Abstract
Purpose:
Adrenocortical carcinoma (ACC) is a hallmark cancer in families with Li Fraumeni syndrome (LFS) caused by mutations in the TP53 gene. The prevalence of germline TP53 mutations in children diagnosed with ACC ranges from 50–97%. Although existing criteria advocate for TP53 testing in all patients with ACC regardless of age at diagnosis, the overall prevalence of germline mutations in patients diagnosed with ACC has not been well studied.
Patients and Methods:
A total of 114 patients with confirmed ACC evaluated in the University of Michigan Endocrine Oncology Clinic were prospectively offered genetic counseling and TP53 genetic testing, regardless of age at diagnosis or family history. Ninety-four of the 114 patients met with a genetic counselor (82.5%), with 53 of 94 (56.4%) completing TP53 testing; 9.6% (nine of 94) declined testing. The remainder (32 of 94; 34%) expressed interest in testing but did not pursue it for various reasons.
Results:
Four of 53 patients in this prospective, unselected series were found to have a TP53 mutation (7.5%). The prevalence of mutations in those diagnosed over age 18 was 5.8% (three of 52). There were insufficient data to estimate the prevalence in those diagnosed under age 18. None of these patients met clinical diagnostic criteria for classic LFS. Three of the families met criteria for Li Fraumeni-like syndrome; one patient met no existing clinical criteria for LFS or Li Fraumeni-like syndrome. Three of the four patients with mutations were diagnosed with ACC after age 45.
Conclusions:
Genetic counseling and germline testing for TP53 should be offered to all patients with ACC. Restriction on age at diagnosis or strength of the family history would fail to identify mutation carriers.
Adrenocortical carcinoma (ACC) is a rare, aggressive tumor that, when diagnosed in children, prompts a genetic evaluation for Li Fraumeni syndrome (LFS) due to germline TP53 mutations (1). LFS is an autosomal dominant cancer predisposition syndrome with elevated risks of premenopausal breast cancer, sarcoma, lymphoma, and ACC (2, 3). Strict criteria exist for the clinical diagnosis of classic LFS (2) and for the clinical diagnosis of the less stringent Li Fraumeni-like syndrome (4, 5). The Chompret genetic testing criteria (6, 7) were drafted to guide clinicians on the appropriate patients in whom to offer a genetic evaluation for LFS. The criteria advocate for TP53 genetic testing in all individuals diagnosed with ACC, regardless of age at diagnosis or strength of the family history. However, this recommendation was based primarily on studies of children with ACC and has not been validated by an independent cohort that includes adult ACC patients.
The prevalence of LFS among children with ACC has been reviewed in several series and is estimated to be 50–80% dependent on the study population (8, 9). A TP53 mutation, p.R337H, with a population frequency of 0.3% in Brazil was found in up to 97% of Brazilian pediatric ACC patients (10). In a U.S. series, Gonzalez et al. (11) reviewed clinical histories of patients referred to a CLIA (Clinical Laboratory Improvement Amendments of 1988) -certified lab for TP53 gene sequencing. Of the 21 patients with ACC, 14 (67%) had identifiable TP53 mutations. Twelve of 14 mutation carriers (80%) were diagnosed under age 18 (range, 0.5–38 yr). Based on the clinical information provided by the referral center, two TP53 mutation carriers met classic LFS criteria, and four patients met Eeles Li Fraumeni-like criteria. Recently, Herrmann et al. (12) sequenced TP53 in a subset of the adult patients enrolled in the German ACC registry and reported a TP53 mutation prevalence of 3.9%. This study was the first to specifically analyze adult-onset ACC; however, their analysis was restricted to an undefined subset of all patients enrolled in the registry (12).
The University of Michigan Endocrine Oncology Program is a specialized center with expertise in the diagnosis, treatment, and management of ACC, which serves as a referral center for ACC patients nationally and internationally. This multidisciplinary clinic incorporates clinicians with expertise in endocrinology, endocrine surgery, medical oncology, radiation oncology, pathology, and genetics. In 2009, we began routinely offering genetic counseling and TP53 testing to clinic patients with the goal of better defining genetic risk in this predominantly adult population with ACC.
Patients and Methods
Patients presenting to the University of Michigan Endocrine Oncology Program between December 1, 2009, and October 31, 2011, with a diagnosis of ACC were included in this analysis. Patients were offered a genetics consultation as part of their visit and met with a genetic counselor who obtained a four-generation cancer genetics pedigree. Based on Chompret genetic testing criteria (6), all patients were offered germline TP53 genetic testing with sequencing and large rearrangement analysis with multiplex ligation probe analysis or gene dosage analysis through a CLIA-certified clinical laboratory.
Families were classified based on published clinical diagnostic criteria. “Typical LFS cancers” and “LFS-related malignancies” for this study include all cancers cited by Tinat et al. (6) as belonging to the LFS tumor spectrum: soft tissue sarcoma, osteosarcoma, brain tumor, breast cancer, ACC, leukemia, lung cancer, and choroid plexus tumor. Criteria are: 1) classic LFS defined as proband with a sarcoma diagnosed before age 45 yr, a first-degree relative with any cancer before age 45 yr, and a first- or second-degree relative with any cancer before age 45 yr or a sarcoma at any age (2); 2) Birch Li Fraumeni-like syndrome, defined as a proband with any childhood cancer or sarcoma, brain tumor, or ACC diagnosed before age 45 yr, and a first- or second-degree relative with a typical LFS cancer at any age, and a first- or second-degree relative with any cancer before age 60 yr (4, 5); 3) Eeles Li Fraumeni-like syndrome defined as two first- or second-degree relatives with LFS-related malignancies at any age (4, 5); or 4) no clinical criteria met. Each family was included in only one of the four diagnostic categories corresponding with the most stringent criteria met.
Permission for research was obtained from the Institutional Review Board at the University of Michigan, Ann Arbor, Michigan (HUM00043430).
Results
A total of 114 patients with ACC were evaluated in the University of Michigan Endocrine Oncology Program during the study period. Forty-two patients were male (36.8%), and 72 were female (63.2%). The majority of patients were Caucasian (87.7%), with a small number being African-American (7.9%) or Asian (2.6%). Average age at ACC diagnosis was 45.5 yr, with a range of 3 to 82 yr at diagnosis. Ninety-eight percent of patients were diagnosed after age 18 (Table 1).
Table 1.
Characteristics of patients presenting to University of Michigan Endocrine Oncology Program
| No. evaluated in EndoOnc program (% of total population) | No. counseled (% of evaluated) | No. tested for germline TP53 mutation (% of counseled) | No. not tested for germline TP53 mutation (% of counseled) | No. positive for germline TP53 mutation (% of tested) | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 72 (63.2) | 61 (84.7) | 33 (54.1) | 28 (45.9) | 3 (9.1) |
| Male | 42 (36.8) | 33 (78.6) | 20 (60.6) | 13 (39.4) | 1 (5.0) |
| Race | |||||
| Caucasian | 100 (87.7) | 82 (82.0) | 48 (58.5) | 34 (41.5) | 3 (6.3) |
| African-American | 9 (7.9) | 8 (88.9) | 4 (50.0) | 4 (50.0) | 1 (25.0) |
| Asian | 3 (2.6) | 2 (66.7) | 0 | 2 (100) | 0 |
| Other | 2 (1.8) | 2 (100) | 1 (50.0) | 1 (50.0) | 0 |
| Total | 114 | 94 (82.5) | 53 (56.4) | 41 (43.6) | 4 (7.5) |
| Mean age (yr) at diagnosis (range) | 45.5 (3–82) | 44.7 (3–82) | 45.5 (3–82) | 43.7 (17–76) | 42.5 (3–68) |
| Median age (yr) at diagnosis | 46.5 | 46.0 | 47.0 | 43.0 | 49.5 |
| Age at diagnosis (yr) | |||||
| ≤17 | 2 (1.8) | 2 (100) | 1 (50.0) | 1 (50.0) | 1 (100) |
| 18–39 | 35 (30.7) | 28 (80.0) | 13 (46.4) | 15 (53.6) | 0 |
| ≥40 | 77 (67.5) | 64 (83.1) | 39 (60.9) | 25 (39.1) | 3 (7.7) |
Of the 114 patients evaluated in the University of Michigan Endocrine Oncology Program, 94 (82.5%) opted to meet with a genetic counselor. After completion of a four-generation cancer genetics pedigree, 58 families met criteria for clinical diagnoses of Li Fraumeni-like syndrome, 12 met Birch criteria (12.8%), and 46 met Eeles criteria (48.9%). No patients met classic LFS criteria (Table 2).
Table 2.
Most stringent LFS clinical diagnostic criteria met by the 94 families who received genetic counseling
| Counseled | Tested for germline TP53 mutation | Not tested for germline TP53 mutation | Positive for germline TP53 mutation | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| N | 94 | 53 | 41 | 4 | ||
| Classic LFS clinical diagnostic criteria | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 100 |
| Birch LFL clinical diagnostic criteria | 12 (12.8) | 8 (15.1) | 4 (9.8) | 0 (0) | 0 | 83.7 |
| Eeles LFL clinical diagnostic criteria | 46 (48.9) | 28 (52.8) | 18 (43.9) | 3 (75.0) | 75 | 49 |
| No clinical diagnostic criteria met | 36 (38.3) | 17 (32.1) | 19 (46.3) | 1 (25.0) | — | — |
| Chompret genetic testing criteria | 94 (100) | 53 (100) | 41 (100) | 4 (100) | 100 | 0 |
Data are expressed as number (percentage). All 94 independent probands met the Chompret genetic testing criteria. LFL, Li Fraumeni-like syndrome.
Fifty-three (56.4%) patients pursued TP53 genetic testing, and 9.6% (nine of 94) declined the testing. Thirty-two patients (34%) expressed interest, but did not ultimately pursue testing. Reasons for not pursuing genetic testing included the patients' desire to consider the option of testing, including discussing with family members (34.4%; 11 of 32), wanting to verify insurance coverage, or wanting to pursue genetic testing within their local insurance network (56.3%; 18 of 32). Three patients were denied insurance coverage for TP53 genetic testing (9.4%; three of 32). Average age at diagnosis of the individuals who did (45.5 yr) and did not pursue genetic testing (43.7 yr) did not differ significantly (P = 0.55; t test). Four individuals were positive for a TP53 mutation (7.5%; four of 53).
Patient A: positive for TP53 c.814G>A;p.V272M
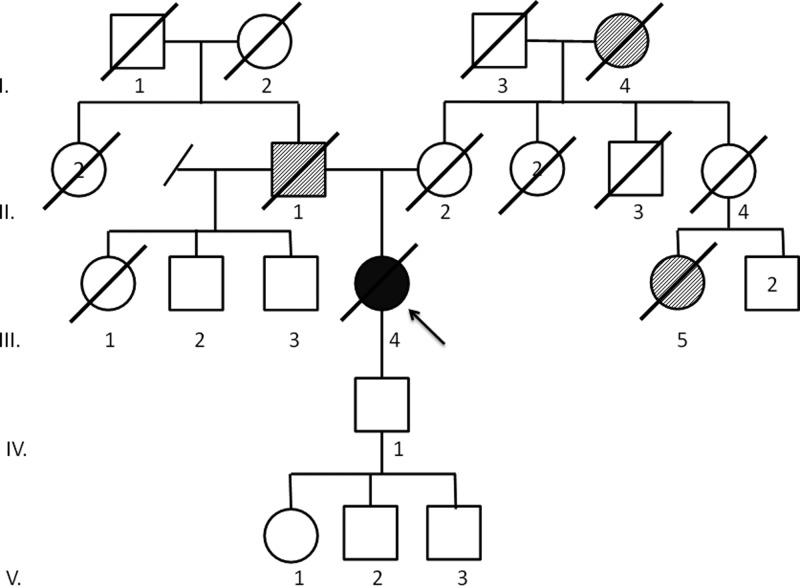
This is a missense mutation that is in a highly conserved codon. This mutation has been previously reported in a family with LFS and as a somatic mutation (13). The patient was a Caucasian female diagnosed with stage III ACC at age 68, presenting as Cushing's syndrome. Biochemical cortisol and dehydroepiandrosterone sulfate excess was confirmed. The patient underwent surgery for a 7.2-cm, 161-g high-grade ACC, with lung metastases being diagnosed 2.5 months after surgery. After treatment with mitotane and six cycles of etoposide, doxorubicin, and cisplatin, the patient experienced disease progression and ultimately died 20 months after initial diagnosis. Her family history was notable for a father who died at age 86 of a brain tumor, a maternal first cousin who died at age 69 of breast cancer, and a maternal grandmother who died of lung cancer at an advanced age (Fig. 1).
Fig. 1.
Family history of patient A. Proband (III.4) diagnosed with ACC at age 68 yr and died at 70. Father (II.1) died at 86 of a brain tumor. Mother (II.2) died at 84. Maternal first cousin (III.5) died at 69 of breast cancer. Maternal grandmother (I.4) died of lung cancer at an advanced age. Paternal half-brothers (III.2, III.3) are living at 79 and 83. Son (IV.1) is living at 50 and grandchildren (V.1, V.2, V.3) are living at 23, 25, and 26.
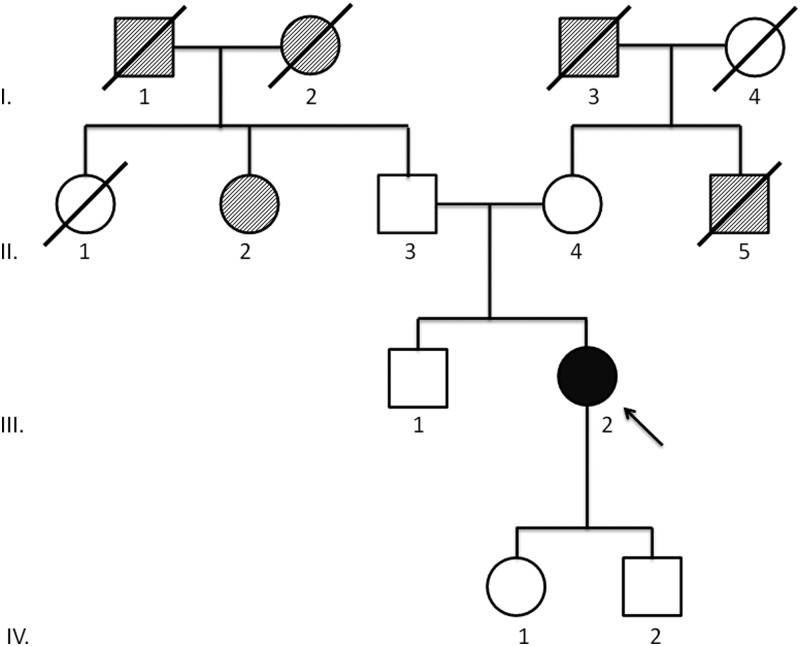
Patient B: positive for TP53 c.542G>A;p.R181H
This missense mutation is in a fairly well-conserved codon. It has been reported in a family with LFS, and mutations at the same codon have been reported in LFS (13). This patient is a Caucasian female initially diagnosed with bilateral adrenal tumors at age 52. Pathological specimen revealed a high-grade 7-cm left-sided ACC and a contralateral pheochromocytoma. The patient did not show any clinical symptoms of hormone secretion by the ACC. Eight months after surgery, the patient was diagnosed with liver metastases and underwent initial treatment with eight cycles of mitotane, etoposide, doxorubicin, and cisplatin. Due to further progression, she was included in a double-blind trial using a targeted tyrosine kinase inhibitor. Three years later, she was diagnosed with glioblastoma multiforme and underwent surgery, followed by treatment with irinotecan, avastin, and carboplatin. She is currently alive with stage IV ACC 4 yr after initial diagnosis. Her medical history is further complicated by a clinical diagnosis of neurofibromatosis type 1 (NF1) diagnosed at age 11, characterized by a history of multiple cutaneous neurofibromas, Lisch nodules, and multiple café au lait macules. After the genetic consultation, this diagnosis was confirmed with identification of a novel missense mutation (c.6881T>G;p.L2294R) in the NF1 gene. Her family history is notable for late-onset pancreatic and colon cancer on the maternal side and a paternal family history of late-onset renal, breast, and colon cancers. No other family members are known to have clinical diagnoses of NF1 (Fig. 2).
Fig. 2.
Family history of patient B. Proband (III.2) was diagnosed with ACC at age 52 yr, contralateral pheochromocytoma at 52, and glioblastoma multiforme at 55. Parents (II.3, II.4) are living at 86 and 84. Paternal aunt (II.2) was diagnosed with a renal cancer. Paternal grandparents (I.1, I.2) were diagnosed with late-onset colon and breast cancer, respectively. Maternal uncle (II.5) died at 75 of pancreas cancer. Maternal grandfather (I.3) died at 81 of colon cancer. Brother (III.1) is living at 59. Daughter (IV.1) and son (IV.2) are living at 29 and 24, respectively.
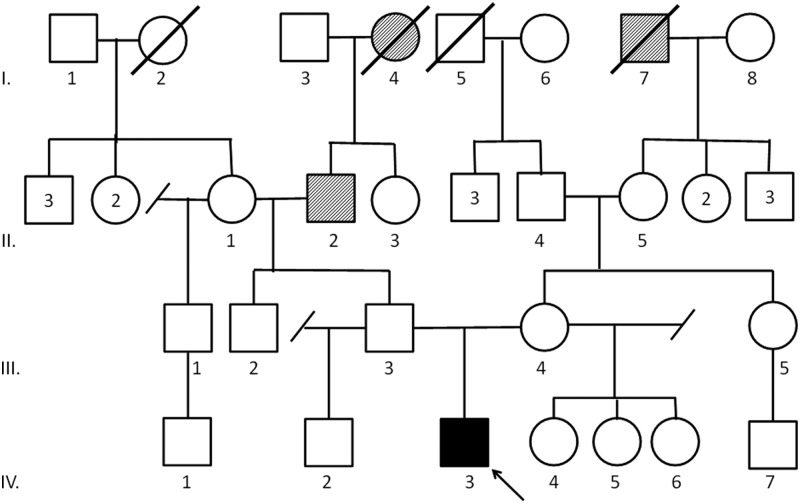
Patient C: positive for TP53 c.473G>A;p.R158H
This missense mutation is in a completely conserved codon and has been previously reported in eight families with LFS (13). This Caucasian male was diagnosed with ACC at age 3. The patient initially presented with precocious puberty due to dehydroepiandrosterone sulfate and testosterone excess. He underwent surgery for a 2-cm, 9-g low-grade ACC. He is currently alive without evidence of disease 17 months after initial diagnosis. His medical history is otherwise unremarkable. His family history is notable for a paternal family history of prostate and renal cancer. The patient's mother, maternal half-sisters, and paternal half-brother tested negative for the identified TP53 mutation (Fig. 3).
Fig. 3.
Family history of patient C. Proband (IV.3) was diagnosed with ACC at age 3 yr. Paternal grandfather (II.2) was diagnosed with prostate cancer at 57. Paternal great grandmother (I.4) was diagnosed with renal cancer at 69. Maternal great grandfather (I.7) died of lung cancer. Mother (III.4) at age 26 has tested negative for the TP53 mutation. Father (III.3) at age 27 has declined genetic testing. Paternal half-brother (IV.2), age 2, and maternal half-sisters (IV.4-IV.6), ages 9, 2, and 1, have tested negative for the TP53 mutation.
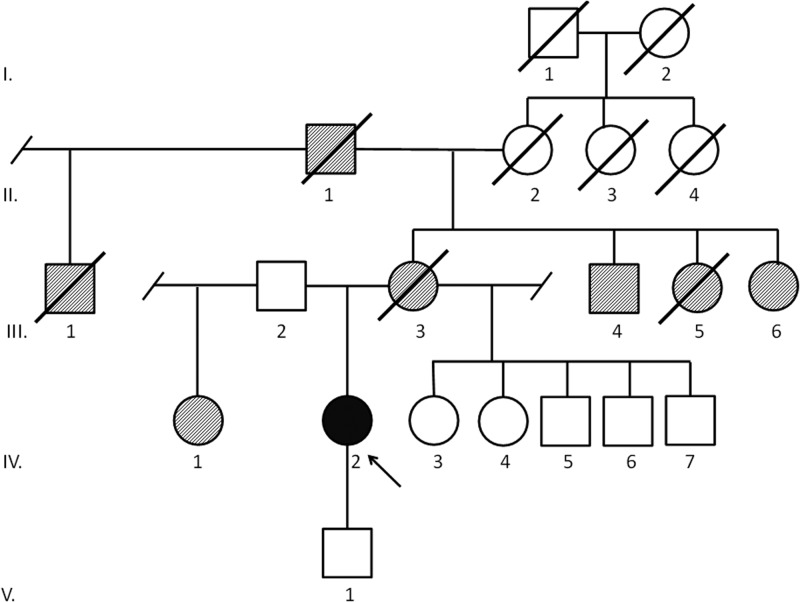
Patient D: positive for a novel variant of uncertain significance in TP53, c.G>T 207bp upstream from exon 1
This African-American female was diagnosed with ACC at age 47. The patient initially presented with symptoms compatible with Cushing syndrome. However, an initial biochemical workup was not performed. She underwent initial surgery and had a locoregional recurrence as well as liver metastases 2 yr later. Pathological specimen of repeat surgery revealed a low-grade ACC. After resection, the patient received 46-Gy adjuvant radiation to the tumor bed. Three years and 8 months later, the patient had a repeat retroperitoneal recurrence inferior to the kidney, treated with surgery and radiation therapy (50 Gy). She currently is alive without evidence of disease 8 yr and 7 months after initial diagnosis. The medical history is otherwise remarkable for a colon adenoma. Her family history is significant for ovarian cancer diagnosed in her mother at age 55, a “bone” cancer diagnosed in a maternal aunt, endometrial cancer diagnosed in a second maternal aunt, and lung cancer diagnosed in a maternal uncle and maternal grandfather (Fig. 4). Immunohistochemistry analysis in the ACC tumor noted absent TP53 expression.
Fig. 4.
Family history of patient D. Proband (IV.2) diagnosed with ACC at age 48 yr. Mother (III.3) was diagnosed with ovarian cancer at 55. Maternal uncle (III.4) was diagnosed with lung cancer at 70, maternal aunt (III.5) died at 65 of “bone” cancer, maternal aunt (III.6) was diagnosed with endometrial cancer at 53. Maternal grandfather (II.1) was diagnosed with lung cancer at 64, maternal half uncle (III.1) died at 75 of prostate cancer. Father's (III.2) health history knowledge is limited. Paternal half-sister (IV.1) diagnosed with throat cancer at 67. Maternal half siblings (IV.3–IV.7) are living at 49, 46, 47, 44, and 43.
None of the TP53-positive patients met clinical diagnostic criteria for classic LFS. Three patients (A, B, and D) met Eeles Li Fraumeni-like clinical diagnostic criteria (Table 2). The average age at diagnosis did not significantly differ between those positive for germline TP53 mutations (42.5 yr) and those found not to have a mutation (45.6 yr) (P = 0.68; t test).
Discussion
ACC is a rare aggressive tumor with a Surveillance Epidemiology and End Results (SEER) estimated prevalence of 0.72 cases per 1,000,000 individuals (14). ACC has been described in patients with inherited cancer predisposition syndromes, most commonly LFS, a rare inherited condition caused by germline mutations in the TP53 gene (15). Individuals with LFS are at highly elevated risk of cancer, with a lifetime risk of almost 100% in women and 73% in men, the gender difference thought to be accounted for by the high incidence of female breast cancer in LFS (16).
Additionally, one of the hallmark features of LFS is the diagnosis of pediatric cancers (17). It is estimated that 50–80% of children diagnosed with ACC have a germline TP53 mutation (8). In certain pediatric ACC populations, the risk of TP53 mutations is higher. Ribeiro et al. (10) sequenced the coding region of TP53 in 27 patients with ACC and nine patients with adrenal cortical adenomas in Brazil. The patients average age at diagnosis was 3 yr (range, 4 months to 13.5 yr). Thirty-five of these 36 patients (97%) had an identical TP53 mutation (p.R337H). Latronico et al. (18) examined the prevalence of this p.R337H mutation in Brazilian patients with benign and malignant adrenal tumors and identified the mutation in 14 of 18 children (77.7%) and five of 37 adults (13.5%). These results strongly implicate TP53 p.R337H as the leading cause of ACC in the population in Brazil. However, beyond the p.R337H mutation, the link between TP53 mutations and adult-onset ACC has not been as well studied.
The Chompret criteria (6, 7) advocate for TP53 genetic testing for all patients diagnosed with ACC regardless of age at diagnosis or family history. Despite this recommendation, the overall prevalence of LFS in patients with ACC, including patients with adult-onset ACC, is not well described. A recent study sequenced TP53 in the germline of 103 of the 400 (25.8%) patients with ACC enrolled in the German ACC registry (12). Their analysis specifically focused on an undefined subset of patients with adult-onset ACC and identified four patients with germline TP53 mutations (3.9%). The median age at diagnosis in this group was 50 yr, with a range of 18–78 yr. Three of the four positive patients were diagnosed with ACC under age 40. When analysis was restricted to the 23 ACC patients with an age at diagnosis under 40 yr, the incidence of germline TP53 mutations in this cohort was 13% (three of 23).
Our study is the first to prospectively examine an unselected series of patients presenting to a specialized center for ACC with a large national and international referral basis. Patients were offered TP53 genetic testing regardless of age at ACC diagnosis or strength of the family history. The prevalence of TP53 mutations in this population was 7.4% (four of 53). The average age at diagnosis was 44.7 yr in the entire study cohort and 42.5 yr in those positive for TP53 mutations. When pediatric ACC cases were excluded (n = 1), the prevalence of TP53 mutations in the adult population was 5.8% (three of 52). Interestingly, none of these families met classic LFS clinical diagnostic criteria. Three of the four patients met Eeles Li Fraumeni-like syndrome clinical diagnostic criteria. Three of four patients were diagnosed at age 47 or later, above the suggested 40-yr-old age restriction proposed by Herrmann et al. (12) and above the upper age limit for cancer diagnosis in a proband based on the classic LFS diagnostic criteria (2, 12).
The Chompret criteria have previously been shown to have an “acceptable” sensitivity in identifying patients with LFS. In 105 families identified in The Netherlands who met Chompret criteria, 22 (21%) had TP53 mutations (sensitivity, 92%; specificity, 47%) (19). A French series identified TP53 mutations in 67 of 232 families meeting Chompret criteria (sensitivity, 82%; specificity, 58%) (20). A series from the United States identified TP53 mutations in 69 of 195 families who met these criteria (sensitivity, 92%; specificity, 53%) (11). In our prospective series, none of the eight patients meeting Birch criteria for Li Fraumeni-like syndrome had an identifiable TP53 mutation (sensitivity, 0%; specificity, 83.7%). Three of the 28 patients meeting Eeles Li Fraumeni-like criteria had an identifiable TP53 mutation (sensitivity, 75%; specificity, 49%). The limitation of using family history alone to guide TP53 genetic testing is the high de novo rate of germline mutations that has been described as high as 24% (16). Using the Chompret genetic testing criteria, with no age restrictions for diagnosis of ACC, was the best tool for identifying patients with TP53 mutations. Herrmann et al. (12) proposed modifying the Chompret criteria to restrict age at ACC diagnosis to under age 40 yr. Using this age restriction would have failed to identify three of the four mutation carriers in our cohort, arguing against using age restrictions to limit TP53 testing in patients with ACC. When focusing only on those patients diagnosed with ACC at an age greater than 40, three of 39 patients tested positive for a mutation (7.7%).
With advances in genetic technology, the ability to recognize individuals with underlying inherited cancer predisposition syndromes has increased. As genetic testing becomes more fully integrated with healthcare, we are recognizing that few families meet the strict clinical diagnostic criteria originally developed to define the cancer syndrome of interest. For example, a proportion of families with Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome), an inherited colorectal and endometrial cancer predisposition syndrome, fail to meet the strict clinical diagnostic criteria known as the Amsterdam I/II criteria (21). It is suggested that all patients with colorectal or endometrial cancer be screened for tumor features suggestive of Lynch syndrome-associated tumors, regardless of their family history or age at diagnosis (21, 22). Similarly, it is recommended that women with estrogen receptor-negative, progesterone receptor-negative, and HER2/neu-negative breast cancers (triple negative breast cancer) be screened for mutations in the BRCA1 and BRCA2 genes responsible for hereditary breast and ovarian cancer syndrome, regardless of the strength of the family history (23). As genetic technology continues to improve, we are less restricted by family history alone to guide genetic risk assessment and can use tumor pathology or tumor features. The same proves true with LFS. Given the high de novo rate and the variable expressivity and penetrance of TP53 mutations, families with LFS can be overlooked when using only the strength of the family history in the genetic risk assessment. The presence of a deleterious mutation in a cancer predisposition gene allows us to rely on genotype rather than strictly family cancer phenotype in making the diagnosis of an inherited cancer predisposition syndrome. Using the diagnosis of a rare LFS-associated cancer allowed for the diagnosis within these families.
Increasing the uptake of genetic testing will identify more variants of uncertain significance (VUS), especially in poorly studied ethnic groups in which the spectrum of germline mutations is less well defined. Determining whether a VUS is a deleterious mutation or a benign polymorphism requires many lines of evidence, including epidemiological, genetic, and molecular confirmation. This process may span years to decades. Indeed, definitive evidence may never occur in a timeframe to impact medical management for the patient of interest. Despite this limitation, these VUS cannot be ignored. Molecular information must be considered in conjunction with the family history to determine appropriate clinical recommendations for the patient and their family. This is the case for patient D, where a novel VUS in TP53 was identified. Given the diagnosis of ACC and the positive family history of cancer, this patient meets the Eeles clinical diagnostic criteria for a diagnosis of Li Fraumeni-like syndrome and is being followed with a working diagnosis of LFS.
This study substantiates the recommendation to test all persons diagnosed with ACC for TP53 mutations regardless of age at diagnosis or strength of the family cancer history. Relying solely on the Chompret criteria, we were able to demonstrate that the prevalence of TP53 mutations in this diverse, prospective cohort, 7.5%, was higher than the 3.9% prevalence in a selected cohort of patients enrolled in the German ACC registry, although not statistically different (P = 0.57) (12). This remains consistent even when restricting the age at diagnosis to greater than age 18 (three of 52; 5.8%; P = 0.70) (Table 1). Given the primarily adult population, there were insufficient data to estimate the prevalence in those patients diagnosed under age 18 (Table 1).
The diagnostic and prognostic value of surveillance screening for tumor development in patients with known mutations in TP53 is supported by recent data from Villani et al. (24) who screened 18 of 33 LFS-positive patients with a specific annual screening protocol and identified 10 tumors in seven asymptomatic patients, suggesting a benefit in screening individuals with LFS. Masciari et al. (25) screened 15 patients with TP53 mutations or obligate carrier status and identified cancer in three asymptomatic individuals using F18-fluorodeoxyglucose-positron emission tomography/computed tomography scans. Although demonstrating a potential screening benefit, there are concerns about using F18-fluorodeoxyglucose-positron emission tomography/computed tomography scans as a screening modality for individuals with very elevated risks of cancers. The National Comprehensive Cancer Network has also developed a guideline for screening in patients with LFS (26). Continued collaborative work is necessary to develop the appropriate screening regimen for individuals with LFS.
The identification of an inherited risk of genetic TP53 mutations in 7.5% of patients with ACC suggests that incorporating genetic counseling and testing into the treatment algorithm of all ACC patients can help guide medical management of these patients and identify at-risk family members. This should be explored further and in conjunction with the development and evolution of LFS cancer-screening protocols.
Acknowledgments
The authors thank Leon Raskin, Ph.D. (Vanderbilt University Department of Medicine) for his contribution to the molecular analysis of the TP53 variant.
This work was supported by National Institutes of Health/National Cancer Institute Grants P30 CA014089 (to S.B.G.) and P30 CA046592 (to S.B.G.) and National Institutes of Health Grant T32-DK007245 (to T.E.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ACC
- Adrenocortical carcinoma
- LFS
- Li Fraumeni syndrome
- NF1
- neurofibromatosis type 1
- VUS
- variant(s) of uncertain significance.
References
- 1. Fraumeni JF, Jr, Miller RW. 1967. Adrenocortical neoplasms with hemihypertrophy, brain tumors, and other disorders. J Pediatr 70:129–138 [DOI] [PubMed] [Google Scholar]
- 2. Li FP, Fraumeni JF, Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, Miller RW. 1988. A cancer family syndrome in twenty-four kindreds. Cancer Res 48:5358–5362 [PubMed] [Google Scholar]
- 3. Nichols KE, Malkin D, Garber JE, Fraumeni JF, Jr, Li FP. 2001. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev 10:83–87 [PubMed] [Google Scholar]
- 4. Birch JM, Hartley AL, Tricker KJ, Prosser J, Condie A, Kelsey AM, Harris M, Jones PH, Binchy A, Crowther D. 1994. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 54:1298–1304 [PubMed] [Google Scholar]
- 5. Eeles RA. 1995. Germline mutations in the TP53 gene. Cancer Surveys 25:101–124 [PubMed] [Google Scholar]
- 6. Tinat J, Bougeard G, Baert-Desurmont S, Vasseur S, Martin C, Bouvignies E, Caron O, Bressac-de Paillerets B, Berthet P, Dugast C, Bonaiti-Pellie C, Stoppa-Lyonnet D, Frebourg T. 2009. 2009 Version of the Chompret criteria for Li Fraumeni syndrome. J Clin Oncol 27:e108–e109; author reply e110 [DOI] [PubMed] [Google Scholar]
- 7. Chompret A, Abel A, Stoppa-Lyonnet D, Brugiéres L, Pagés S, Feunteun J, Bonaïti-Pellié C. 2001. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet 38:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varley JM, McGown G, Thorncroft M, James LA, Margison GP, Forster G, Evans DG, Harris M, Kelsey AM, Birch JM. 1999. Are there low-penetrance TP53 alleles? Evidence from childhood adrenocortical tumors. Am J Hum Genet 65:995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varley JM. 2003. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 21:313–320 [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro RC, Sandrini F, Figueiredo B, Zambetti GP, Michalkiewicz E, Lafferty AR, DeLacerda L, Rabin M, Cadwell C, Sampaio G, Cat I, Stratakis CA, Sandrini R. 2001. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci USA 98:9330–9335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, Han JH, Lowstuter K, Longmate J, Sommer SS, Weitzel JN. 2009. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 27:1250–1256 [DOI] [PubMed] [Google Scholar]
- 12. Herrmann LJ, Heinze B, Fassnacht M, Willenberg HS, Quinkler M, Reisch N, Zink M, Allolio B, Hahner S. 2012. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 97:E476–E485 [DOI] [PubMed] [Google Scholar]
- 13. Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. 2002. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat 19:607–614 [DOI] [PubMed] [Google Scholar]
- 14. Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. 2006. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg 30:872–878 [DOI] [PubMed] [Google Scholar]
- 15. Varley JM, Evans DG, Birch JM. 1997. Li-Fraumeni syndrome—a molecular and clinical review. Br J Cancer 76:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chompret A, Brugières L, Ronsin M, Gardes M, Dessarps-Freichey F, Abel A, Hua D, Ligot L, Dondon MG, Bressac-de Paillerets B, Frébourg T, Lemerle J, Bonaïti-Pellié C, Feunteun J. 2000. P53 Germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 82:1932–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lustbader ED, Williams WR, Bondy ML, Strom S, Strong LC. 1992. Segregation analysis of cancer in families of childhood soft-tissue-sarcoma patients. Am J Hum Genet 51:344–356 [PMC free article] [PubMed] [Google Scholar]
- 18. Latronico AC, Pinto EM, Domenice S, Fragoso MC, Martin RM, Zerbini MC, Lucon AM, Mendonca BB. 2001. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J Clin Endocrinol Metab 86:4970–4973 [DOI] [PubMed] [Google Scholar]
- 19. Ruijs MW, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, Kluijt I, Sijmons RH, Aalfs CM, Wagner A, Ausems MG, Hoogerbrugge N, van Asperen CJ, Gomez Garcia EB, Meijers-Heijboer H, Ten Kate LP, Menko FH, van't Veer LJ. 2010. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet 47:421–428 [DOI] [PubMed] [Google Scholar]
- 20. Bougeard G, Sesboüé R, Baert-Desurmont S, Vasseur S, Martin C, Tinat J, Brugières L, Chompret A, de Paillerets BB, Stoppa-Lyonnet D, Bonaïti-Pellié C, Frébourg T. 2008. Molecular basis of the Li-Fraumeni syndrome: an update from the French LFS families. J Med Genet 45:535–538 [DOI] [PubMed] [Google Scholar]
- 21. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, Lockman J, LaJeunesse J, Comeras I, de la Chapelle A. 2008. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 26:5783–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, La Jeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, Penzone P, Lombardi J, Dunn P, Cohn DE, Copeland L, Eaton L, Fowler J, Lewandowski G, Vaccarello L, Bell J, Reid G, de la Chapelle A. 2006. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66:7810–7817 [DOI] [PubMed] [Google Scholar]
- 23. Hartman AR, Kaldate RR, Sailer LM, Painter L, Grier CE, Endsley RR, Griffin M, Hamilton SA, Frye CA, Silberman MA, Wenstrup RJ, Sandbach JF. 2012. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer 118:2787–2795 [DOI] [PubMed] [Google Scholar]
- 24. Villani A, Tabori U, Schiffman J, Shlien A, Beyene J, Druker H, Novokmet A, Finlay J, Malkin D. 2011. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. Lancet Oncol 12:559–567 [DOI] [PubMed] [Google Scholar]
- 25. Masciari S, Van den Abbeele AD, Diller LR, Rastarhuyeva I, Yap J, Schneider K, Digianni L, Li FP, Fraumeni JF, Jr, Syngal S, Garber JE. 2008. F18-fluorodeoxyglucose-positron emission tomography/computed tomography screening in Li-Fraumeni syndrome. JAMA 299:1315–1319 [DOI] [PubMed] [Google Scholar]
- 26. 2012. NCCN guidelines. Version 1.2012: Li Fraumeni syndrome. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf