google Scholar]
- 2. Hang B. (2004) Repair of exocyclic DNA adducts: rings of complexity. BioEssays 26, 1195–1208 [DOI] [PubMed] [Google Scholar]
- 3. Leonard N. J. (1984) Etheno-substituted nucleotides and coenzymes: fluorescence and biological activity. CRC Crit. Rev. Biochem. 15, 125–199 [DOI] [PubMed] [Google Scholar]
- 4. Krzyzosiak W. J., Biernat J., Ciesiolka J., Gornicki P., Wiewiorowski M. (1979) Further studies on adenosine and cytidine reaction with chloroacetaldehyde, a new support for the carbinolamine structure of the stable reaction intermediate and its relevance to the reaction mechanism and tRNA modification. Pol. J. Chem. 53, 243–252 [Google Scholar]
- 5. Kuśmierek J. T., Singer B. (1982) Chloroacetaldehyde-treated ribo- and deoxyribopolynucleotides. 1. Reaction products. Biochemistry 21, 5717–5722 [DOI] [PubMed] [Google Scholar]
- 6. Borys E., Mroczkowska-Słupska M. M., Kuśmierek J. T. (1994) The induction of adaptive response to alkylating agents in Escherichia coli reduces the frequency of specific C→T mutations in chloroacetaldehyde-treated M13 glyU phage. Mutagenesis 9, 407–410 [DOI] [PubMed] [Google Scholar]
- 7. Maciejewska A. M., Ruszel K. P., Nieminuszczy J., Lewicka J., Sokołowska B., Grzesiuk E., Kuśmierek J. T. (2010) Chloroacetaldehyde-induced mutagenesis in Escherichia coli: the role of AlkB protein in repair of 3,N4-ethenocytosine and 3,N4-α-hydroxyethanocytosine. Mutat. Res. 684, 24–34 [DOI] [PubMed] [Google Scholar]
- 8. Krwawicz J., Arczewska K. D., Speina E., Maciejewska A., Grzesiuk E. (2007) Bacterial DNA repair genes and their eukaryotic homologues: 1. Mutations in genes involved in base excision repair (BER) and DNA-end processors and their implication in mutagenesis and human disease. Acta Biochim. Pol. 54, 413–434 [PubMed] [Google Scholar]
- 9. Aravind L., Koonin E. V. (2001) The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol. 2, RESEARCH0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falnes P. Ø., Johansen R. F., Seeberg E. (2002) AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature 419, 178–182 [DOI] [PubMed] [Google Scholar]
- 11. Trewick S. C., Henshaw T. F., Hausinger R. P., Lindahl T., Sedgwick B. (2002) Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419, 174–178 [DOI] [PubMed] [Google Scholar]
- 12. Duncan T., Trewick S. C., Koivisto P., Bates P. A., Lindahl T., Sedgwick B. (2002) Reversal of DNA alkylation damage by two human dioxygenases. Proc. Natl. Acad. Sci. U.S.A. 99, 16660–16665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koivisto P., Duncan T., Lindahl T., Sedgwick B. (2003) Minimal methylated substrate and extended substrate range of Escherichia coli AlkB protein, a 1-methyladenine-DNA dioxygenase. J. Biol. Chem. 278, 44348–44354 [DOI] [PubMed] [Google Scholar]
- 14. Mishina Y., Yang C. G., He C. (2005) Direct repair of the exocyclic DNA adduct 1,N6-ethenoadenine by the DNA repair AlkB proteins. J. Am. Chem. Soc. 127, 14594–14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frick L. E., Delaney J. C., Wong C., Drennan C. L., Essigmann J. M. (2007) Alleviation of 1,N6-ethanoadenine genotoxicity by the Escherichia coli adaptive response protein AlkB. Proc. Natl. Acad. Sci. U.S.A. 104, 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delaney J. C., Smeester L., Wong C., Frick L. E., Taghizadeh K., Wishnok J. S., Drennan C. L., Samson L. D., Essigmann J. M. (2005) AlkB reverses etheno DNA lesions caused by lipid oxidation in vitro and in vivo. Nat. Struct. Mol. Biol. 12, 855–860 [DOI] [PubMed] [Google Scholar]
- 17. Delaney J. C., Essigmann J. M. (2004) Mutagenesis, genotoxicity, and repair of 1-methyladenine, 3-alkylcytosines, 1-methylguanine, and 3-methylthymine in alkB Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 101, 14051–14056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koivisto P., Robins P., Lindahl T., Sedgwick B. (2004) Demethylation of 3-methylthymine in DNA by bacterial and human DNA dioxygenases. J. Biol. Chem. 279, 40470–40474 [DOI] [PubMed] [Google Scholar]
- 19. Falnes P. Ø. (2004) Repair of 3-methylthymine and 1-methylguanine lesions by bacterial and human AlkB proteins. Nucleic Acids Res. 32, 6260–6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aas P. A., Otterlei M., Falnes P. O., Vågbø C. B., Skorpen F., Akbari M., Sundheim O., Bjørås M., Slupphaug G., Seeberg E., Krokan H. E. (2003) Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421, 859–863 [DOI] [PubMed] [Google Scholar]
- 21. Falnes P. Ø., Bjørås M., Aas P. A., Sundheim O., Seeberg E. (2004) Substrate specificities of bacterial and human AlkB proteins. Nucleic Acids Res. 32, 3456–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ougland R., Zhang C. M., Liiv A., Johansen R. F., Seeberg E., Hou Y. M., Remme J., Falnes P. Ø. (2004) AlkB restores the biological function of mRNA and tRNA inactivated by chemical methylation. Mol. Cell 16, 107–116 [DOI] [PubMed] [Google Scholar]
- 23. Yi C., Jia G., Hou G., Dai Q., Zhang W., Zheng G., Jian X., Yang C. G., Cui Q., He C. (2010) Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase. Nature 468, 330–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maciejewska A. M., Sokołowska B., Nowicki A., Kuśmierek J. T. (2011) The role of AlkB protein in repair of 1,N6-ethenoadenine in Escherichia coli cells. Mutagenesis 26, 401–406 [DOI] [PubMed] [Google Scholar]
- 25. Chenna A., Iden C. R. (1993) Characterization of 2′-deoxycytidine and 2′-deoxyuridine adducts formed in reactions with acrolein and 2-bromoacrolein. Chem. Res. Toxicol. 6, 261–268 [DOI] [PubMed] [Google Scholar]
- 26. Borys-Brzywczy E., Arczewska K. D., Saparbaev M., Hardeland U., Schär P., Kuśmierek J. T. (2005) Mismatch dependent uracil/thymine-DNA glycosylases excise exocyclic hydroxyethano and hydroxypropano cytosine adducts. Acta Biochim. Pol. 52, 149–165 [PubMed] [Google Scholar]
- 27. Yu B., Hunt J. F. (2009) Enzymological and structural studies of the mechanism of promiscuous substrate recognition by the oxidative DNA repair enzyme AlkB. Proc. Natl. Acad. Sci. U.S.A. 106, 14315–14320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt M. W., Baldridge K. K., Boatz J. A., Elbert S. T., Gordon M. S., Jensen J. J., Koseki S., Matsunaga N., Nguyen K. A., Su S., Windus T. L., Dupuis M., Montgomery J. A. (1993) General atomic and molecular electronic structure system. J. Comput. Chem. 14, 1347–1363 [Google Scholar]
- 29. Hertwig R. H., Koch W. (1997) On the parameterization of the local correlation functional: what is Becke-3-LYP? Chem. Phys. Lett. 268, 345–351 [Google Scholar]
- 30. Francl M. M., Pietro W. J., Hehre W. J., Binkley J. S., Gordon M. S., DeFrees D. J., Pople J. A. (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 77, 3654–3665 [Google Scholar]
- 31. Poznański J., Najda A., Bretner M., Shugar D. (2007) Experimental (13C NMR) and theoretical (ab initio molecular orbital calculations) studies on the prototropic tautomerism of benzotriazole and some derivatives symmetrically substituted on the benzene ring. J. Phys. Chem. A 111, 6501–6509 [DOI] [PubMed] [Google Scholar]
- 32. Wasik R., Łebska M., Felczak K., Poznański J., Shugar D. (2010) Relative role of halogen bonds and hydrophobic interactions in inhibition of human protein kinase CK2α by tetrabromobenzotriazole and some C(5)-substituted analogues. J. Phys. Chem. B 114, 10601–10611 [DOI] [PubMed] [Google Scholar]
- 33. Nilges M., Clore G. M., Gronenborn A. M. (1988) Determination of three-dimensional structures of proteins from interproton distance data by dynamical simulated annealing from a random array of atoms. Circumventing problems associated with folding. FEBS Lett. 239, 129–136 [DOI] [PubMed] [Google Scholar]
- 34. Nilges M., Kuszewski J., Brunger A. T. (1991) Computational Aspects of the Study of Biological Macromolecules by NMR (Hoch J. C., ed) pp. 451–455, Plenum Press, New York [Google Scholar]
- 35. Krieger E., Joo K., Lee J., Lee J., Raman S., Thompson J., Tyka M., Baker D., Karplus K. (2009) Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 77, Suppl. 9, 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., Olson A. J. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 [Google Scholar]
- 37. Duan Y., Wu C., Chowdhury S., Lee M. C., Xiong G., Zhang W., Yang R., Cieplak P., Luo R., Lee T., Caldwell J., Wang J., Kollman P. (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 24, 1999–2012 [DOI] [PubMed] [Google Scholar]
- 38. Saparbaev M., Laval J. (1998) 3,N4-Ethenocytosine, a highly mutagenic adduct, is a primary substrate for Escherichia coli double-stranded uracil-DNA glycosylase and human mismatch-specific thymine-DNA glycosylase. Proc. Natl. Acad. Sci. U.S.A. 95, 8508–8513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jurado J., Maciejewska A., Krwawicz J., Laval J., Saparbaev M. K. (2004) Role of mismatch-specific uracil-DNA glycosylase in repair of 3,N4-ethenocytosine in vivo. DNA Repair 3, 1579–1590 [DOI] [PubMed] [Google Scholar]
- 40. O'Neill R. J., Vorob'eva O. V., Shahbakhti H., Zmuda E., Bhagwat A. S., Baldwin G. S. (2003) Mismatch uracil glycosylase from Escherichia coli: a general mismatch or a specific DNA glycosylase? J. Biol. Chem. 278, 20526–20532 [DOI] [PubMed] [Google Scholar]
- 41. Saparbaev M., Kleibl K., Laval J. (1995) Escherichia coli, Saccharomyces cerevisiae, rat and human 3-methyladenine DNA glycosylases repair 1,N6-ethenoadenine when present in DNA. Nucleic Acids Res. 23, 3750–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen H., Laurent S., Bédu S., Ziarelli F., Chen H. L., Cheng Y., Zhang C. C., Peng L. (2006) Studying the signaling role of 2-oxoglutaric acid using analogs that mimic the ketone and ketal forms of 2-oxoglutaric acid. Chem. Biol. 13, 849–856 [DOI] [PubMed] [Google Scholar]
- 43. Ho R. Y., Mehn M. P., Hegg E. L., Liu A., Ryle M. J., Hausinger R. P., Que L., Jr. (2001) Resonance Raman studies of the iron(II)-α-keto acid chromophore in model and enzyme complexes. J. Am. Chem. Soc. 123, 5022–5029 [DOI] [PubMed] [Google Scholar]
- 44. Kim M. Y., Zhou X., Delaney J. C., Taghizadeh K., Dedon P. C., Essigmann J. M., Wogan G. N. (2007) AlkB influences the chloroacetaldehyde-induced mutation spectra and toxicity in the pSP189 supF shuttle vector. Chem. Res. Toxicol. 20, 1075–1083 [DOI] [PubMed] [Google Scholar]
- 45. Leiros I., Nabong M. P., Grøsvik K., Ringvoll J., Haugland G. T., Uldal L., Reite K., Olsbu I. K., Knaevelsrud I., Moe E., Andersen O. A., Birkeland N. K., Ruoff P., Klungland A., Bjelland S. (2007) Structural basis for enzymatic excision of N1-methyladenine and N3-methylcytosine from DNA. EMBO J. 26, 2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gozdek A., Stankiewicz-Drogoń A., Poznański J., Boguszewska-Chachulska A. M. (2008) Circular dichroism analysis for multidomain proteins: studies of the irreversible unfolding of hepatitis C virus helicase. Acta Biochim. Pol. 55, 57–66 [PubMed] [Google Scholar]
- 47. Poznanski J., Wszelaka-Rylik M., Zielenkiewicz W. (2004) Concentration dependencies of NaCl salting of lysozyme by calorimetric methods. Thermochim. Acta 409, 25–32 [Google Scholar]
- 48. Zielenkiewicz W., Marcinowicz A., Poznanski J., Cherenok S., Kalchenko V. (2005) Complexation of isoleucine by phosphorylated calix 4 arene in methanol followed by calorimetry, NMR and UV-VIS spectroscopies, and molecular modeling methods. J. Mol. Liq. 121, 8–14 [Google Scholar]
- 49. Smith R. A., Williamson D. S., Cohen S. M. (1989) Identification of 3,N4-propanodeoxycytidine 5′-monophosphate formed by the reaction of acrolein with deoxycytidine 5′-monophosphate. Chem. Res. Toxicol. 2, 267–271 [DOI] [PubMed] [Google Scholar]
- 50. Westbye M. P., Feyzi E., Aas P. A., Vågbø C. B., Talstad V. A., Kavli B., Hagen L., Sundheim O., Akbari M., Liabakk N. B., Slupphaug G., Otterlei M., Krokan H. E. (2008) Human AlkB homolog 1 is a mitochondrial protein that demethylates 3-methylcytosine in DNA and RNA. J. Biol. Chem. 283, 25046–25056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ringvoll J., Moen M. N., Nordstrand L. M., Meira L. B., Pang B., Bekkelund A., Dedon P. C., Bjelland S., Samson L. D., Falnes P. Ø., Klungland A. (2008) AlkB homologue 2-mediated repair of ethenoadenine lesions in mammalian DNA. Cancer Res. 68, 4142–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nair U., Bartsch H., Nair J. (2007) Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic. Biol. Med. 43, 1109–1120 [DOI] [PubMed] [Google Scholar]