Abstract
Objective:
This study investigated the disparity between muscle metabolic rate and mitochondrial metabolism in human muscle of sedentary vs. active individuals.
Research Design and Methods:
Chronic activity level was characterized by a physical activity questionnaire and a triaxial accelerometer as well as a maximal oxygen uptake test. The ATP and O2 fluxes and mitochondrial coupling (ATP/O2 or P/O) in resting muscle as well as mitochondrial capacity (ATPmax) were determined in vivo in human vastus lateralis muscle using magnetic resonance and optical spectroscopy on 24 sedentary and seven active subjects. Muscle biopsies were analyzed for electron transport chain content (using complex III as a representative marker) and mitochondrial proteins associated with antioxidant protection.
Results:
Sedentary muscle had lower electron transport chain complex content (65% of the active group) in proportion to the reduction in ATPmax (0.69 ± 0.07 vs. 1.07 ± 0.06 mm sec−1) as compared with active subjects. This lower ATPmax paired with an unchanged O2 flux in resting muscle between groups resulted in a doubling of O2 flux per ATPmax (3.3 ± 0.3 vs. 1.7 ± 0.2 μm O2 per mm ATP) that reflected mitochondrial uncoupling (P/O = 1.41 ± 0.1 vs. 2.1 ± 0.3) and greater UCP3/complex III (6.0 ± 0.7 vs. 3.8 ± 0.3) in sedentary vs. active subjects.
Conclusion:
A smaller mitochondrial pool serving the same O2 flux resulted in elevated mitochondrial respiration in sedentary muscle. In addition, uncoupling contributed to this higher mitochondrial respiration. This finding resolves the paradox of stable muscle metabolism but greater mitochondrial respiration in muscle of inactive vs. active subjects.
A sedentary lifestyle is associated with lower aerobic capacity and mitochondrial enzyme activity (1–3). In addition, mitochondria appear to be uncoupled in nonathletes based on the ability of exercise (4) to improve coupling of mitochondria, to reduce a key uncoupling protein (UCP), UCP3 (5), and to elevate exercise efficiency (6) from nonathletes. Taken together, these results indicate that mitochondria are reduced in content and altered in function with physical inactivity. Paradoxically, no consistent difference is reported in resting metabolic rate between sedentary and active individuals despite properties that point to greater mitochondrial energy expenditure in less active subjects (7, 8). One possibility is that the loss of mitochondrial content means fewer mitochondria are available to meet cell energy demands, resulting in higher respiration of mitochondria in inactive subjects. A test of the hypothesis that fewer mitochondria respire more in muscle of sedentary vs. active individuals requires direct comparison of muscle cell and mitochondrial metabolism.
Noninvasive tools now permit this direct measurement of both muscle and mitochondrial energy fluxes in human muscle in vivo (9, 10). A pairing of magnetic resonance and optical spectroscopic approaches yields the cell ATP and O2 (ATP/O2) fluxes as well as mitochondrial coupling (P/O) and capacity (ATPmax) (11, 12). These measurements have demonstrated that muscle energy fluxes decline, but mitochondrial uncoupling increases in elderly tissue of both animals and humans (11, 12). Combining these in vivo measures with the assays of mitochondrial properties from muscle biopsies will allow us to test whether a direct measure of cell energy fluxes can resolve the paradox of reduced mitochondrial efficiency without a change in the resting metabolism in the muscle from sedentary vs. active subjects.
Here we evaluated muscle and mitochondrial energy fluxes in vivo in muscle of healthy, young subjects screened for their level of chronic physical activity using an activity questionnaire, triaxial accelerometer, and maximal oxygen uptake (VO2max) test. We used parallel noninvasive spectroscopic tools to measure muscle cell energy fluxes to yield mitochondrial capacity and coupling in vastus lateralis muscle in vivo (10). Biopsies taken at the same site as the in vivo measures were analyzed for electron transport complexes as a measure of mitochondrial content as well as mitochondrial proteins associated with anti-oxidant protection.
Materials and Methods
Study population
All patients signed informed written consent that was approved by the Pennington Biomedical Research Center Human Subjects Institutional Review Board and in accord with the Declaration of Helsinki. Volunteers were healthy males aged 20–40 yr, with a body mass index of 20–30 kg m−2, not having diabetes, and taking no medications. Subjects in this study were recruited based on their level of habitual physical activity. Physical activity level was calculated from a 7-d physical activity recall questionnaire and a triaxial accelerometer worn for at least 4 d. Physical activity index (total daily energy expenditure/resting metabolic rate) was calculated using both methods, and daily activity level was determined from accelerometer data. Sedentary healthy controls (n = 24) had an activity index of less than 1.4 and no bout of high physical activity/exercise exceeding 30 min. Active individuals (n = 7) had an activity index greater than 1.6 and a VO2max of 40 ml kg−1 · min−1 or greater.
Subjects underwent a physical examination and medical history as well as routine fasting blood work including a complete blood count, glucose, insulin, and lipids. Volunteers with chronic illnesses such as heart disease, hypothyroidism, renal, and lung and liver diseases were excluded. The use of β-blockers and other drugs known to affect body weight or adrenergic tone were also exclusionary.
Body composition
Body weight was measured in a gown after voiding and waist circumference measured using a standardized protocol. Height was measured on a calibrated stadiometer using Pennington Biomedical Research Center standard protocols.
Mitochondrial function in vivo
Our protocol combined optical spectroscopy and magnetic resonance spectroscopy to measure the contents and dynamics of key metabolic compounds as previously described in detail (10).
Magnetic resonance methods
Phosphorus spectra were collected as described previously (13) on a 3T GE Signa magnet (GE, Milwaukee, WI) using a 4- or 6-cm 31P-tuned surface coil positioned over the distal vastus lateralis. The free-induction decays were line broadened with the half-height width of the resting phosphocreatine (PCr) peak and Fourier-transformed into spectra. PCr, inorganic phosphate, and ATP peak areas in the fully relaxed spectra were determined by integration using Varian Unity INOVA software (Varian Instruments, Palo Alto, CA). For dynamic PCr determinations during ischemia or exercise, metabolite concentrations were determined relative to the γ-ATP peak during the experiment using the fit-to-standard algorithm (14). The chemical shift of inorganic phosphate relative to PCr in each spectrum was used to calculate pH (15).
Mitochondrial energy fluxes in resting muscle
A detailed description of the procedure for measuring resting ATP flux was given in our original papers on this method in human muscle (16) (see Supplemental Fig. 2). Briefly, we perturbed [PCr] using ischemia (18 min) induced by a tourniquet to block blood flow and eliminate O2 delivery. The rate of PCr breakdown in anoxic muscle measures the ATP flux, which represents the basal ATP demand of the cell, and the flux that must be met by mitochondrial oxidative phosphorylation (9, 10). The glycolytic ATP contribution was determined from the change in pH and PCr during ischemia (17) and found to be less than 10% of total flux as previously reported (11). Validation of this approach in vivo is given elsewhere (18).
ATPmax in vivo
We determined the oxidative ATPmax using a simple exercise protocol in the magnetic resonance spectroscopy magnet, as presented in our published method (19) and as shown in Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Confirmation that ATPmax is a good measure of phosphorylation capacity comes from the close agreement with an independent determination based on the mitochondrial content of the same muscle (20) and the association with the oxidative enzyme activity of healthy muscle (21, 22).
Optical spectroscopy
Two 10-mm fiber optic bundles with a fixed separation distance of 3.0 cm were positioned adjacent to one another over the vastus lateralis. Care was taken to ensure full contact between the probes and the skin throughout the experiment. These probes were fed into a Horiba Jobin Yvon optical spectrograph (Edison, NJ). One fiber with a round-end-to-round-end configuration (model BN-4622; Multimode Fiber Optics Inc., East Hanover, NJ) carrying white light from a 250-W tungsten-halogen light source (model LSH-T250) illuminated the tissue. The remaining fiber (model BN-4621; Multimode Fiber Optics) with a round end (on skin surface) to slit end (to spectrograph) carried reflected light to a spectrograph mounted to a 1024 × 256 matrix backilluminated, deep depleted, thermoelectrically cooled charge-coupled device (CCD) detector. A 300-g/mm grating coupled to a 320-mm focal length spectrograph was used to obtain spectra over a range of wavelengths in the visible-near infrared region (center wavelength 675 nm). We used a 100-msec exposure time and a 1.0 sec delay between scans, resulting in a data acquisition rate of approximately 1 spectra per 1.2 sec. Our approach for developing calibration sets and for the partial least squares analysis of the spectra has been described in detail for mouse hindlimb muscle in vivo (23).
Determining O2 uptake
The O2 uptake was calculated from the rate of deoxygenation of hemoglobin (Hb)-O2 and myoglobin (Mb)-O2 during ischemia, as shown in Supplemental Fig. 2 and as previously described (9, 23). These deoxygenation rates were calibrated using myoglobin concentration determined by gel electrophoresis of extracts from muscle biopsy material as described below (see Biopsy section). Because a blood pressure tourniquet was used to block blood flow and likely pushed blood out of the muscle, the Hb level was determined using a mass-balance approach that used the rate of desaturation of O2 carriers to determine the relative Hb/Mb content as follows:
where Δ%Mb-O2 and Δ%Hb-O2 are the slopes of the lines for the change in saturation of myoglobin and hemoglobin during ischemia for %Mb-O2 greater than 50%.
Mitochondrial coupling
The ratio of phosphorylation to oxidation (ATP/O2) divided by two yields the biochemical convention for coupling: P/O. The P/O measurement was reproducible on average for an individual to within ±6% or ±0.07 absolute units of P/O (n = 4).
Biopsy
After an overnight fast, local anesthesia consisting of lidocaine/bupivacaine was injected and allowed to take effect after which skeletal muscle samples were collected from the vastus lateralis using the Bergstrom technique with suction (Propper Manufacturing Co., Long Island City, NY). At the bedside, samples were rapidly cleaned and blotted dry before snap freezing in liquid nitrogen for Western blotting. Myoglobin was quantified by separation on an 18% Tris-glycine Criterion gel (Bio-Rad Laboratories, Hercules CA) using horse Mb as a standard run on each gel. The gels were stained with Coomassie blue, imaged, and quantified using NIH Image (National Institutes of Health, Bethesda, MD). For the protein analysis, muscle was homogenized by Kontes Duall tissue grinders in radioimmunoprecipitation assay buffer with protease inhibitor and phosphatase inhibitor cocktails (Sigma, St. Louis, MO). For the electron transport complexes (OXPHOS), 20 μg of protein of each sample was run on a 12.5% Criterion Tris-HCl gel (Bio-Rad Laboratories) and transferred to a polyvinyl difluoride membrane (Millipore, Billerica, MA) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1000, ab9484; Abcam, Cambridge, MA) as the loading control. For the remaining protein, 15 μg of protein per sample was run on a 10% SDS-PAGE gel (Bio-Rad Laboratories) and transferred to a polyvinyl difluoride membrane (Thermo Scientific, Rockford, IL). Membranes were incubated with antibodies against UCP3 (ab3477; Abcam), superoxide dismutase (SOD)-2 (sc-30080; Santa Cruz Biotechnology, Santa Cruz, CA), subunit core 2 of complex III (MS304; MitoSciences, Eugene, OR), OXPHOS (1:208, MS601; MitoSciences, Eugene, OR), or GAPDH (MCA2427; AbD Serotec, Raleigh, NC) at 4 C overnight and then probed with goat antirabbit IgG IRDye 800CW or goat antimouse IgG IRDye 680 (926-32211 and 926-32220; LI-COR, Lincoln, NE). The bands were visualized using an Odyssey 9120 infrared imaging system (LI-COR) and quantified using Odyssey application software, version 3.0 (LI-COR). UCP3, SOD2, and adenine nucleotide translocase were run on the same Western blot. Raw densitometry data can be found in Supplemental Fig. 3. GAPDH is commonly used for a loading control in protein analyses of human muscle, and no difference between the groups was found (Supplemental Fig. 4).
Statistical analysis
The analyses were performed and graphs were made using JMP version 4.0.4 (Statistical Analysis Software, Cary, NC) and GraphPad Prism, version 5.0 (GraphPad Software Inc., La Jolla, CA). Data were analyzed using unpaired Student t tests when comparing the sedentary and active groups. A Shapiro-Wilk test was used to confirm the normality of the data. With normally distributed data, a Pearson correlation was used, otherwise a Spearman correlation was used. Significant differences were defined for P ≤ 0.05.
Results
Aerobic, muscle, and mitochondrial oxidative phosphorylation capacities
The physical characteristics of the subjects were similar but the groups were physiologically distinct (Table 1). Sedentary subjects showed VO2max, ATPmax, and complex III (CIII) in muscle that were all approximately 65% of the levels found in active muscle (individual data appear in Supplemental Fig. 5). Table 2 contains a separate analysis of the five complexes of the electron transport chain and shows a similar reduction on average of complexes I-IV to 48–64% of the levels found in active muscle. Complex V is the ATP synthase, which is not involved in oxidation and was unchanged between the two groups.
Table 1.
Physical and physiological characteristics of the subjects in this study
| Height (cm) | Weight (kg) | BMI (kg m−2) | Age (yr) | VO2max [ml (kg min)−1] | ATPmax (mm sec−1) | CIII/GAPDH | |
|---|---|---|---|---|---|---|---|
| Active | 179 ± 1 | 76.4 ± 3.3 | 23.8 ± 1.0 | 23 ± 1 | 49.9 ± 1.2a | 1.07 ± 0.06a | 0.148 ± 0.022a |
| Sedentary | 177 ± 1 | 81.8 ± 2.4 | 26.0 ± 0.6 | 26 ± 1 | 32.5 ± 1.0 | 0.69 ± 0.03 | 0.094 ± 0.007 |
Values are means ± sem.
P < 0.05.
Table 2.
ETC properties of muscle tissue taken from the site of the in vivo measurements determined from Western blotting using OXPHOS antibody
| Groups | ETC complexes |
||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Active | 5.31 ± 0.70 | 2.52 ± 0.27 | 2.48 ± 0.21 | 7.16 ± 1.09 | 1.63 ± 0.22 |
| Sedentary | 2.62 ± 0.31 | 1.63 ± 0.17 | 1.51 ± 0.20 | 4.12 ± 0.52 | 1.59 ± 0.27 |
| Sedentary/active | 0.49a | 0.64a | 0.61a | 0.58a | 0.97 |
Values are means ± sem.
P < 0.05.
Mitochondrial uncoupling and higher ATP and O2 flux per mitochondrion with inactivity
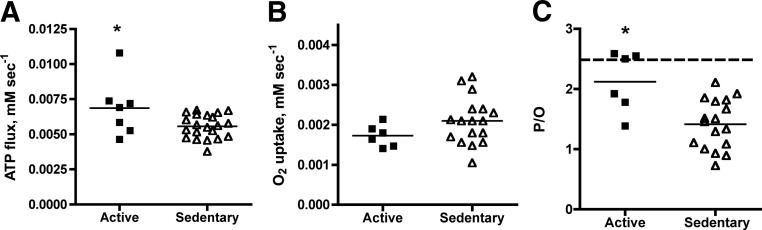
Figure 1 shows reduced ATP flux but no significant change in O2 flux in muscle of sedentary vs. active subjects. The differences in number of subjects in each panel reflect the data that met our quality control criteria. The stable muscle cell O2 flux between groups is in agreement with reports that whole-body resting metabolism was not different between sedentary and active individuals. The ratio of these muscle cell energy fluxes reveal a reduced ATP flux per O2 (low P/O), indicating uncoupling of oxidative phosphorylation in muscle of the sedentary group. This mitochondrial uncoupling is consistent with in vitro measures of mitochondrial inefficiency in biopsy material isolated from muscle of untrained subjects (3, 4, 24). In contrast, the active group had a higher P/O that corresponded to the theoretical value for well-coupled mitochondria (Fig. 1C, dashed line). The significant difference in P/O between the groups (P = 0.001) is maintained if the subject with the high ATP flux value in Fig. 1A is dropped from the active group. The variation in P/O corresponded with the range of the key independently determined physiological measurements as shown by significant correlations with VO2max (r2 = 0.26), ATPmax (r2 = 0.37), and CIII (r2 = 0.51) as presented in Supplemental Fig. 5. These measurements demonstrate at the cell level the disparity in the resting metabolic rate vs. mitochondrial metabolism reported previously in sedentary vs. active muscle (7, 8).
Fig. 1.
ATP flux (A), O2 flux (B), and mitochondrial coupling (C) in individuals from the active (■) and sedentary (▵) groups. The horizontal dashed line in panel C represents the theoretical value for mitochondrial coupling with glucose as substrate (28). *, P < 0.05. Sample sizes for active and sedentary groups, respectively, are: n = 7 and 21 (A); n = 6 and 17 (B); and n = 6 and 17 (C).
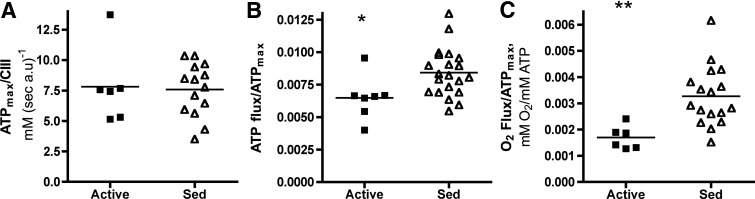
Expression of these fluxes per content of CIII reveals that ATPmax was lower in proportion to mitochondrial content in sedentary muscle (Fig. 2). The similarity in ATPmax/CIII between groups indicates that the functional capacity per mitochondria was not significantly different between sedentary vs. active muscle. However, ATP flux per ATPmax was significantly higher in muscle of sedentary vs. active subjects [0.0084 ± 0.0004 (n = 7) vs. 0.0064 ± 0.0006 (n = 21), P < 0.021], which demonstrates that mitochondria work at a higher fraction of their capacity in the less active muscle. This flux excludes glycolytic ATP supply, but a separate determination indicated no difference between groups and only a small contribution to total ATP supply by glycolysis (<7.5%) as also reported in leg and hand muscles (11).
Fig. 2.
Muscle energy fluxes normalized to mitochondrial content or capacity in vastus lateralis of active (■) and sedentary (▵) subjects: ATPmax/CIII (A), ATP flux/ATPmax (B), and O2 flux/ATPmax (C). *, P < 0.03; **, P < 0.004. Sample sizes for active and sedentary groups, respectively, are: n = 6 and 14 (A); n = 7 and 21 (B); and n = 6 and 17 (C).
Mitochondrial respiration was also significantly elevated in the sedentary group as evidenced by the near doubling of O2 flux/ATPmax [0.0033 ± 0.0003 (n = 6) vs. 0.0017 ± 0.0002 mm O2 (mm ATP)−1 (n = 17), respectively; P < 0.004] relative to the active group. This elevation in O2 flux/ATPmax is consistent with the in vitro and in vivo effects of uncouplers on oxidative phosphorylation that increase respiration (9, 25).
Elevated UCP3 protein with mitochondrial uncoupling
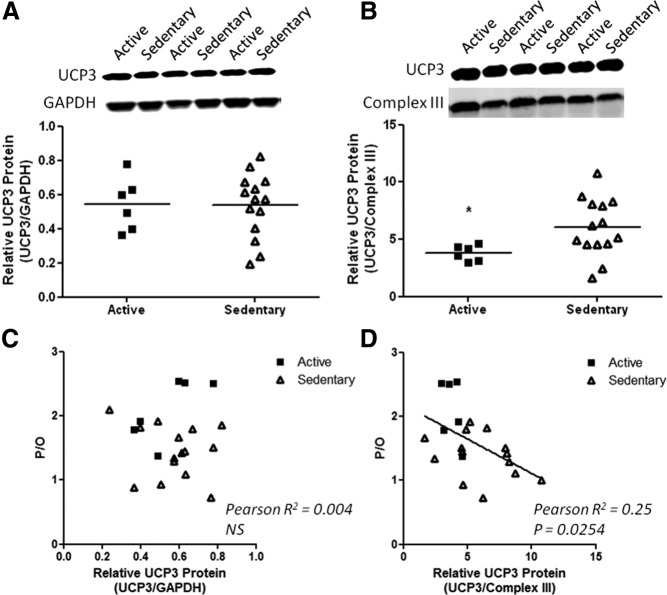
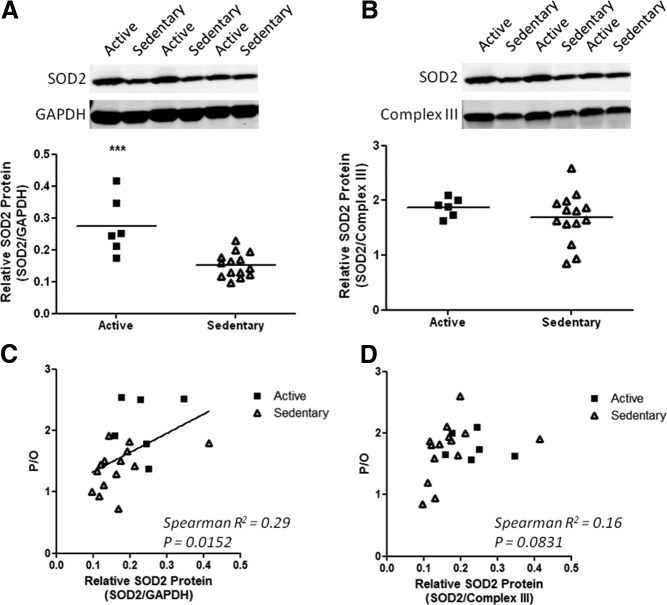
To determine whether the changes in mitochondrial metabolism were reflected at the protein level, two channels that are activated by oxidative stress to facilitate H+ movement across the inner membranes and an antioxidant enzyme were measured. UCP3 and SOD2 levels were determined on biopsy samples from individuals that had complete physiological data sets with successful measurements for six samples from active and 13 for sedentary subjects. Higher UCP3 per CIII of the electron transport chain was found in sedentary subjects compared with active subjects (Fig. 3). The UCP3 was proportional to the extent of uncoupling as shown by the negative correlation between UCP3/CIII and P/O (P = 0.025, Fig. 3D). In contrast, SOD2 per CIII protein was not significantly different, but SOD2 per GAPDH was lower in the sedentary group (Fig. 4). Thus, SOD2 content was lower in proportion to the reduction in CIII content. The total SOD2 level varied with coupling as shown by the positive correlation between SOD2 and P/O (P = 0.029, Fig. 4C). No change was found in adenine nucleotide translocase either per CIII or in total content (Supplemental Fig. 6).
Fig. 3.
UCP3 protein content per cell (A) and per CIII of the electron transport chain (B) in vastus lateralis of active (■) and sedentary (▵) subjects. *, P < 0.05. Relationship between mitochondrial coupling and total UCP3 (C) or UCP3 per CIII of the electron transport chain (D) indicates increased uncoupling protein level with mitochondrial uncoupling among the subjects of the study.
Fig. 4.
SOD2 protein content per cell (A) and per CIII of the electron transport chain (B) in vastus lateralis of active (■) and sedentary (▵) subjects. ***, P < 0.001. Relationship between mitochondrial coupling and total SOD2 (C) or SOD2 per CIII (D) among the subjects of the study is shown.
Discussion
This study investigated whether a disparity exists between muscle and mitochondrial metabolism in physically inactive subjects (7, 8). Our direct measurement of cell energy metabolism in vivo confirmed in muscle the result reported for whole-body resting metabolic rate that resting metabolism did not differ with chronic activity (7, 8). These measurements also revealed that mitochondria were significantly uncoupled in vivo in sedentary subjects, thereby also confirming the paradox of inefficient mitochondria but stable cell metabolism in inactive vs. active subjects. Reconciling these two findings was the reduced mitochondrial function (in vivo ATPmax) and content [CIII of the electron transport chain (ETC)] in sedentary muscle, which resulted in substantially greater O2 flux per CIII in muscle of these subjects. Thus, fewer mitochondria respire more in sedentary muscle to meet the same cell energy demand as found in active muscle. The reduced mitochondrial content in sedentary muscle therefore explains why mitochondrial respiration is elevated at the same muscle metabolism compared with active muscle (7, 8).
Muscle and mitochondrial capacities
The reduced CIII level in sedentary vs. active groups in Table 1 is supported by separate measures of the other complexes of the ETC involved in oxidation (Table 2). The packing of the complexes of the ETC is found to be uniform per inner mitochondrial membrane surface area, which is relatively constant per mitochondrial content in mammalian muscle (for discussion, see Ref. 26). Thus, a change in ETC content is used as a measure of reduced mitochondrial content and capacity. This lower ETC content provides the key insight into the basis of the disparity between cell and mitochondrial metabolism in the sedentary group. Reduced ETC content mirrored the lower relative level of VO2max and mitochondrial functional capacity (ATPmax) compared with the active group (Tables 1 and 2). The reduced ATPmax in parallel with the lower CIII at the muscle level resulted in no difference in ATPmax/CIII (Fig. 2) between groups. This agreement supports the notion that ETC content is proportional to the functional capacity of mitochondria in healthy individuals. Thus, the functional capacity per ETC was maintained, but the ETC content and ATPmax in the sedentary group were both approximately 60% of the active muscle level.
Muscle and mitochondrial metabolism
The lower ETC content was greater than the difference in ATP flux between the two groups (Fig. 1), resulting in a significantly higher mitochondrial ATP flux (ATP flux/ATPmax) in the inactive subjects (Fig. 2). Similarly, although muscle O2 flux was not different between the groups, the O2 flux per ATPmax was doubled in sedentary vs. active muscle. This finding confirms at the muscle level the disparity reported in resting whole-body metabolic rate vs. mitochondrial metabolism of isolated mitochondria (7, 8). Part of the explanation for this paradoxical disparity in muscle vs. mitochondrial metabolism is the lower ETC content, which required the remaining mitochondria to generate 30% more ATP flux (greater ATP flux/ATPmax, Fig. 2B) to meet the cell energy demand. The rest of the explanation is that the O2 flux/ATPmax was elevated more than that expected from ATP flux/ATPmax in sedentary relative than in active muscle. This higher than expected mitochondrial respiration reflects uncoupling of oxidative phosphorylation (low P/O), which is apparent in Fig. 1 for the sedentary subjects.
Mitochondrial coupling
Energy uncoupling resulting from the doubling of O2 flux relative to the mitochondrial capacity (O2 flux/ATPmax) in sedentary muscle agrees with results of isolated mitochondrial preparations from muscle of inactive individuals (4). This elevation of O2 flux is the classic response to uncoupling as found with activation of UCP1 in brown fat for thermogenesis (27). Higher respiration is elicited in vivo in mouse muscle treated with chemical uncoupling agents [e.g. dinitrophenol (9)] or exposed to the oxidizing agent, paraquat (25). In contrast, coupling in the active group (P/O = 2.1) was close to the theoretical limit for oxidative phosphorylation (2.3–2.5) (28) and in agreement with values from isolated fibers [P/O = 2.6 (29)] and mitochondria (30) derived from vastus lateralis muscle biopsies. Also in agreement are in vivo whole-body measures [P/O = 2.5 (31)] and direct noninvasive studies of exercising gastrocnemius muscle [P/O = 2.1 (32)] and resting first dorsal interosseous muscle [P/O = 2.7 (11)]. These results indicate that mitochondria are qualitatively different in inactive subjects based on a lower P/O and higher respiration of mitochondria (O2 flux/ATPmax) relative to active muscle.
Mechanisms of uncoupling
An additional qualitative difference in mitochondria from sedentary subjects is the elevated UCP3/CIII (Fig. 3). Transport channels present in the inner membrane (e.g. UCP3, adenine nucleotide transporter) are reported to regulate H+ movement and the ATP generation per O2 uptake (28, 33). Figure 3 shows that UCP3/CIII is significantly higher in inactive subjects and correlated with the degree of uncoupling, whereas no change was found in the mitochondrial antioxidant enzyme, SOD2, per CIII (Fig. 4). Studies in human muscle have found an association between higher UCP3 and lower exercise efficiency (7, 34) consistent with uncoupling in inactive subjects (24). However, the level of UCP3 alone may not be sufficient to cause uncoupling based on studies in which the protein level changes without evidence of alterations in mitochondrial coupling (35).
Limitations
A potential limitation of this study is that we did not reaffirm the finding that whole-body resting metabolic rate was also similar between groups as found for muscle metabolism. Linking mitochondria to muscle to whole-body respiration is an important goal for future studies.
Conclusions
Here we resolve the paradox at the muscle level between stable resting metabolic rate but elevated mitochondrial metabolism reported in sedentary subjects. The resolution lies in part in the lower mitochondrial content that requires higher mitochondrial flux to meet cell energy needs but also reflected additional respiration due to uncoupling of mitochondria in sedentary subjects. Insight into this uncoupling comes from the higher UCP3 per CIII of the ETC in muscle of inactive subjects, indicating greater membrane channel content. Thus, reduced mitochondrial capacity and content with inactivity are accompanied by an increase in mitochondrial respiration and uncoupling as well as in UCP3/CIII relative to active subjects. The end result is that mitochondria work harder to meet the same cell energy demands in sedentary vs. active subjects.
Acknowledgments
We acknowledge the expert technical assistance of Randy Neiderhofer, Randall Dean, Conrad Earnest, Stephanie Anaya, Laura Roan, Zhengyu Zhang, and our research volunteers. Author contributions include the following: K.E.C., S.B., S.R.C., and S.R.S. conceived and designed the experiments; all of the authors contributed to the collection, analysis, and interpretation of the data; and K.E.C., C.E.A., S.B., S.R.C., S.A.J., D.J.M., and S.R.S. drafted or revised the paper.
This work was supported by the Novartis Clinical Innovation Fund, Takeda Pharmaceuticals North America, National Institutes of Health Grant R01-AG030226 (to S.R.S.); National Institutes of Health Grant P30-DK072476 and P20-RR021945; and Grants RC2-AG036606 and R01-AR41928 (to K.E.C.).
Current Address for S.R.S.: Translational Research Institute for Metabolism and Diabetes, Florida Hospital and Sanford-Burnham Medical Research Institute, 301 East Princeton Street, Orlando, Florida 32804.
Current Address for C.A.: Faculty of Physical Education and Health, University of Toronto, 55 Harbord Street, WSB 2008, Toronto, Ontario, Canada M5S 2W6.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATPmax
- Mitochondrial phosphorylation capacity
- ATP/O2
- mitochondrial coupling
- CCD
- charge-coupled device
- CIII
- complex III
- ETC
- electron transport chain
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Hb
- hemoglobin
- Mb
- myoglobin
- PCr
- phosphocreatine
- P/O
- mitochondrial coupling
- SOD
- superoxide dismutase
- UCP
- uncoupling protein
- VO2max
- maximal oxygen uptake.
References
- 1. Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. 1995. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol 78:2033–2038 [DOI] [PubMed] [Google Scholar]
- 2. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. 1992. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47:B71–B76 [DOI] [PubMed] [Google Scholar]
- 3. Zoll J, Sanchez H, N′Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B. 2002. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 543:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernström M, Tonkonogi M, Sahlin K. 2004. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol 554:755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schrauwen P, Russell AP, Moonen-Kornips E, Boon N, Hesselink MK. 2005. Effect of 2 weeks of endurance training on uncoupling protein 3 content in untrained human subjects. Acta Physiol Scand 183:273–280 [DOI] [PubMed] [Google Scholar]
- 6. Schrauwen P, Hesselink M. 2003. Uncoupling protein 3 and physical activity: the role of uncoupling protein 3 in energy metabolism revisited. Proc Nutr Soc 62:635–643 [DOI] [PubMed] [Google Scholar]
- 7. Schrauwen P, Troost FJ, Xia J, Ravussin E, Saris WH. 1999. Skeletal muscle UCP2 and UCP3 expression in trained and untrained male subjects. Int J Obes Relat Metab Disord 23:966–972 [DOI] [PubMed] [Google Scholar]
- 8. Broeder CE, Burrhus KA, Svanevik LS, Wilmore JH. 1992. The effects of aerobic fitness on resting metabolic rate. Am J Clin Nutr 55:795–801 [DOI] [PubMed] [Google Scholar]
- 9. Marcinek DJ, Schenkman KA, Ciesielski WA, Conley KE. 2004. Mitochondrial coupling in vivo in mouse skeletal muscle. Am J Physiol Cell Physiol 286:C457–C463 [DOI] [PubMed] [Google Scholar]
- 10. Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE. 2008. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods 46:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. 2007. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 104:1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. 2005. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol 569:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Burk DH, Zhang Z, Gupta A, Kjems L, Smith SR. 2011. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab 96:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heineman FW, Eng J, Berkowitz BA, Balaban RS. 1990. NMR spectral analysis of kinetic data using natural lineshapes. Magn Reson Med 13:490–497 [DOI] [PubMed] [Google Scholar]
- 15. Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. 1983. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med 1:77–94 [PubMed] [Google Scholar]
- 16. Blei ML, Conley KE, Kushmerick MJ. 1993. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465:203–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. 1997. Activation of glycolysis in human muscle in vivo. Am J Physiol: Cell Physiol [Erratum (1999) 1276(1993 Pt 1991):section C] 273:C306–C315 [DOI] [PubMed] [Google Scholar]
- 18. Kemper WF, Lindstedt SL, Hartzler LK, Hicks JW, Conley KE. 2001. From the cover: shaking up glycolysis: sustained, high lactate flux during aerobic rattling. Proc Natl Acad Sci USA 98:723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE. 2003. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 553:589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conley KE, Jubrias SA, Esselman PC. 2000. Oxidative capacity and aging in human muscle. J Physiol 526:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. 1993. Relationship between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75:813–819 [DOI] [PubMed] [Google Scholar]
- 22. Paganini AT, Foley JM, Meyer RA. 1997. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol 272:C501–C510 [DOI] [PubMed] [Google Scholar]
- 23. Marcinek DJ, Ciesielski WA, Conley KE, Schenkman KA. 2003. Oxygen regulation and limitation to cellular respiration in mouse skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 285:H1900–H1908 [DOI] [PubMed] [Google Scholar]
- 24. Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. 2011. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13:149–159 [DOI] [PubMed] [Google Scholar]
- 25. Siegel MP, Kruse SE, Knowels G, Salmon A, Beyer R, Xie H, Van Remmen H, Smith SR, Marcinek DJ. 2011. Reduced coupling of oxidative phosphorylation in vivo precedes electron transport chain defects due to mild oxidative stress in mice. PLoS ONE 6:e26963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwerzmann K, Hoppeler H, Kayar SR, Weibel ER. 1989. Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci USA 86:1583–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicholls DG, Rial E. 1999. A history of the first uncoupling protein, UCP1. J Bioenerg Biomembr 31:399–406 [DOI] [PubMed] [Google Scholar]
- 28. Brand MD. 2005. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33:897–904 [DOI] [PubMed] [Google Scholar]
- 29. Kuznetsov AV, Kunz WS, Saks V, Usson Y, Mazat JP, Letellier T, Gellerich FN, Margreiter R. 2003. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal Biochem 319:296–303 [DOI] [PubMed] [Google Scholar]
- 30. Mogensen M, Bagger M, Pedersen PK, Fernström M, Sahlin K. 2006. Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J Physiol 571:669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flatt JP, Pahud P, Ravussin E, Jequier E. 1984. An estimate of the P:O ration in man. Trends Biochem Sci 9:466–468 [Google Scholar]
- 32. Cettolo V, Cautero M, Tam E, Francescato MP. 2007. Mitochondrial coupling in humans: assessment of the P/O2 ratio at the onset of calf exercise. Eur J Appl Physiol 99:593–604 [DOI] [PubMed] [Google Scholar]
- 33. Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. 2010. Mitochondrial proton and electron leaks. Essays Biochem 47:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Majerczak J, Rychlik B, Grzelak A, Grzmil P, Karasinski J, Pierzchalski P, Pulaski L, Bartosz G, Zoladz JA. 2010. Effect of 5-week moderate intensity endurance training on the oxidative stress, muscle specific uncoupling protein (UCP3) and superoxide dismutase (SOD2) contents in vastus lateralis of young, healthy men. J Physiol Pharmacol 61:743–751 [PubMed] [Google Scholar]
- 35. Hesselink MK, Greenhaff PL, Constantin-Teodosiu D, Hultman E, Saris WH, Nieuwlaat R, Schaart G, Kornips E, Schrauwen P. 2003. Increased uncoupling protein 3 content does not affect mitochondrial function in human skeletal muscle in vivo. J Clin Invest 111:479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]