Abstract
Objective:
The most difficult thyroid tumors to be diagnosed by cytology and histology are conventional follicular carcinomas (cFTCs) and oncocytic follicular carcinomas (oFTCs). Several microRNAs (miRNAs) have been previously found to be consistently deregulated in papillary thyroid carcinomas; however, very limited information is available for cFTC and oFTC. The aim of this study was to explore miRNA deregulation and find candidate miRNA markers for follicular carcinomas that can be used diagnostically.
Design:
Thirty-eight follicular thyroid carcinomas (21 cFTCs, 17 oFTCs) and 10 normal thyroid tissue samples were studied for expression of 381 miRNAs using human microarray assays. Expression of deregulated miRNAs was confirmed by individual RT-PCR assays in all samples. In addition, 11 follicular adenomas, two hyperplastic nodules (HNs), and 19 fine-needle aspiration samples were studied for expression of novel miRNA markers detected in this study.
Results:
The unsupervised hierarchical clustering analysis demonstrated individual clusters for cFTC and oFTC, indicating the difference in miRNA expression between these tumor types. Both cFTCs and oFTCs showed an up-regulation of miR-182/-183/-221/-222/-125a-3p and a down-regulation of miR-542-5p/-574-3p/-455/-199a. Novel miRNA (miR-885-5p) was found to be strongly up-regulated (>40-fold) in oFTCs but not in cFTCs, follicular adenomas, and HNs. The classification and regression tree algorithm applied to fine-needle aspiration samples demonstrated that three dysregulated miRNAs (miR-885-5p/-221/-574-3p) allowed distinguishing follicular thyroid carcinomas from benign HNs with high accuracy.
Conclusions:
In this study we demonstrate that different histopathological types of follicular thyroid carcinomas have distinct miRNA expression profiles. MiR-885-5p is highly up-regulated in oncocytic follicular carcinomas and may serve as a diagnostic marker for these tumors. A small set of deregulated miRNAs allows for an accurate discrimination between follicular carcinomas and hyperplastic nodules and can be used diagnostically in fine-needle aspiration biopsies.
Follicular thyroid carcinomas of conventional type (cFTCs) and oncocytic (Hürthle) type (oFTCs) are the most common types of thyroid malignancies after papillary carcinoma (1). These tumors represent a diagnostic challenge, especially in a preoperative setting in which common histological criteria for malignancy, such as capsular penetration or vascular invasion, cannot be assessed (2). Therefore, most of fine-needle aspiration biopsies (FNABs) from follicular carcinoma nodules are diagnosed as indeterminate by cytological evaluation, hindering patient management. Genetic alterations such as mutations in RAS genes and PAX8/PPARγ are known to occur in follicular carcinomas, and they have been introduced into clinical practice to facilitate an accurate preoperative and postoperative diagnosis (3–5). However, approximately 30% of all follicular carcinomas and more than 80% of oFTCs do not harbor any known mutations (6, 7). Therefore, a discovery of additional molecular markers can be useful for improvement of diagnosis in these tumors.
MicroRNAs (miRNAs) are small noncoding RNAs that negatively regulate gene expression either through inhibition of mRNA translation or by promoting mRNA degradation. They are pivotal regulators of various cellular processes including proliferation, differentiation, apoptosis, survival, motility, and morphogenesis. Being centrally involved in these processes, it is not surprising that they play a key role in human cancer (8–14). Both the loss and gain of miRNA function contributes to cancer development through either up-regulation or silencing of target genes, therefore allowing them to function as tumor suppressors or as oncogenes.
Some data suggest that miRNA profiles allow the reliable identification of the cell origin of tumors (15). However, it remains to be fully understood whether variable tumor types originating from the same cell type have different miRNA profiles. The most recent World Health Organization classification of thyroid tumors designates oncocytic thyroid adenomas and carcinomas as a variant of follicular tumors (1). However, some histological features and mutational profiles (16) are different between these tumor types.
miRNA signatures have been detected in hematological and solid human malignancies (17–19) including thyroid tumors (20, 21). Several miRNAs (miR-146, miR-221, miR-222, miR-155, miR-31, miR-21) have been reported to be consistently deregulated in papillary thyroid carcinomas (22–26) and have demonstrated to have a reasonable diagnostic accuracy for detection of malignancy in FNABs and surgically removed tumors (26–28). In addition, miRNA profiling has been proven to be a valuable tool to predict the outcome in papillary thyroid cancer (25, 29). Recently several studies indicated the importance of miRNAs in thyroid cell proliferation and in mediation of thyroid cell growth induced by TSH (30, 31) Yet only limited information is available for miRNA expression in follicular carcinomas (23, 32). The aim of this study was to analyze a large series of follicular carcinomas for miRNA expression using an expanded miRNA panel and to find novel candidate miRNA markers that can be used diagnostically.
Materials and Methods
Thyroid samples
Totally, 61 thyroid samples (38 follicular carcinomas, 21 cFTCs, and 17 oFTCs), 11 follicular adenomas (FA) (three of conventional and eight of oncocytic type), 10 normal thyroid tissues, and two hyperplastic nodules were analyzed. Thirty tissues were snap frozen and 31 were formalin fixed and paraffin embedded (FFPE) tissues. Snap-frozen tissue from surgically removed thyroid samples was collected at the Department of Pathology, University of Pittsburgh Medical Center, following the institutional review board approval. FFPE tissues were received from the University Hospital Zurich and surrounding pathology institutes, approved by the Cantonal Research Ethics Board (STV 28-2006). All tumors were classified according to widely accepted diagnostic histologic criteria (1). In addition, 19 FNABs collected into nucleic acids preservative solution (Roche Molecular Biochemicals, Mannheim, Germany) and frozen at −80 C for routine mutational testing at the Molecular Anatomic Pathology laboratory, University of Pennsylvania Medical Center, were analyzed for the expression of individual miRNAs.
RNA isolation
Each FFPE tissue specimen was stained with hematoxylin and eosin to ensure that characteristic features of thyroid tumor were present. Areas with high density and purity (>80%) of tumor cells were marked for microdissection of adjacent sections to minimize contamination from surrounding healthy thyroid tissue or infiltrating cells. Overlapping areas in up to six adjacent slides were manually microdissected from 10- to 15-μm unstained histological sections under the guidance of a hematoxylin and eosin-stained slide using an Olympus SZ61 stereo microscope (Olympus, Hamburg, Germany). Each frozen tissue sample was embedded into optimum cutting temperature medium (Thermo Shandon, Pittsburgh, PA), sectioned, stained, and evaluated for tumor purity under the microscope. Total RNA was extracted from snap-frozen surgical specimens using Trizol reagent (Invitrogen, Life Technologies, Carlsbad, CA) as previously described (33) and from FFPE tissue samples with the RecoverAll kit (Ambion, Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Total nucleic acids were isolated from FNABs using the MagNA Pure Compact RNA isolation kit (Roche Molecular Biochemicals, Mannheim, Germany). RNA quantity was assessed with a Spectrophotometer (NanoDrop 1000; Thermo Scientific, Waltham, MA). mRNA quality was assessed by amplification of GAPDH and KRT7 housekeeping genes, whereas miRNA quality was assessed by the amplification of small nuclear RNAs, RNU44 and U6 snRNA.
miRNA expression analysis
Quantitation of mature miRNA expression levels in thyroid tumors and normal thyroid tissue was performed using TaqMan human microarray assays (Applied Biosystems, Life Technologies, Carlsbad, CA), which was designed to detect 381 human miRNAs. The array was performed on an ABI 7900 platform (Applied Biosystems, Life Technologies). To assure a reproducibility of the method, one tumor sample (FC7) was assayed three times using different concentrations of RNA (6, 30, 150 ng). A high correlation (average r = 0.91) in miRNA expression levels was found between the three runs. Total RNA from frozen samples and from FFPE tissues was reverse transcribed using a high-capacity cDNA archive kit (Applied Biosystems, Life Technologies) followed by amplification on an ABI 7900 real-time PCR system (Applied Biosystems, Life Technologies). Endogenous controls, RNU44 and RNU48 (Applied Biosystems, Life Technologies), were used for the normalization of RNA input and nonhuman miRNA ath-miR159a was used as a negative control. Expression of individual miRNAs was analyzed using the TaqMan individual miRNA assays (Applied Biosystems, Life Technologies) according to the manufacturer's instructions.
To assess for differences in miRNA preservation between frozen and FFPE tissue, miRNA expression was measured within each tissue type. Most of the miRNAs were similarly expressed between frozen and FFPE tissues, demonstrating a high correlation between the data sets (r = 0.903). However, some differences were observed for miRNAs expressed at later cycles of amplification. Therefore, all strongly up-regulated or down-regulated miRNAs were validated by individual RT-PCR reactions, and only concordant miRNAs and miRNAs validated in both frozen and FFPE data sets were included in this study.
miRNA expression levels were calculated by relative quantitation using DataAssist version 3.0 software (Applied Biosystems, Life Technologies) and the fold-expression changes were determined by the 2−ΔΔCT method (34). The maximum allowed the cycle threshold value for calculations was 38. Outliers among replicates were excluded and P values were adjusted using the Benjamin-Hochberg false discovery rate. The data are presented as the fold change of miRNA expression in tumors relatively to normal thyroid tissues after normalization to endogenous controls, RNU44 and U6 snRNA.
Statistical analysis
DataAssist version 3.0 software (Applied Biosystems, Life Technologies) was used to calculate agglomerative hierarchical clustering and RQ plots between thyroid specimens. Classification and regression tree analysis was performed with SPSS 17 (SPSS Inc., Chicago, IL). In silico analysis of miRNA targets was performed with microRNA Data Integration Portal, which integrated multiple prediction databases to assure accurate miRNA-target relationships as described (35–37). The Genemania Network (36) was used to determine physical interaction between predicted target genes.
Results
miRNA expression profiles of cFTCs and oFTCs
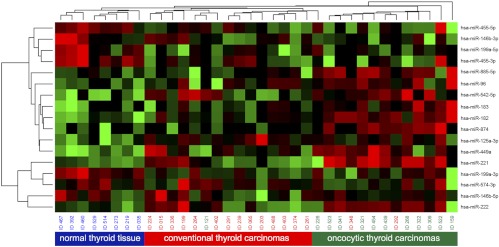
Initially, 38 follicular carcinomas (21 cFTCs and 17 oFTCs) and 10 normal tissues were studied for the expression of 381 miRNAs using a PCR-based array approach. To determine whether different histopathological types of follicular thyroid carcinomas have distinct miRNA profiles, the unsupervised hierarchical clustering analysis of miRNA expression was performed. It revealed three individual clusters: one for normal thyroid tissue, one for conventional follicular carcinomas, and one for oncocytic follicular carcinomas (Fig. 1). These individual clusters support distinct biological mechanisms involved in the development of these tumors and their histopathological differences.
Fig. 1.
Unsupervised hierarchical clustering analysis (Euklidian distance, average linkage) of follicular carcinomas and normal thyroid tissue based on miRNA expression. Oncocytic thyroid carcinomas (green), conventional thyroid carcinomas (red), and normal thyroid tissue (blue) form three distinct clusters.
Next, we searched for individual miRNAs that had the highest levels of deregulation in cFTCs and oFTCs. We found miR-182, -183, -221, and -222 being up-regulated in both cFTCs and oFTCs compared with normal thyroid tissue. However, the levels of expression were different (Table 1). oFTCs expressed these miRNAs at a higher level, ranging from 10- to 30-fold as compared with 6- to 9-fold in cFTCs. Other up-regulated miRNAs in both tumor types were miR-96, miR-874, and miR-449a (Table 1). Many miRNAs were significantly down-regulated in both cFTCs and oFTCs including miR-542-5p, -574-3p, -455-3p, -455-5p, -199a-5p, -199a-3p, and 125a-3p (Table 1). Interestingly, two miRNAs that are known to be strongly up-regulated in papillary thyroid carcinomas, miR-146b-5p and miR-146b-3p, were also overexpressed at low level (2- to 4-fold) in cFTCs but not in oFTCs (Table 1).
Table 1.
MicroRNAs dysregulated in conventional and oncocytic (Hürthle cell) follicular carcinomas
| miRNA | Conventional FTC (mean fold change) | Oncocytic FTC (mean fold change) |
|---|---|---|
| hsa-miR-182 | 8.97 | 9.47 |
| hsa-miR-183 | 6.94 | 22.83 |
| hsa-miR-222 | 6.78 | 8.61 |
| hsa-miR-221 | 6.67 | 28.75 |
| hsa-miR-125a-3p | 9.42 | 6.02 |
| hsa-miR-146b-5p | 4.25 | 1.22 |
| hsa-miR-146b-3p | 2.64 | 1.29 |
| hsa-miR-96 | 2.41 | 4.81 |
| hsa-miR-874 | 2.08 | 8.90 |
| hsa-miR-449a | 1.99 | 4.85 |
| hsa-miR-885-5p | 0.70 | 39.21 |
| hsa-miR-574-3p | 0.72 | 0.58 |
| hsa-miR-542-5p | 0.53 | 0.59 |
| hsa-miR-455-3p | 0.39 | 0.10 |
| hsa-miR-455-5p | 0.38 | 0.36 |
| hsa-miR-199a-5p | 0.25 | 0.23 |
| hsa-miR-199a-3p | 0.20 | 0.33 |
Fold change of 1 indicates no difference in miRNA expression as compared to normal tissue, whereas a fold change less than 1 reflects a down-regulation and more than 1 reflects an up-regulation of the specific miRNA. Novel miRNA (miR-885-5p) strongly up-regulation only in oFTC has shown in bold.
Expression of miR-885-5p
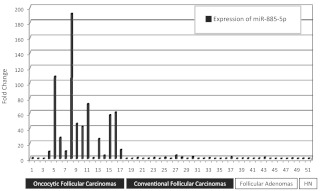
While exploring miRNAs differentially expressed between cFTCs and oFTCs, mir-885-5p was noticed as highly up-regulated in oFTCs. Expression of this miRNA was validated with an individual RT-PCR assay. Fifteen of 17 oFTCs (88%) demonstrated overexpression of miR-885-5p at levels ranging from 4- to 191-fold compared with normal thyroid tissue (Fig. 2). In contrast, only one of 21 conventional follicular thyroid carcinomas (FTCs) (4.8%) showed an up-regulation of miR-885-5p. These results were validated in an independent set of 11 follicular adenomas (eight oncocytic and three conventional types) and two hyperplastic nodules (Fig. 2). miR-885-5p was not up-regulated in follicular adenomas and hyperplastic nodules with the exception of one oncocytic FA.
Fig. 2.
Validation of miR-885-5p expression by individual RT-PCR assays in oFTCs, cFTCs, follicular adenomas, and hyperplastic nodules (HN). Strong up-regulation of miR-885-5p was detected only in oFTCs.
A search for predicted target genes for miR-885-5p using a combination of different prediction databases via microRNA Data Integration Portal software (35) revealed several genes that are associated with mitochondrial oxidative stress including the HTRA2 gene. In addition, the Genemania Network (36) determined a physical interaction between HTRA2 and NDUFA13 (GRIM19), a gene involved in the mitochondrial respiratory chain (38–40). This suggested that miR-885-5p may play a pathogenetic role in the development of oFTCs, whose hallmark is an accumulation of abundant mitochondria.
Diagnostic utility of deregulated miRNAs in fine needle aspiration samples
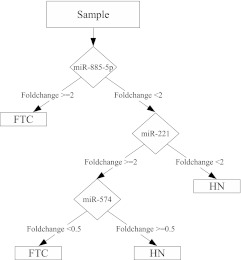
To validate the potential diagnostic utility of deregulated miRNAs found in this study, we analyzed an independent set of 19 FNABs that was derived either from hyperplastic nodules (n = 11) or from nodules diagnosed as FTC after surgery (n = 8). Seven of these FNAB samples were from oncocytic FTCs and one from conventional FTC. All these FNAB samples were diagnosed as indeterminate by cytological evaluation and tested negative for BRAF, RAS, RET/PTC and PAX8/PPARγ mutations as previously described (3). Expression of up-regulated miRNAs (miR-182/183/221/222/146-5p/146-3p/885-5p) and down-regulated miRNAs (miR-542-5p/455-3p/445-5p/574-3p/199a-5p/199a-3p) were determined by individual RT-PCR assays. As a result, only a small subset of miRNAs demonstrated consistent difference in expression between benign and malignant FNAB samples. Classification and regression tree algorithm revealed that a 94% diagnostic accuracy can be achieved using only miR-885–5p and a 100% diagnostic accuracy using miR-885-5p, -221 and -574-3p (Fig. 3).
Fig. 3.
Classification and regression tree analysis of miRNA expression for discrimination between FTCs and hyperplastic nodules (HN) revealed the following diagnostic algorithm. Three miRNAs (miR-885-5p, miR-221, and miR-574) were sufficient to achieve a 100% diagnostic accuracy in FNABs derived from between benign hyperplastic nodules and follicular carcinomas.
Discussion
In this study, we evaluated miRNA expression in a large series of follicular thyroid carcinomas using miRNAs array approach with subsequent validation of deregulated miRNAs by individual assays. We demonstrated that two histopathological types of follicular carcinomas (cFTC and oFTC) have distinct miRNA expression profiles. We also identified miR-885–5p as a novel miRNA marker of oFTC. In addition, a limited set of miRNAs (miR-221, miR-885-5p, miR-574-3p) allowed us to discriminate between benign hyperplastic nodules and FTCs in clinically collected FNAB samples with high accuracy.
It is currently known that miRNAs are expressed in a tissue specific manner according to the cell of origin (15). Nonetheless, the picture is not clear whether this also holds true for different kinds of tumors arising from the same cell type. We tested two closely related histopathological types of follicular carcinoma for expression of 381 miRNAs. The miRNA profiles were substantially different between conventional and oncocytic follicular carcinomas because they formed an individual cluster on unsupervised clustering analysis. It provided more evidence to support the notion that oncocytic tumors represent a distinct class of thyroid tumors and that miRNA profiles are indeed able to separate even closely related tumors.
Despite the difference in miRNA expression between conventional and oncocytic follicular carcinomas, we were able to identify miRNAs that showed similar pattern of deregulation in both tumor types. For example, miR-182, -183, -221, -222, -96, -874, and -449a were up-regulated and miR-542-5p, -574-3p, -455, and -199a were down-regulated in both tumor types compared with normal thyroid tissue. Some of these miRNAs have been previously reported in thyroid cancer. For example, miR-221 and miR-222 are known to be strongly up-regulated in papillary thyroid carcinoma and at a lower level in dedifferentiated thyroid tumors (20, 21, 23, 24). These two miRNAs are well-known oncogenic miRs, and several important genes have been reported to be their functional targets; among them are CDKN1B (p27Kip1) and KIT (41, 42). Their overexpression has also been directly linked to response to chemotherapy to breast, prostate, and lung cancer (43). Up-regulation of these miRNAs have been previously reported in FTCs by Nikiforova et al. (23) but not by Weber et al. (32), which can be explained by differences in the methodologies used and the selection of tumor samples. In this study, we confirmed the up-regulation of miR-221 and miR-222 in follicular carcinomas of both oncocytic and conventional types. It suggests that miR-221 and miR-222 are the only miRNAs that are found to be consistently up-regulated in different types of thyroid cancer including papillary and follicular carcinoma and can be used as markers for thyroid malignancy.
Most interestingly, we identified a novel miR-885-5p that has never been linked to follicular thyroid carcinomas. miR-885-5p was highly up-regulated in oFTCs but not in conventional FTCs. In addition, this miRNA was not up-regulated in follicular adenomas and hyperplastic nodules, making it a promising diagnostic marker. The miR-885-5p gene is located within the intron of the ATP2B2 gene at chromosomal band 3p25.3. This chromosomal region is frequently deleted in conventional follicular carcinomas (44), which might be one of the explanations for the absence of miR-885-5p expression in conventional FTCs. Our search of target genes for miR-885-5p identified the HtrA serine peptidase 2 (HTRA2) gene as the most promising candidate. Its protein is localized in the mitochondrial membrane and can physically interact with NDUFA13 (GRIM19) (36, 45), a gene known to be involved in oncocytic thyroid tumorigenesis (38, 46). This suggests that miR-885-5p may play a pathogenetic role in the development of oFTCs, whose hallmark is an accumulation of abundant mitochondria, and awaits experimental confirmation.
All deregulated miRNAs in surgically removed follicular carcinomas were evaluated for diagnostic use in FNABs derived from benign and malignant thyroid nodules. Using a series of well-characterized FNAB samples, we provided evidence for high diagnostic potential of a limited panel of miRNAs for the diagnosis of follicular carcinoma in FNAB samples. It appears that only a small number of miRNAs (miR-221, miR-885-5p, miR-574) is sufficient to differentiated FTCs from benign nodules with high diagnostic accuracy. Some miRNAs with high levels of deregulation in surgically removed specimens did not show diagnostic utility in fine-needle aspiration samples. For example, up-regulated miR-182 and miR-183 were found to be overexpressed in hyperplastic nodules and down-regulated miR-199a-3p, -199a-5p, and -455-5p was also down-regulated in hyperplastic nodules. Although our findings need to be further confirmed in a larger series of samples, they lay the foundation for the use of miRNA profiling as an effective diagnostic tool for the preoperative assessment of thyroid nodules.
Acknowledgments
This work was supported by the National Institutes of Health Grant R01 CA88041 (to Y.E.N.) and by the Fondation pour la Recherche Nuovo-Soldati, the Gertrud-Hagmann-Stiftung für Malignomforschung, and the Research Support Foundation (to M.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cFTC
- FTC of a conventional type
- FA
- follicular adenoma
- FFPE
- formalin fixed and paraffin embedded
- FNAB
- fine-needle aspiration biopsy
- FTC
- follicular thyroid carcinoma
- miRNA
- microRNA
- oFTC
- oncocytic (Hürthle) type of FTC.
References
- 1. DeLellis R, Lloyd R, Heitz P, Eng C, eds. 2004. Pathology and genetics of tumours of endocrine organs. Lyon, France: IARC Press [World Health Organization classification of tumours] [Google Scholar]
- 2. Nikiforov YE. 2012. Thyroid tumors: classification, staging and general considerations. In: Nikiforov Y, Biddinger PW, Thompson LDR, eds. Diagnostic pathology and molecular genetics of the thyroid. Baltimore: Lippincott Williams &Wilkins; 108–118 [Google Scholar]
- 3. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT, Coyne C, Johnson JT, Stewart AF, Nikiforova MN. 2011. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 96:3390–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. 2009. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 5. Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, Di Santo A, Caruso G, Carli AF, Brilli L, Montanaro A, Pacini F. 2010. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab 95:1365–1369 [DOI] [PubMed] [Google Scholar]
- 6. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW, 2nd, Tallini G, Kroll TG, Nikiforov YE. 2003. RAS point mutations and PAX8-PPAR γ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 88:2318–2326 [DOI] [PubMed] [Google Scholar]
- 7. Sobrinho-Simoes M, Eloy C, Magalhaes J, Lobo C, Amaro T. 2011. Follicular thyroid carcinoma. Mod Pathol 24(Suppl 2):S10–S18 [DOI] [PubMed] [Google Scholar]
- 8. Lynam-Lennon N, Maher SG, Reynolds JV. 2009. The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos Soc 84:55–71 [DOI] [PubMed] [Google Scholar]
- 9. Garzon R, Calin GA, Croce CM. 2009. MicroRNAs in Cancer. Annu Rev Med 60:167–179 [DOI] [PubMed] [Google Scholar]
- 10. Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. 2004. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 5:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. 2005. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 353:1793–1801 [DOI] [PubMed] [Google Scholar]
- 12. Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. 2006. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9:189–198 [DOI] [PubMed] [Google Scholar]
- 13. Raponi M, Dossey L, Jatkoe T, Wu X, Chen G, Fan H, Beer DG. 2009. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res 69:5776–5783 [DOI] [PubMed] [Google Scholar]
- 14. Busacca S, Germano S, De Cecco L, Rinaldi M, Comoglio F, Favero F, Murer B, Mutti L, Pierotti M, Gaudino G. 2010. MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol 42:312–319 [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. 2005. MicroRNA expression profiles classify human cancers. Nature 435:834–838 [DOI] [PubMed] [Google Scholar]
- 16. Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. 2002. PAX8-PPARγ rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol 26:1016–1023 [DOI] [PubMed] [Google Scholar]
- 17. Barbarotto E, Schmittgen TD, Calin GA. 2008. MicroRNAs and cancer: profile, profile, profile. Int J Cancer 122:969–977 [DOI] [PubMed] [Google Scholar]
- 18. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. 2006. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103:2257–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. 2002. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 99:15524–15529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. 2005. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA 102:19075–19080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M, Negrini M, Croce CM, Fusco A. 2006. MicroRNA deregulation in human thyroid papillary carcinomas. Endocrine Relat Cancer 13:497–508 [DOI] [PubMed] [Google Scholar]
- 22. Schwertheim S, Sheu SY, Worm K, Grabellus F, Schmid KW. 2009. Analysis of deregulated miRNAs is helpful to distinguish poorly differentiated thyroid carcinoma from papillary thyroid carcinoma. Horm Metab Res 41:475–481 [DOI] [PubMed] [Google Scholar]
- 23. Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. 2008. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab 93:1600–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tetzlaff MT, Liu A, Xu X, Master SR, Baldwin DA, Tobias JW, Livolsi VA, Baloch ZW. 2007. Differential expression of miRNAs in papillary thyroid carcinoma compared to multinodular goiter using formalin fixed paraffin embedded tissues. Endocr Pathol 18:163–173 [DOI] [PubMed] [Google Scholar]
- 25. Yip L, Kelly L, Shuai Y, Armstrong MJ, Nikiforov YE, Carty SE, Nikiforova MN. 2011. MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. Ann Surg Oncol 18:2035–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen YT, Kitabayashi N, Zhou XK, Fahey TJ, 3rd, Scognamiglio T. 2008. MicroRNA analysis as a potential diagnostic tool for papillary thyroid carcinoma. Mod Pathol 21:1139–1146 [DOI] [PubMed] [Google Scholar]
- 27. Nikiforova MN, Chiosea SI, Nikiforov YE. 2009. MicroRNA expression profiles in thyroid tumors. Endocr Pathol 20:85–91 [DOI] [PubMed] [Google Scholar]
- 28. Menon MP, Khan A. 2009. Micro-RNAs in thyroid neoplasms: molecular, diagnostic and therapeutic implications. J Clin Pathol 62:978–985 [DOI] [PubMed] [Google Scholar]
- 29. Chou CK, Chen RF, Chou FF, Chang HW, Chen YJ, Lee YF, Yang KD, Cheng JT, Huang CC, Liu RT. 2010. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAF(V600E) mutation. Thyroid 20:489–494 [DOI] [PubMed] [Google Scholar]
- 30. Akama T, Sue M, Kawashima A, Wu H, Tanigawa K, Suzuki S, Hayashi M, Yoshihara A, Ishido Y, Ishii N, Suzuki K. 2012. Identification of microRNAs that mediate thyroid cell growth induced by TSH. Mol Endocrinol 26:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leone V, D'Angelo D, Rubio I, de Freitas PM, Federico A, Colamaio M, Pallante P, Medeiros-Neto G, Fusco A. 2011. MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting CCND2, CXCR4, and SDF-1α. J Clin Endocrinol Metab 96:E1388–E1398 [DOI] [PubMed] [Google Scholar]
- 32. Weber F, Teresi RE, Broelsch CE, Frilling A, Eng C. 2006. A limited set of human microRNA is deregulated in follicular thyroid carcinoma. J Clin Endocrinol Metab 91:3584–3591 [DOI] [PubMed] [Google Scholar]
- 33. Nikiforova MN, Caudill CM, Biddinger P, Nikiforov YE. 2002. Prevalence of RET/PTC rearrangements in Hashimoto's thyroiditis and papillary thyroid carcinomas. Int J Surg Pathol 10:15–22 [DOI] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 35. Shirdel EA, Xie W, Mak TW, Jurisica I. 2011. NAViGaTing the micronome—using multiple microRNA prediction databases to identify signalling pathway-associated microRNAs. PLoS One 6:e17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montojo J, Zuberi K, Rodriguez H, Kazi F, Wright G, Donaldson SL, Morris Q, Bader GD. 2010. GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics 26:2927–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. 2010. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38:W214–W220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sobrinho-Simões M, Preto A, Rocha AS, Castro P, Máximo V, Fonseca E, Soares P. 2005. Molecular pathology of well-differentiated thyroid carcinomas. Virchows Arch 447:787–793 [DOI] [PubMed] [Google Scholar]
- 39. Fusco A, Viglietto G, Santoro M. 2005. Point mutation in GRIM-19: a new genetic lesion in Hurthle cell thyroid carcinomas. Br J Cancer 92:1817–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Máximo V, Lima J, Soares P, Silva A, Bento I, Sobrinho-Simões M. 2008. GRIM-19 in health and disease. Adv Anat Pathol 15:46–53 [DOI] [PubMed] [Google Scholar]
- 41. Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. 2005. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA 102:18081–18086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM, Fusco A. 2007. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer 14:791–798 [DOI] [PubMed] [Google Scholar]
- 43. Sun T, Yang M, Kantoff P, Lee GS. 2009. Role of microRNA-221/-222 in cancer development and progression. Cell Cycle 8:2315–2316 [DOI] [PubMed] [Google Scholar]
- 44. Hunt JL, Yim JH, Carty SE. 2006. Fractional allelic loss of tumor suppressor genes identifies malignancy and predicts clinical outcome in follicular thyroid tumors. Thyroid 16:643–649 [DOI] [PubMed] [Google Scholar]
- 45. Ma X, Kalakonda S, Srinivasula SM, Reddy SP, Platanias LC, Kalvakolanu DV. 2007. GRIM-19 associates with the serine protease HtrA2 for promoting cell death. Oncogene 26:4842–4849 [DOI] [PubMed] [Google Scholar]
- 46. Máximo V, Botelho T, Capela J, Soares P, Lima J, Taveira A, Amaro T, Barbosa AP, Preto A, Harach HR, Williams D, Sobrinho-Simões M. 2005. Somatic and germline mutation in GRIM-19, a dual function gene involved in mitochondrial metabolism and cell death, is linked to mitochondrion-rich (Hurthle cell) tumours of the thyroid. British journal of cancer 92:1892–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]