Abstract
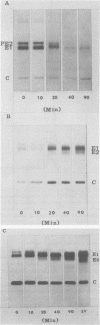
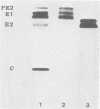
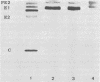
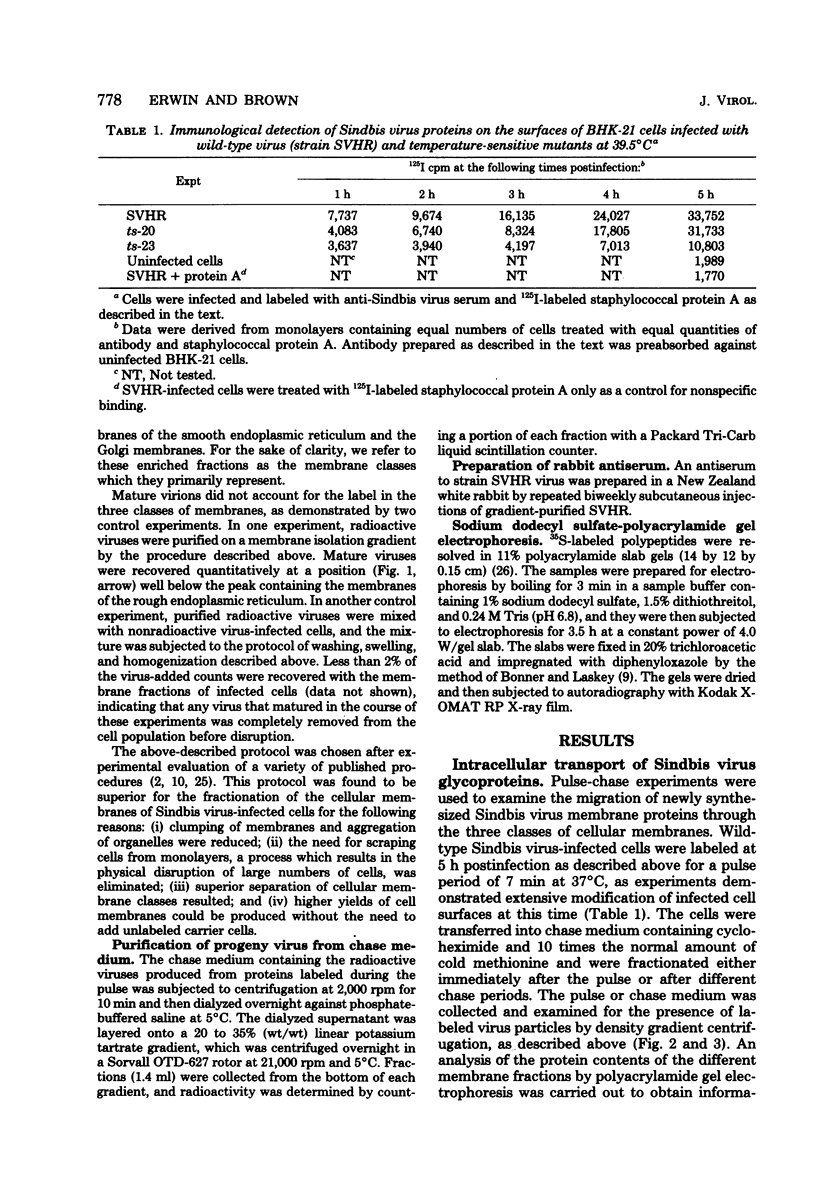
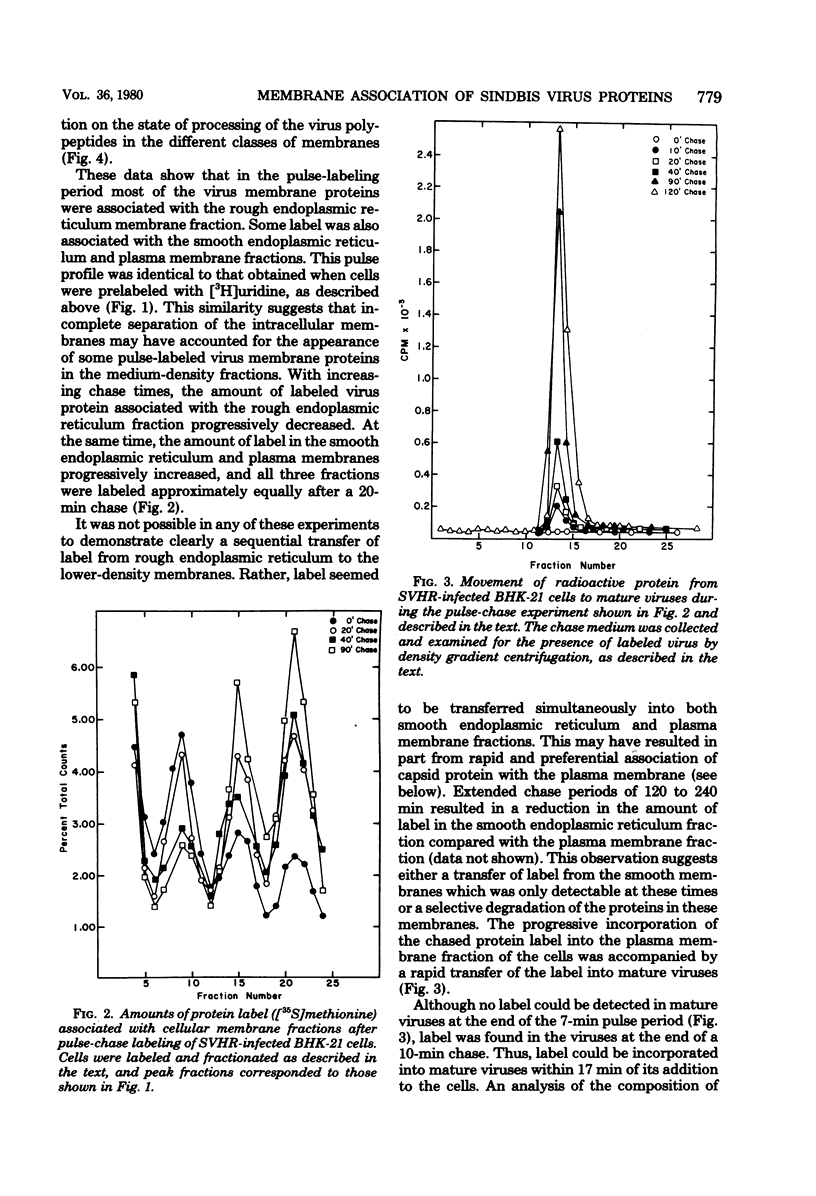
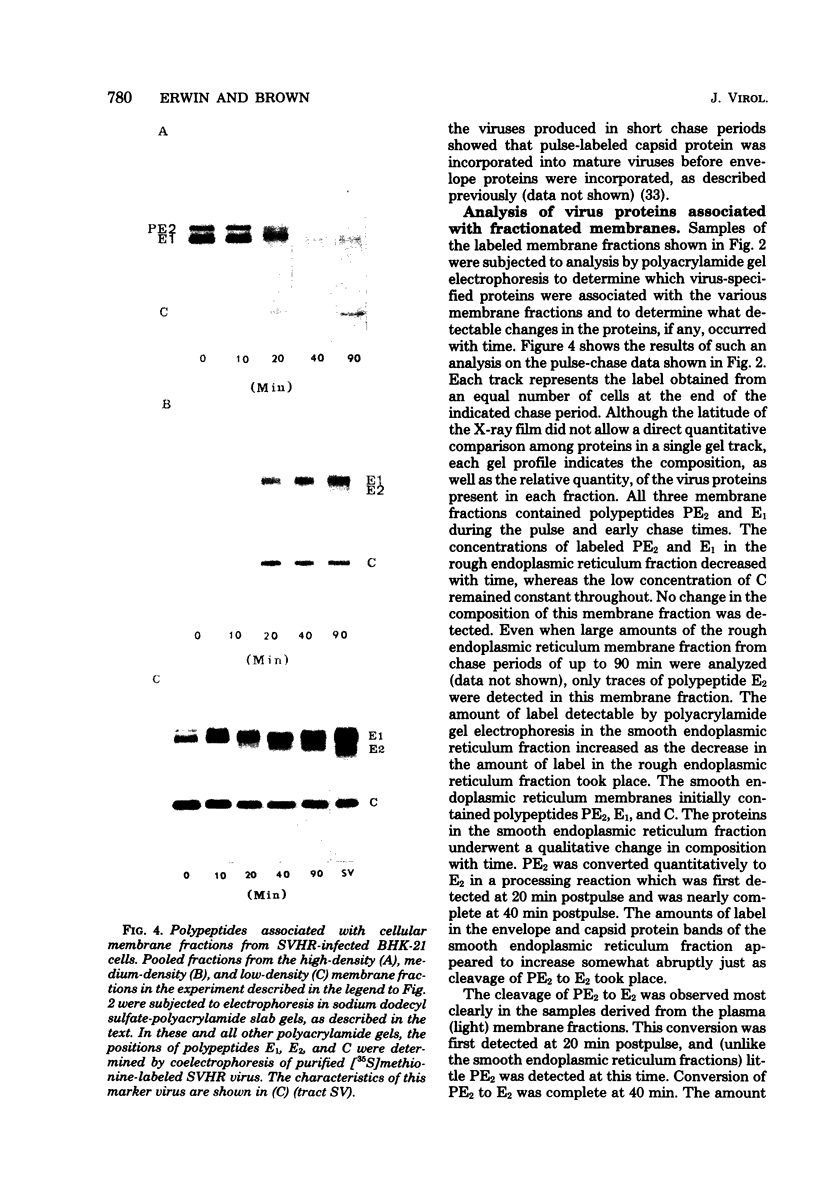
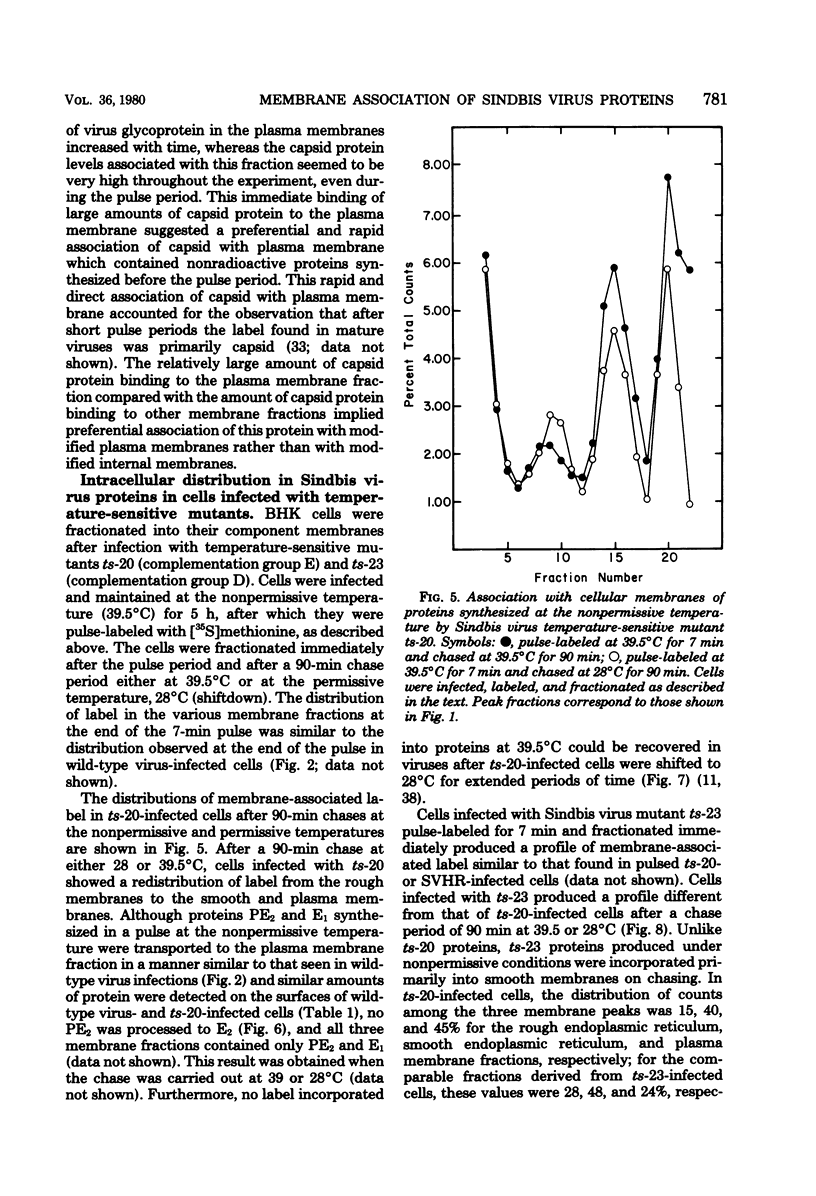
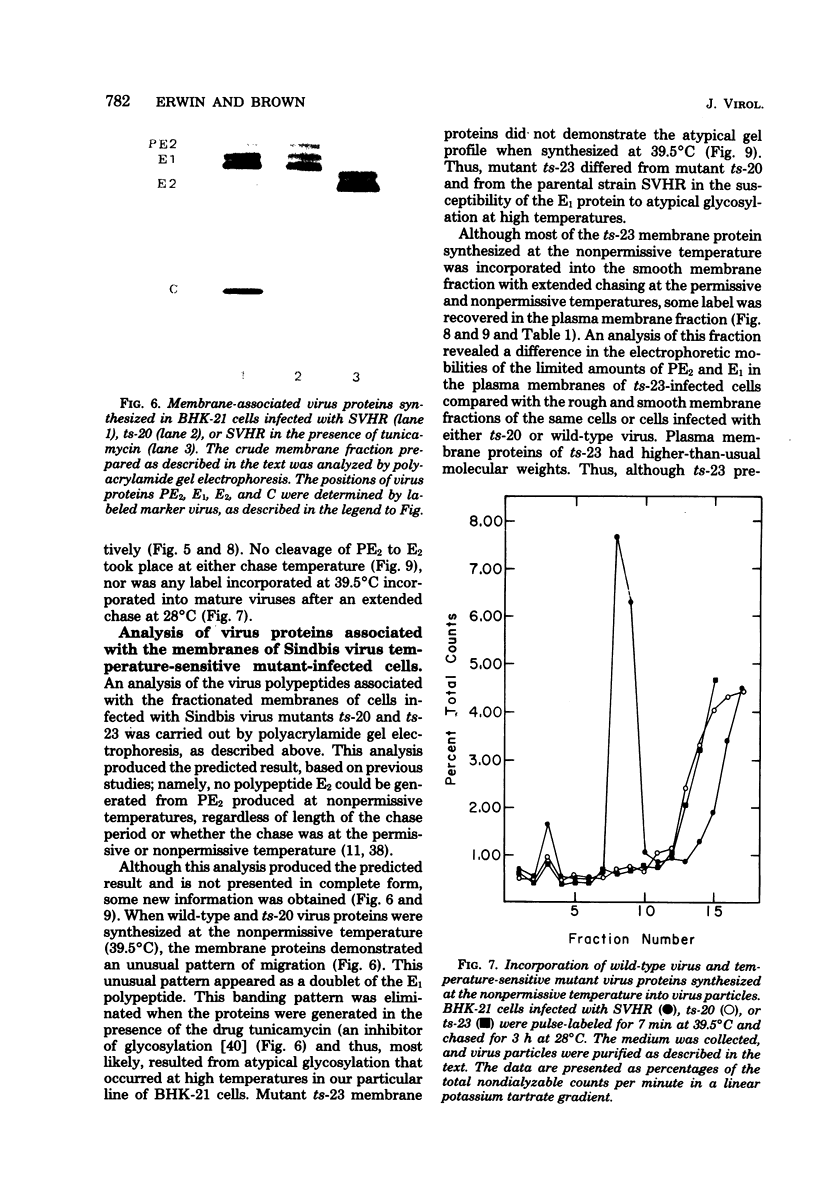
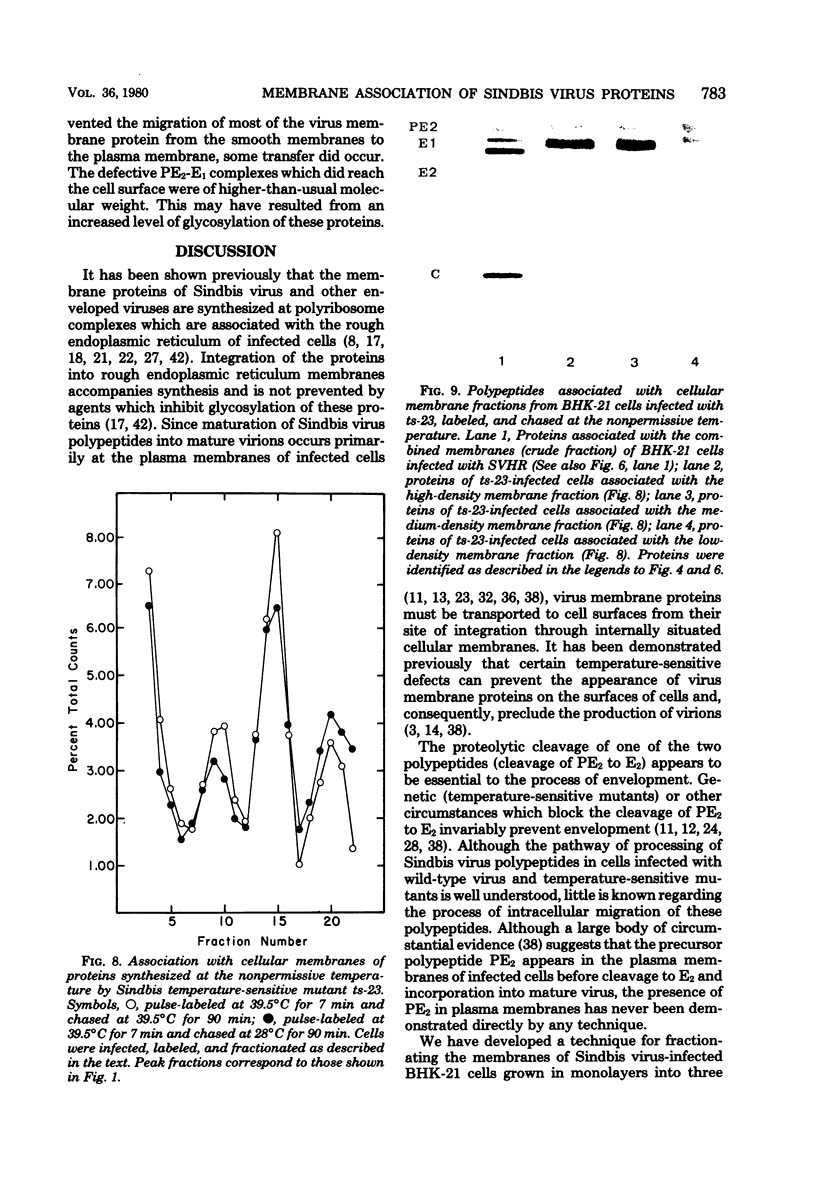
The association of Sindbis virus proteins with cellular membranes during virus maturation was examined by utilizing a technique for fractionating the membranes of BHK-21 cells into three subcellular classes, which were enriched for rough endoplasmic reticulum, smooth endoplasmic reticulum, and plasma membrane. Pulse-chase experiments with wild-type (strain SVHR) virus-infected cells showed that virus envelope proteins were incorporated initially into membranes of the rough endoplasmic reticulum and subsequently migrated to the smooth and plasma membrane fractions. Large amounts of capsid protein were associated with the plasma membrane fraction even at the earliest times postpulse, and relatively little was found associated with the other membranes, suggesting a rapid and preferential association of nucleocapsids with the plasma membrane. We also examined the intracellular processing of the proteins of two temperature-sensitive Sindbis virus mutants in pulse-chase experiments at the nonpermissive temperature. Labeled virus proteins of mutant ts-20 (complementation group E) first appeared in the rough endoplasmic reticulum and were then transported to the smooth and plasma membrane fractions, as in wild-type (strain SVHR) virus-infected cells. In cells infected with ts-23 (complementation group D), the pulse-labeled virus proteins appeared initially in the rough membrane fraction and were transported to the smooth membrane fraction, but only limited amounts reached the plasma membrane. Thus, in ts-23-infected cells, the transport of the virus-encoded proteins from the smooth membranes seemed to be defective. In both ts-20- and ts-23-infected cells the envelope precursor polypeptide PE2 was not processed to E2, and no label was incorporated into free virus at the nonpermissive temperature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Adelman M. R., Blobel G., Sabatini D. D. An improved cell fractionation procedure for the preparation of rat liver membrane-bound ribosomes. J Cell Biol. 1973 Jan;56(1):191–205. doi: 10.1083/jcb.56.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. W., Jr, Waite M. R. Envelope antigens of Sindbis virus in cells infected with temperature-sensitive mutants. J Virol. 1977 Feb;21(2):788–791. doi: 10.1128/jvi.21.2.788-791.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S., Blobel G. Absence of a cleavable signal sequence in Sindbis virus glycoprotein PE2. J Biol Chem. 1979 Dec 25;254(24):12261–12264. [PubMed] [Google Scholar]
- Bonatti S., Cancedda R., Blobel G. Membrane biogenesis. In vitro cleavage, core glycosylation, and integration into microsomal membranes of sindbis virus glycoproteins. J Cell Biol. 1979 Jan;80(1):219–224. doi: 10.1083/jcb.80.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Garoff H., Schwarz R. T. Glycosylation is not necessary for membrane insertion and cleavage of Semliki Forest virus membrane proteins. Nature. 1978 Aug 3;274(5670):487–490. doi: 10.1038/274487a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Grimes W. J., Burge B. W. Modification of Sindbis virus glycoprotein by host-specified glycosyl transferases. J Virol. 1971 Mar;7(3):309–313. doi: 10.1128/jvi.7.3.309-313.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Association of vesicular stomatitis virus proteins with HeLa cell membranes and released virus. J Virol. 1976 Dec;20(3):637–645. doi: 10.1128/jvi.20.3.637-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Glycosylation of vesicular stomatitis virus glycoprotein in virus-infected HeLa cells. J Virol. 1976 Dec;20(3):646–657. doi: 10.1128/jvi.20.3.646-657.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Scupham R. K., Pfeil J. A., Wan K., Sagik B. P., Bose H. R. Interaction of Sindbis virus glycoproteins during morphogenesis. J Virol. 1977 Feb;21(2):778–787. doi: 10.1128/jvi.21.2.778-787.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., McQuain C. O., Simpson D. Assembly of viral membranes: maturation of the vesicular stomatitis virus glycoprotein in the presence of tunicamycin. J Virol. 1978 Oct;28(1):368–374. doi: 10.1128/jvi.28.1.368-374.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Vance D. E. Biochemical evidence that Semliki Forest virus obtains its envelope from the plasma membrane of the host cell. J Biol Chem. 1976 Sep 25;251(18):5544–5550. [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Lodish H. F., Robbins P. W. Requirements for the insertion of the Sindbis envelope glycoproteins into the endoplasmic reticulum membrane. J Cell Biol. 1979 Apr;81(1):154–162. doi: 10.1083/jcb.81.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lockart R. Z., Jr Maturation defects in temperature-sensitive mutants of Sindbis virus. J Virol. 1968 Jul;2(7):728–737. doi: 10.1128/jvi.2.7.728-737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H. Temperature-sensitive behavior of hemagglutinin in a temperature-sensitive mutant virion of Sindbis. J Virol. 1969 Oct;4(4):547–548. doi: 10.1128/jvi.4.4.547-548.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Hexagonal glycoprotein arrays from Sindbis virus membranes. J Virol. 1978 Nov;28(2):578–583. doi: 10.1128/jvi.28.2.578-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]