Background: The ATP-binding reaction was hypothesized to be the main torque-generating step for V1-ATPase.
Results: Upon mechanical manipulation, the ATP-binding reaction of V1-ATPase showed weaker angle dependence than that of F1-ATPase.
Conclusion: The ATP-binding reaction is not the main torque-generating step in V1.
Significance: This external manipulation technique should be applied to other reaction steps of ATP hydrolysis to get the whole view of the mechanochemical coupling mechanism in V1.
Keywords: Enzyme Kinetics, H+-ATPase, Molecular Motors, Single-molecule Biophysics, Vacuolar ATPase
Abstract
V1-ATPase is a rotary motor protein that rotates the central shaft in a counterclockwise direction hydrolyzing ATP. Although the ATP-binding process is suggested to be the most critical reaction step for torque generation in F1-ATPase (the closest relative of V1-ATPase evolutionarily), the role of ATP binding for V1-ATPase in torque generation has remained unclear. In the present study, we performed single-molecule manipulation experiments on V1-ATPase from Thermus thermophilus to investigate how the ATP-binding process is modulated upon rotation of the rotary shaft. When V1-ATPase showed an ATP-waiting pause, it was stalled at a target angle and then released. Based on the response of the V1-ATPase released, the ATP-binding probability was determined at individual stall angles. It was observed that the rate constant of ATP binding (kon) was exponentially accelerated with forward rotation, whereas the rate constant of ATP release (koff) was exponentially reduced. The angle dependence of the koff of V1-ATPase was significantly smaller than that of F1-ATPase, suggesting that the ATP-binding process is not the major torque-generating step in V1-ATPase. When V1-ATPase was stalled at the mean binding angle to restrict rotary Brownian motion, kon was evidently slower than that determined from free rotation, showing the reaction rate enhancement by conformational fluctuation. It was also suggested that shaft of V1-ATPase should be rotated at least 277° in a clockwise direction for efficient release of ATP under ATP-synthesis conditions.
Introduction
The vacuolar proton pumps, V-ATPases (VoV1 type), are part of the ATPase/ATP synthase superfamily and share a common rotary catalytic mechanism with F0F1-ATPase (1–3). VoV1 consists of two rotary motors: the membrane-embedded Vo subunit and the water-soluble V1 subunit, each driven by a proton flux to create a proton-motive force and by ATP hydrolysis, respectively. In cells, Vo and V1 bind to one another via the central rotor stalk and peripheral stalks and interconvert the energy liberated from ATP hydrolysis and proton translocation down the proton motive force into rotation of the central stalk (4, 5). Although VoV1 is primarily known as an ATP-driven proton pump that acidifies the inside of vacuoles in eukaryotic cells, VoV1 also catalyzes ATP synthesis driven by the proton motive force in archaea and some eubacteria such as Thermus thermophilus.
The V1 domain from T. thermophilus, termed V1-ATPase,2 has been extensively investigated as a model enzyme for the bacterial type V1 due to its conformational stability and ease of biochemical handling (6–8). V1-ATPase is composed of a hexameric stator ring of A3B3 subunits and the central rotary shaft of DF subunits. The A3B3 ring possesses three catalytic sites on each A-B interface, primarily on the A subunit. The D subunit is embedded inside the central cavity of the A3B3 ring, whereas the F subunit binds to a protruding segment of subunit D (9).
Although V1-ATPase has several rotation features in common with F1-ATPase, such as rotation in the counterclockwise direction and a 120° stepping rotation, the rotational mechanism of V1-ATPase is distinct from that of F1-ATPase (10). One prominent difference between the two types of ATPase is that V1-ATPase does not show any rotational substep (7), whereas the elementary 120° step of F1-ATPase is composed of 80° and 40° substeps (11). Although the process by which the three catalytic A subunits participate in driving the unidirectional rotation remains unclear, it has been suggested that V1-ATPase executes all of the elementary reaction steps, that is, ATP binding, ATP hydrolysis, and product release, each at a pausing position. This hypothesis implies that each elementary reaction step is responsible for contributing to the 120° rotation, in contrast to the torque generation mechanism of F1-ATPase, in which individual reaction steps induce either of the 80° or 40° substeps. Another difference between these two ATPases is that the torque of V1-ATPase is ∼35 pN·nm, which is slightly smaller than that of F1-ATPase (40 pN·nm) (10, 12). Comparative research on V1-ATPase and F1-ATPase could clarify the common working principles and unique mechanisms of these proteins.
We have previously conducted single-molecule stalling experiments to investigate how F1-ATPase modulates the chemical equilibrium and reaction rate of ATP-binding and ATP-hydrolysis steps via the rotation of the central rotary shaft (13). Although both reactions were exponentially enhanced during the forward rotation, the degree of reaction enhancement was distinctive; the ATP-binding rate was largely accelerated during the forward rotation, whereas the reaction enhancement of the ATP-hydrolysis step was only slight. The affinity of F1 for ATP was also exponentially enhanced, suggesting that the F1-ATP complex is stabilized upon rotation. This finding suggested that F1 generates a much greater torque during the binding change process than during the hydrolysis step. The torque generated upon ATP binding was quantitatively estimated from the angle dependence of koff.
Single-molecule manipulation with magnetic tweezers was also utilized in the experiment on V1-ATPase to attempt to activate V1-ATPase in the ADP-inhibited form by forcibly rotating the molecule with the magnetic tweezers (14). When rotated over +110°, V1-ATPase resumed active rotation. The activation probability was notably dependent on the angular displacement from the inhibitory pausing position, as observed in the mechanical activation of F1-ATPase in the ADP-inhibited form (15). This observation suggests that V1-ATPase also possesses the ability to modulate the catalytic reaction upon rotation, similar to that of F1-ATPase. In the present study, we attempted to verify this hypothesis by examining how the ATP-binding process is modulated upon rotation of V1-ATPase. Interestingly, V1-ATPase displayed demonstrably weaker angle dependence of ATP binding than F1-ATPase, suggesting a smaller contribution of the ATP-binding process for torque generation than that observed for F1-ATPase. The results have been discussed in the light of the current understanding of the mechanochemical coupling mechanisms of V1-ATPase and F1-ATPase.
EXPERIMENTAL PROCEDURES
Rotation Assay
Sample preparation and experimental procedures were performed essentially as described in our previous study (14). Wild-type V1 that had a minimal modification for the single-molecule rotation assay, A(His-10/C28S/C508S)3B(C264S)3D(E48C/Q55C)F, was prepared and examined in the rotation assay. Streptavidin-coated magnetic beads (Thermo Scientific) were used as rotational markers. The beads showed relatively great diversity in diameter. The small particles (φ ≈ 200 nm) were selectively observed due to the low frequency of physical interaction with the glass surface. Rotation of the bead was observed under a phase contrast microscope (IX70; Olympus, Tokyo, Japan) by using a 100× objective lens. Images were captured with a charge-coupled device camera (FC300M; Takenaka System Co., Kyoto, Japan) and recorded at 30 frames/sec. A magnetic tweezers system was mounted on the specimen stage of the microscope and controlled with custom-designed software (Celery, Library, Tokyo, Japan). Analysis of rotation was also performed using the custom-designed software (Celery). All the experiments were carried out at a temperature range of 23–25 °C.
RESULTS
V1-ATPase was immobilized on the glass surface through His tags introduced at the N termini of the A subunits of the stator A3B3 ring. Rotation of V1-ATPase was observed by attaching a streptavidin-coated magnetic bead to the D subunit. The magnetic beads were used also as the handle for manipulation by the magnetic tweezers. The rotation assay was conducted under ATP-limiting conditions (1 or 1.5 μm), well below the Michaelis-Menten constant (Km) of the rotation assay with magnetic beads (8.1 μm) (14). Under these conditions, V1-ATPase demonstrated a 120° stepping rotation (Fig. 1A). The mean times of the ATP-waiting pause were 0.57 and 0.32 s at 1 and 1.5 μm, respectively (Fig. 1B). Note that the mean time for catalysis on V1-ATPase was 2.5 ms (7), which was much shorter than the ATP-waiting dwell or the mean time for the 120° rotation of the beads, so that the catalytic pause was obscured and undetectable in this condition. In a recent study, we reported that V1-ATPase spontaneously lapses into two types of inhibitory state, pausing rotation; V1-ATPase undergoes a second-scale inhibitory pause during continuous rotation that is terminated with a long and stable pause. Under ATP-limiting conditions, the occurrence frequency of the second-scale inhibitory pause is <0.4% of the total pause and is hence negligible (14). The observation of the rotation was terminated when the long pause appeared.
FIGURE 1.
Rotation of V1 molecule at 1 μm ATP. A, trajectory of a single V1 molecule at 1 μm ATP. Inset, X-Y trajectory of the same V1 molecule for which the trajectory was shown. B, dwell time histogram of V1 at 1 μm ATP. Data are well fitted with a single exponential, providing the time constant as 0.57 s. The number of molecules was 3, and the number of trials used for dwell time analysis was 654.
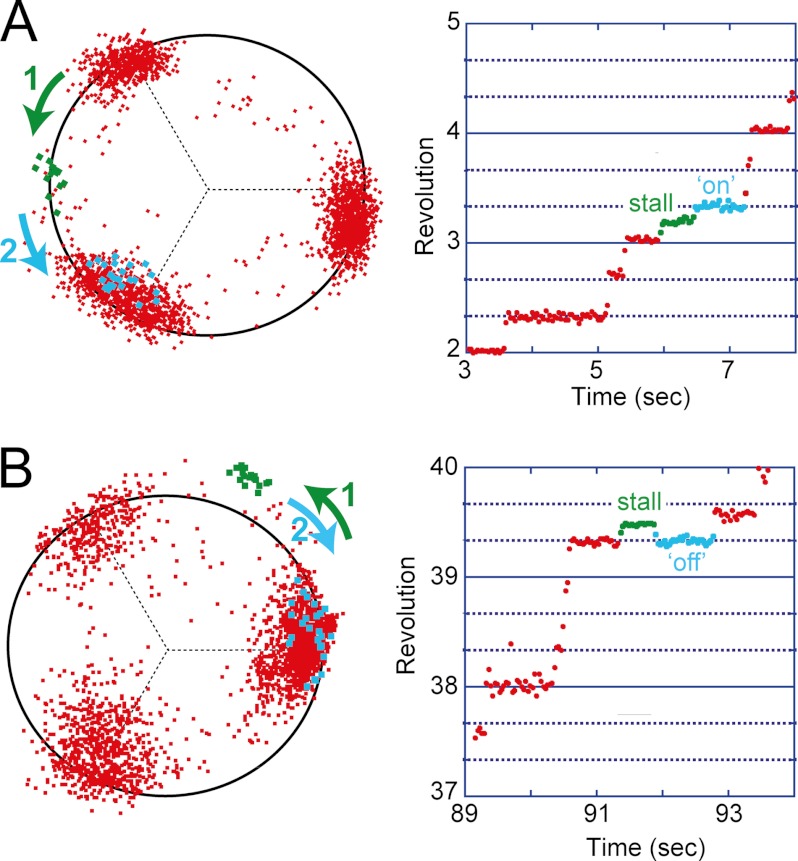
When V1-ATPase showed the ATP-waiting pause, the magnetic tweezers were turned on to stall molecules at the target angle. After a defined period elapsed, the magnetic tweezers were turned off to release the molecule. V1-ATPase essentially demonstrated two distinct responses. In the first response, V1-ATPase made a 120° step to the next ATP-waiting angle immediately after release (Fig. 2A). Because V1-ATPase is unable to induce rotation unless it binds to ATP, this means that V1-ATPase had already bound to ATP when released from the magnetic tweezers. This response was termed the “on” event. In the second response, V1-ATPase simply rotated back to the original ATP-waiting angle (Fig. 2B), termed the “off” event. In the “off” event, the molecules demonstrated a spontaneous 120° step to the next ATP-waiting angle. The histogram of the waiting time until the next spontaneous 120° step displayed nearly the same distribution as that observed of the ATP-waiting time during free rotation (Fig. 3A). This suggests that V1-ATPase returned to the ATP-waiting state in the off event. We also analyzed the ATP-waiting time of the next step after an on event. The waiting time histogram also displayed the same distribution as that observed during the free rotation (Fig. 3B). Thus, the stall-and-release manipulation affected neither the catalytic nor the kinetic properties of V1-ATPase, suggestive of the high robustness of V1-ATPase. The probability of ATP binding was measured as the probability of an on event, Pon. V1-ATPase also displayed unclassifiable behaviors, such as pausing for an unusually long period after returning to the original ATP-waiting angle (likely to be caused by the ADP-inhibited form). However, the occurrence rate of minor behaviors was very low (<5%); therefore, they were omitted from the analysis.
FIGURE 2.
V1 showed two general behaviors upon mechanical manipulation with magnetic tweezers. The left figures display the X-Y trajectories, and the right figures display the revolution versus time graphs. A, on event. When the molecule entered into the ATP-waiting pause, we switched on the magnetic tweezers to generate a magnetic field and rotated the magnetic bead and therefore the shaft to the target angle. Here, the bead was rotated almost 60° from the original waiting angle, stalled there for 0.5 s, and then released. Upon release, the shaft proceeded directly to the next ATP-waiting angle. This behavior implies that V1 was bound ATP at the time of release, which generated the torque necessary for advancing to the next step. B, off event. The same molecule from A was stalled again at 60° from another ATP-waiting angle. Here, shaft returned to the original (0°) angle, indicating that V1 was not bound ATP at the time of release.
FIGURE 3.
Dwell time analysis of spontaneous ATP binding immediately after an off event (A) or an on event (B). To determine the effect of mechanical manipulation on kinetics of V1-ATPase, dwell time analysis was performed for both off and on events.
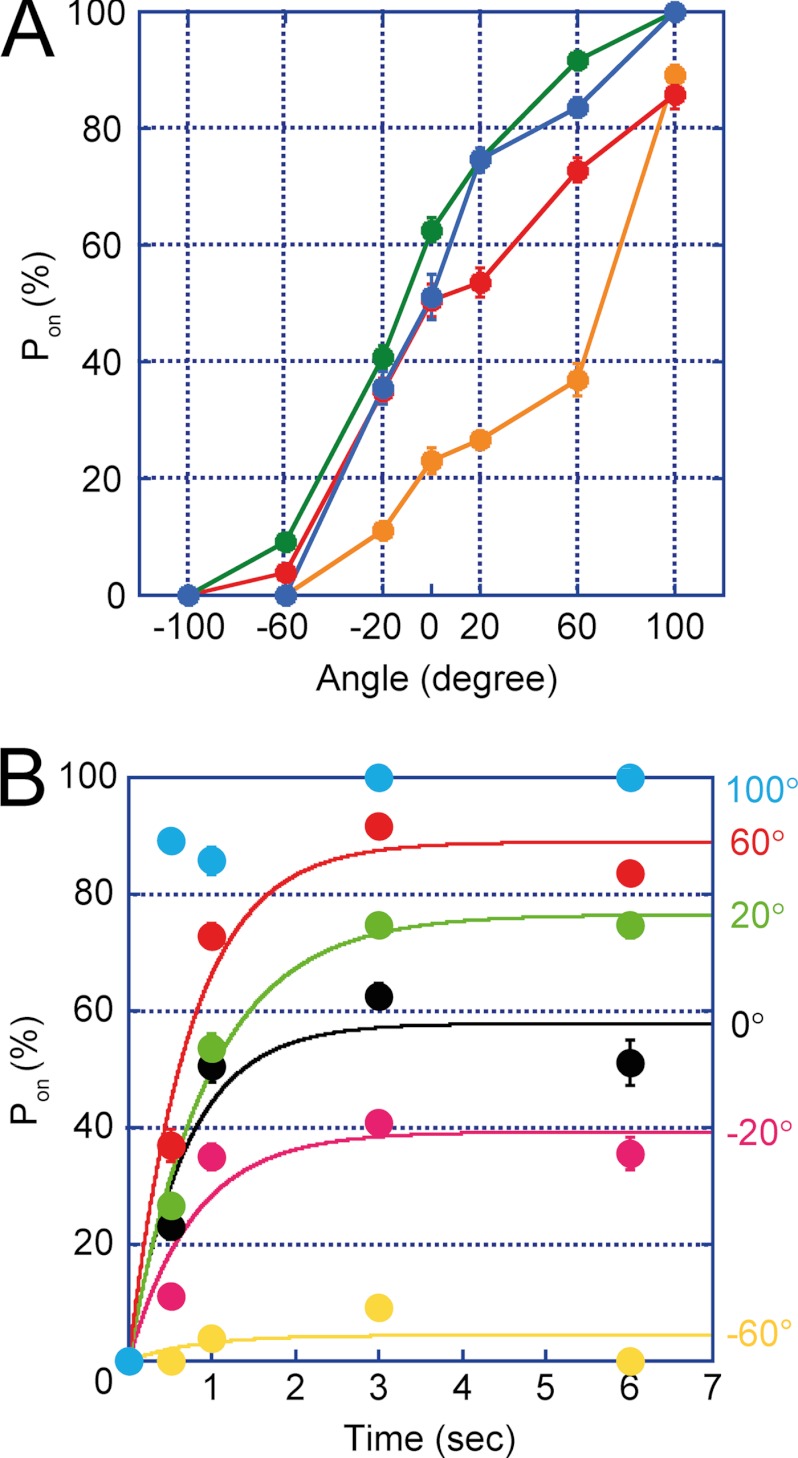
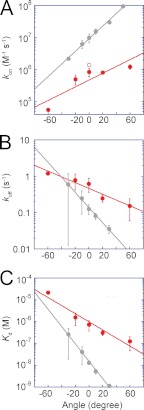
The Pon obtained at limiting concentrations of ATP (1 μm) was plotted against the stall angle (Fig. 4A). It is evident from the graph that Pon significantly depended on the stall angle. Here, we defined 0° as the mean angle for ATP-waiting pause and the “plus” direction as the rotational one (i.e. counterclockwise). When the data points were replotted against the stall time, the time course of Pon was obtained for individual stall angles (Fig. 4B). The values of Pon at −100° were too low to provide a reliable time-dependent increment of Pon; therefore, these data points were omitted. The time courses displayed simple saturation curves, suggesting that the single event, i.e. ATP binding, triggers rotation. This supports the above expectation that the time constant of other reactions such as ATP-hydrolysis step is too short to affect Pon. Note that all of the time courses displayed plateau levels below 100%, except for the +100° stall. This result implies that the ATP-binding event is reversible; during a long period of stalling, V1-ATPase releases ATP into the medium. This process was nearly identical to the stall-and-release experiment of F1-ATPase (13). The time courses were fitted on the basis of a reversible reaction scheme to determine the rate constant of ATP binding (kon) as well as the rate constant of ATP release (koff). The dissociation constant of ATP (Kd) was also determined as the ratio of koff to kon. The kinetic parameters were plotted in semi-log plots (red data in Fig. 5). In Fig. 5A, kon from free rotation was also designated (red open circle). It is evident that kon exponentially increased upon V1-ATPase rotation, whereas koff was exponentially reduced (Fig. 5, A and B), resulting in an exponential reduction in Kd (Fig. 5C). From a −60° to +60° rotation, kon increased by ∼22-fold, whereas koff decreased by 8-fold, resulting in an decrease in Kd of ∼173-fold. For comparison, kinetic parameters of F1-ATPase were also included in Fig. 5 (gray data) (13).
FIGURE 4.
Angle and time dependence of the ATP-binding event in V1. A, angle dependence of ATP binding. The stalling experiment was performed in the range of −120° to +120° for the stalling times of 0.5 (orange), 1 (red), 3 (blue), and 6 s (green). Each data point was obtained from the analysis of 17–201 trials by using a total of 20 molecules. B, time course of ATP binding. The same dataset from A was used to demonstrate the stall time dependence of ATP binding. Data were fitted according to a reversible reaction scheme. The error bars represent the S.D.
FIGURE 5.
Rate constants determined from the time course of ATP binding. kon (A), koff (B), and Kd (C) were fitted with single exponentials. Data of F1-ATPase were also included for comparison (gray-colored) (13). In both V1 and F1, angle dependences are apparent for all the kinetic parameters. Open circle in kon graph (A) designates the kon value in case of free rotation. From the slope of koff, the torque of ATP binding for V1-ATPase was calculated to yield 4 pN·nm per radian, which is smaller than 11 pN·nm per radian of F1-ATPase.
DISCUSSION
In our previous study, the rate constants kon, koff, and Kd were determined in the stall-and-release experiments of F1-ATPase (gray circles and lines; Fig. 5) (13). A comparison of V1-ATPase and F1-ATPase data suggests that the ATP-binding process does not contribute to torque generation in V1-ATPase as much as it does in F1-ATPase. One of the distinctive features of V1-ATPase data is that the ATP-binding site of V1-ATPase has significantly lower affinity to ATP than the ATP-binding site of F1-ATPase (6). In kinetic terms, the kon of V1-ATPase is lower than that of F1-ATPase over all stall angles, whereas koff and Kd are higher. However, the individual data points do not provide any indication of the contribution of ATP-binding process to torque generation. More important in understanding the contributions to torque generation is the angle dependence of the kinetic parameters. In our previous report on the angle dependence of F1-ATPase (13), we estimated the contributions of ATP binding to torque generation from the angle dependence of koff, comparing it with that of the ATP-hydrolysis step. Torque generated by ATP binding corresponds to the slope of the rotary potential of the ATP-bound state (16). Because −kBT lnkoff (θ) represents the relative energy difference between the ATP-bound state and the transition state of ATP binding/release, the differential function, −kBT (σ(lnk(θ)))/(σθ), indicates the magnitude of torque generated by ATP binding. The assumption behind this estimation is that only the free energy of the ground state changes upon rotation, whereas the activation energy level remains constant. The magnitude of −kBT (σ(lnk(θ)))/(σθ), for V1-ATPase is only 38% that of F1-ATPase. The absolute values of estimated torque generation during the ATP-binding process were 4 pN·nm and 11 pN·nm for V1-ATPase and F1-ATPase, respectively. In practice, these values must be underestimated due to the elasticity of the rotary shaft (17–19). Despite this qualification, the estimates still suggest that the role of ATP binding in torque generation is not as important in V1-ATPase as in F1-ATPase, considering that the torsional rigidity of the rotor shaft estimated from the rotary fluctuation during the pausing state does not greatly differ between V1-ATPase and F1-ATPase (10, 19). The conformational changes upon ATP binding of the A subunit are expected to be small compared with those of the β subunit in F1-ATPase. The structural analysis of the A subunit with or without bound nucleotides is highly anticipated.
Interestingly, the ATP-binding rate determined from free rotation (open circle in Fig. 5A) was slightly (but noticeably) higher than the kon determined from the stalling experiment at 0°. The essentially same observation was reported for F1-ATPase. This observation is attributable to rate enhancement by thermal agitation; the rotary shaft of V1-ATPase always undergoes a thermally agitated rotary fluctuation, and an occasional large rotary fluctuation in the forward direction (counterclockwise) triggers ATP binding.
The present results also have implications regarding ATP synthesis. The Kd determined at 0° was 0.7 μm, which is too low to release ATP under physiological conditions where the ATP concentration is in the millimolar range. The angle dependence of Kd predicts that when rotated more than −162°, the Kd increases to the millimolar range, and V1-ATPase is able to release ATP into the medium. Moreover, the koff determined at 0° was 0.46 s−1, which was also too slow to explain the maximum turnover rate of ATP synthesis (67–73 s−1) (6). The angle dependence of koff suggests that the rotary shaft would have to be rotated more than −277° to achieve the maximum turnover rate. Therefore, the present results suggest that the reaction scheme of ATP synthesis is not simply the reverse reaction of the hydrolysis scheme and that the angular dependence of kinetic and thermodynamic parameters must be taken into account (note that these values are also overestimated, considering the possible elasticity of the rotary shaft). The torsional rigidity of the rotary shaft remains to be clarified for a more precise estimation of the contribution of ATP binding to torque generation.
Acknowledgments
We thank Dr. Kino-oka for discussion and continual encouragement, as well as all members of Noji Laboratory for critical discussions and technical advice, and Dr. Watanabe and Dr. Toei for valuable comments.
This work was supported in part by Grant-in-aid for Scientific Research 18074005 (to H. N.) and by a Special Education and Research Expenses grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to H. N.).
- V1-ATPase
- V1 domain from T. thermophilus
- pN
- piconewtons.
REFERENCES
- 1. Yokoyama K., Imamura H. (2005) Rotation, structure, and classification of prokaryotic V-ATPase. J. Bioenerg. Biomembr. 37, 405–410 [DOI] [PubMed] [Google Scholar]
- 2. Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 [DOI] [PubMed] [Google Scholar]
- 3. Yoshida M., Muneyuki E., Hisabori T. (2001) ATP synthase: a marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2, 669–677 [DOI] [PubMed] [Google Scholar]
- 4. Lee L. K., Stewart A. G., Donohoe M., Bernal R. A., Stock D. (2010) The structure of the peripheral stalk of Thermus thermophilus H+-ATPase/synthase. Nat. Struct. Mol. Biol. 17, 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Numoto N., Hasegawa Y., Takeda K., Miki K. (2009) Inter-subunit interaction and quaternary rearrangement defined by the central stalk of prokaryotic V1-ATPase. EMBO Rep. 10, 1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakano M., Imamura H., Toei M., Tamakoshi M., Yoshida M., Yokoyama K. (2008) ATP hydrolysis and synthesis of a rotary motor V-ATPase from Thermus thermophilus. J. Biol. Chem. 283, 20789–20796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furuike S., Nakano M., Adachi K., Noji H., Kinosita K., Jr., Yokoyama K. (2011) Resolving stepping rotation in Thermus thermophilus H+-ATPase/synthase with an essentially drag-free probe. Nat. Commun. 2, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yokoyama K., Muneyuki E., Amano T., Mizutani S., Yoshida M., Ishida M., Ohkuma S. (1998) V-ATPase of Thermus thermophilus is inactivated during ATP hydrolysis but can synthesize ATP. J. Biol. Chem. 273, 20504–20510 [DOI] [PubMed] [Google Scholar]
- 9. Saijo S., Arai S., Hossain K. M., Yamato I., Suzuki K., Kakinuma Y., Ishizuka-Katsura Y., Ohsawa N., Terada T., Shirouzu M., Yokoyama S., Iwata S., Murata T. (2011) Crystal structure of the central axis DF complex of the prokaryotic V-ATPase. Proc. Natl. Acad. Sci. U.S.A. 108, 19955–19960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imamura H., Takeda M., Funamoto S., Shimabukuro K., Yoshida M., Yokoyama K. (2005) Rotation scheme of V1-motor is different from that of F1-motor. Proc. Natl. Acad. Sci. U.S.A. 102, 17929–17933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimabukuro K., Yasuda R., Muneyuki E., Hara K. Y., Kinosita K., Jr., Yoshida M. (2003) Catalysis and rotation of F1 motor: cleavage of ATP at the catalytic site occurs in 1 ms before 40 degree substep rotation. Proc. Natl. Acad. Sci. U.S.A. 100, 14731–14736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi K., Ueno H., Iino R., Noji H. (2010) Fluctuation theorem applied to F1-ATPase. Phys. Rev. Lett. 104, 218103. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe R., Okuno D., Sakakihara S., Shimabukuro K., Iino R., Yoshida M., Noji H. (2012) Mechanical modulation of catalytic power on F1-ATPase. Nat. Chem. Biol. 8, 86–92 [DOI] [PubMed] [Google Scholar]
- 14. Uner N. E., Nishikawa Y., Okuno D., Nakano M., Yokoyama K., Noji H. (2012) Single-molecule analysis of inhibitory pausing states of V1-ATPase. J. Biol. Chem. 287, 28327–28335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirono-Hara Y., Ishizuka K., Kinosita K., Jr., Yoshida M., Noji H. (2005) Activation of pausing F1 motor by external force. Proc. Natl. Acad. Sci. U.S.A. 102, 4288–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinosita K., Jr., Adachi K., Itoh H. (2004) Rotation of F1-ATPase: how an ATP-driven molecular machine may work. Annu. Rev. Biophys. Biomol. Struct. 33, 245–268 [DOI] [PubMed] [Google Scholar]
- 17. Sielaff H., Rennekamp H., Wächter A., Xie H., Hilbers F., Feldbauer K., Dunn S. D., Engelbrecht S., Junge W. (2008) Domain compliance and elastic power transmission in rotary F0F1-ATPase. Proc. Natl. Acad. Sci. U.S.A. 105, 17760–17765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cherepanov D. A., Junge W. (2001) Viscoelastic dynamics of actin filaments coupled to rotary F-ATPase: curvature as an indicator of the torque. Biophys. J. 81, 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okuno D., Iino R., Noji H. (2010) Stiffness of γ subunit of F1-ATPase. Eur. Biophys. J. 39, 1589–1596 [DOI] [PubMed] [Google Scholar]