Abstract
Context:
Most studies examining associations between circulating vitamin D and disease are based on a single measure of vitamin D, which may not reflect levels over time, particularly because vitamin D concentrations vary by season. Few studies evaluated how well multiple 25-hydroxyvitamin D [25(OH)D] measures track within the same individual over time.
Objective:
This study examined variability and reproducibility of vitamin D by evaluating repeat measurements of plasma 25(OH)D concentrations while accounting for determinants of circulating concentrations including dietary supplement use and latitude of residence from a population of U.S. radiologic technologists.
Design and Participants:
We analyzed circulating 25(OH)D in blood samples taken from 538 men and women from a prospective, nationwide study at two time points within a 1-yr period, most measured in different seasons. Inter- and intra-individual variability, reliability coefficients, and measurement error were examined.
Results:
The spearman rank correlation between two measurements of 25(OH)D concentrations was moderate (r = 0.75, P < 0.001) and did not vary significantly by participant characteristics including age, race, or latitude. The intraclass correlation coefficient was 0.72 (95% confidence interval = 0.68–0.76). The deattenuation factor of plasma 25(OH)D levels was 1.39, suggesting that a single measure of vitamin D on a continuous scale in regression analyses may result in attenuated relationships of about 40%.
Conclusion:
Our results suggest that a single blood sample obtained in spring or fall provides a reasonable average for 25(OH)D over a 1-yr period, but additional studies are needed to estimate variability and agreement in plasma 25(OH)D measurements over longer intervals and younger populations.
Vitamin D is essential in the regulation of calcium homeostasis and bone mineralization. Vitamin D insufficiency may also contribute to the risk of several chronic diseases including diabetes (1), cancer (2–4), and cardiovascular disease (5, 6) and is associated with health conditions such as obesity (7). 25-Hydroxyvitamin D [25(OH)D], the primary circulating form in humans, is derived from diet and exposure to UV radiation (UVR) and is considered the best measure of vitamin D status (8). Mechanistic studies have provided evidence supporting direct beneficial effects of vitamin D on bone health and a lower risk of cardiovascular disease and hypertension (9, 10). Vitamin D has also been shown to inhibit cell proliferation and angiogenesis, induce apoptosis, and enhance immune function (11). Measurement of circulating 25(OH)D concentration is widely used in clinical practice to assess vitamin D status and guide supplementation recommendations (12, 13).
Despite supportive experimental research evidence involving antiinflammatory actions and potential for anticancer therapeutics of vitamin D (14, 15), prospective population-based studies of prediagnostic circulating 25(OH)D and cancer risk have been inconsistent and therefore inconclusive (13). A potential reason for the null results and inconsistencies may be failure of a single measure to capture variability in concentration levels over time. Within-person variability of circulating micronutrient concentrations over time can be caused by changes in daily diet, supplement use, physical activity, and in the case of 25(OH)D, exposure to solar UVR (16–19). A major limitation of previous epidemiologic studies assessing associations of circulating 25(OH)D and risk of various serious diseases is that most studies rely on one measure taken at a single point in time, despite known seasonal variation and behavioral changes affecting circulating 25(OH)D levels (20, 21). A single measure of 25(OH)D may not reflect long-term levels and might therefore introduce measurement error into the study. In addition to variation in vitamin D levels reflecting changing patterns of health behaviors and solar UVR exposure, measurement error may introduce additional random variation, all of which would likely attenuate the true association with health outcomes (16).
Advancing our understanding in this area is becoming critically important, particularly for examining the effects of exposure variability on biomarker-disease associations (18, 22). Furthermore, the assessment of dietary measurement error in nutritional cohort studies has recently heightened interest in employing methods for correcting point estimates in dietary exposure studies (23). Yet few studies have examined variability between 25(OH)D measurements over time and specifically across seasons using the same assay and laboratory (24, 25). These studies were limited to a small geographic area, a single race, or subset to only one sex. The U.S. Radiologic Technologist (USRT) Study is a nationwide study in which blood was collected across seasons from a sample of 538 mostly Black and White men and women with a wide range of geographic latitudes. The present study examines the intra-individual variability and reproducibility of plasma 25(OH)D across two time points of varying seasons within a 1-yr period among a large population sample from the USRT Study.
Subjects and Methods
Participants
Details of the study population are reported elsewhere (26). Briefly, the USRT Study was initiated in 1983 and is comprised of a large cohort of radiologic technologists who resided in the United States and were certified by the American Registry of Radiologic Technologists for at least 2 yr between 1926 and 1982. Participants included a random sample of 10,752 technologists enriched by inclusion of African-Americans who were not in the random sample (supplementary group; n = 2593) because this racial group constitutes a small percentage of the USRT Study population. A total of 4321 respondents provided blood samples in 2008–2009, of whom 1500 had 25(OH)D assays measured. A subset of the 1500 participants (n = 538) provided a second blood sample approximately 6 months later to determine whether vitamin D concentrations varied over this time period in the same individuals; participant characteristics including age, sex, race, body mass index (BMI), vitamin supplement use, and latitude were similar between the two groups (i.e. the 1500 vs. the 538 participants). The final analytic cohort included the 538 subjects for whom two measurements of 25(OH)D were available. Most of the first samples were obtained in months November–April. The USRT Study has been approved annually by the human subjects review boards at the University of Minnesota and the National Cancer Institute. All participants provided written informed consent.
Procedures
Blood samples were shipped overnight with ice packs to the processing laboratory in Frederick, MD. Plasma samples were assayed for 25(OH)D concentration in 23 batches (89–95 samples per batch) at Heartland Assays, Inc. (Ames, IA) using LIAISON chemiluminescent immunoassay in commercially available kits from DiaSorin (Stillwater, MN). We selected quality controls (five per batch) to represent low, medium, and high concentrations; the quality control samples were blinded. Samples from those providing multiple blood draws were randomly distributed across and within batches, with samples from the same person kept in the same batch. The total coefficient of variation (CV) was 8.3, 7.2, and 5.8%, for low, medium, and high levels of 25(OH)D concentrations, with an overall average of 7.1%.
Information was ascertained from self-administered questionnaires at the time of blood collection (2008–2009) on demographics (e.g. age, sex, and race), anthropometry (i.e. weight and height), health-related behaviors during the past 30 d (e.g. current smoker, diet, and supplement use), and other potential determinants of vitamin D status (e.g. season of blood draw and latitude of residential address). Vitamin D supplement intake (international units per day) was assessed from questions on use of multivitamins, vitamin D, and cod liver oil consumption.
Estimates of ground-level UVR erythemal exposure were obtained from the Total Ozone Mapping Spectrometer (TOMS) database maintained by the National Aeronautics and Space Administration (NASA) (http://toms.gsfc.nasa.gov) by linking the participant's residential addresses to the TOMS database. Mean potential ambient UVR exposure was estimated for each participant for the month of questionnaire administration based on the average value for that month measured by NASA satellites during the time period (1978–1993) that the satellites were operational. The geographic resolution for the satellite measurements of ground-level UVR erythemal exposure was 1.0° latitude, 1.25° longitude.
Statistical analyses
The distribution of plasma 25(OH)D concentration levels at the time of each measurement (time A and time B) was compared using box-and-whisker plots; differences between median values at the two time points were analyzed using the nonparametric Wilcoxon signed-rank test for paired observations. Measurement agreement of the two samples obtained at different times from each participant was examined using pairwise comparisons and evaluated using Spearman rank correlation coefficients. Season of blood collection was defined as January–March (winter), April–June (spring), July–September (summer), and October–December (autumn) to examine seasonal variation in 25-OH)D concentration. Time of blood collection was also categorized into two groups to reflect months with shorter days (November–April) and months with longer days (May–October). The two repeated measures were also compared by computing the mean absolute differences of the measurements. Vitamin D status of insufficiency was defined using a threshold concentration of 25(OH)D below 50 nmol/liter (12). κ-Coefficients were calculated to examine agreement between vitamin D status at the two time points.
A mixed-effects linear regression model with a random effect for study subject was used to estimate within- and between-subject variance components of log-transformed 25(OH)D, before and after adjustment for known determinants of 25(OH)D levels, including age, sex, race, smoking status, BMI, latitude, supplement use, ambient UVR exposure, and season of blood draw (28, 29). The 25(OH)D levels were log-transformed before regression analysis to normalize distributions. We additionally adjusted for time of measurement [i.e. first measurement (time A) vs. second measurement (time B)], which was significantly associated with 25(OH)D levels (P = 0.03) in the present study. To provide more details of these statistical methods, the estimates of the total variance from the two models were used to estimate the percent reduction of the total random variation due to addition of the fixed effects. The intraclass correlation coefficient (ICC) was calculated to measure the fraction of total variance that is from the between-subject variance component (30). The deattenuation coefficient is a factor that is used to correct a regression coefficient for bias from measurement error in the corresponding independent variable (18, 31). For instance (see Table 4), we computed a deattenuation factor for circulating vitamin D that, when it is multiplied by the regression coefficient of 25(OH)D levels used in a regression analysis, estimates the true regression coefficient without measurement error in vitamin D. We provide the deattenuation coefficient for 25(OH)D to provide the reader with a sense of the effect that the measurement error may have in regression analysis. We further examined the variance components and deattenuation after accounting for potential periodic variation using a cosinor model approach (32), and the results did not change markedly; therefore, the results from the former approach are reported. Data were analyzed with SAS Software version 9.2 (SAS Institute, Inc., Cary, NC).
Table 4.
Absolute change in vitamin D concentrations by subject characteristics
| Characteristic | No. of subjects | Mean change (nmol/liter) ± sd |
|---|---|---|
| All subjects | 538 | 16.8 ± 16.7 |
| Age at blood draw (yr) | ||
| <60 | 265 | 16.6 ± 17.3 |
| ≥60 | 273 | 17.0 ± 16.1 |
| Gender | ||
| Female | 326 | 16.0 ± 17.4 |
| Male | 212 | 17.9 ± 15.4 |
| Race | ||
| White | 329 | 15.4 ± 14.2 |
| Non-White | 209 | 19.0 ± 19.8 |
| Weight (kg) | ||
| Below median | 261 | 16.1 ± 17.2 |
| Above median | 261 | 17.8 ± 16.4 |
| BMI (kg/m2) | ||
| <25.0 | 124 | 15.4 ± 18.8 |
| 25.0–29.9 | 218 | 17.3 ± 15.0 |
| ≥30 | 168 | 17.4 ± 17.5 |
| Current smoker | ||
| No | 462 | 16.8 ± 17.1 |
| Yes | 63 | 17.3 ± 14.7 |
| Latitude tertiles | ||
| <35° | 144 | 14.5 ± 12.9 |
| 35–42° | 285 | 18.6 ± 19.5 |
| >42° | 109 | 14.9 ± 12.1 |
| Daily supplement use | ||
| No | 252 | 16.2 ± 16.1 |
| Yes | 286 | 17.3 ± 17.2 |
| Ambient UVR exposurea | ||
| <73.7 | 269 | 15.0 ± 14.6 |
| ≥73.7 | 269 | 18.5 ± 18.4 |
| Seasons of blood draws | ||
| Winter-Winter | 12 | 17.2 ± 15.9 |
| Winter-Spring | 35 | 17.6 ± 20.8 |
| Winter-Summer | 287 | 18.3 ± 17.1 |
| Winter-Autumn | 102 | 12.6 ± 10.4 |
| Spring-Spring | 3 | 16.2 ± 14.2 |
| Spring-Summer | 12 | 21.3 ± 31.4 |
| Spring-Autumn | 0 | |
| Summer-Summer | 1 | 4.9 |
| Summer-Autumn | 63 | 17.3 ± 17.7 |
| Autumn-Autumn | 23 | 11.2 ± 11.7 |
| Dark months both times | 53 | 12.7 ± 12.6 |
| Lighter months both times | 1 | 4.9 |
| Mixed (dark and light months) | 484 | 17.2 ± 17.0 |
Months with shorter days are considered dark months (November–April); months with longer days are considered lighter months (May–October).
Below and above the median value of 73.7.
Results
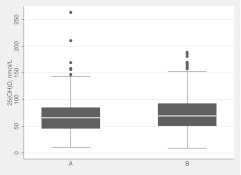
The majority of technologists participating in the vitamin D measurement component of the USRT study were female (60.6%), current nonsmokers (85.9%), and Caucasian (61%); 37% were African-American, and 2% were of other races (Table 1). The mean age of the subjects at date of blood draw was 61.4 yr. Two blood specimens were collected from each of the subjects with an average of 8 months between the samples (median = 7.7 months; 25–75th percentile = 6.6–9.1); 39 subjects (7.2%) had two blood samples taken in the same season. The majority of blood samples (75.3%) were drawn during months with shorter days (November–April) for the first measurement and 65.6% during months with longer days (May–October) for the second measurement. The median value for plasma 25(OH)D levels at the first assessment (shorter days) was lower than the median value at the second measurement (longer days) (65.9 vs. 69.3 nmol/liter, P < 0.0001) (Fig. 1). Median values for log-transformed 25(OH)D were 4.19 and 4.24 for the first and second blood draws, respectively (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Table 1.
Characteristics of 538 subjects for the data analysis
| Characteristic | Total (n = 538) |
|---|---|
| Age at blood draw (yr) | 61.4 ± 8.0 |
| Gender [n (%)] | |
| Female | 326 (60.6) |
| Male | 212 (39.4) |
| Race [n (%)] | 209 (38.8) |
| White | 329 (61.2) |
| Black | 197 (36.6) |
| Other | 12 (2.2) |
| Height (cm) | 168.9 ± 8.8 |
| Weight (kg) | 82.8 ± 18.7 |
| BMI (kg/m2) | 28.9 ± 5.9 |
| Current smoker [n (%)] | |
| No | 462 (85.9) |
| Yes | 63 (11.7) |
| Unknown | 13 (2.4) |
| Season of first blood draw [n (%)] | |
| January–March | 316 (58.7) |
| April–June | 34 (6.3) |
| July–September | 99 (18.4) |
| October–December | 89 (16.5) |
| Months with shorter days (November–April) | 405 (75.3) |
| Months with longer days (May–October) | 133 (24.7) |
| Season of second blood draw [n (%)] | |
| January–March | 132 (24.5) |
| April–June | 19 (3.5) |
| July–September | 265 (49.3) |
| October–December | 122 (22.7) |
| Months with shorter days (November–April) | 185 (34.4) |
| Months with longer days (May–October) | 353 (65.6) |
| Latitude tertiles [n (%)] | |
| <35° | 144 (26.8) |
| 35–42° | 285 (53.0) |
| >42° | 109 (20.2) |
| Total daily supplements (IU) | |
| Missing [n (%)] | 94 (17.5) |
| 0 [n (%)] | 158 (29.4) |
| >0 [n (%)] | 286 (53.2) |
| Ambient UVR exposure | 96.7 ± 67.7 |
| First 25(OH)D measure (nmol/liter) | 67.6 ± 30.7 |
| Second 25(OH)D measure (nmol/liter) | 72.7 ± 32.2 |
| Time between 25(OH)D measures (months) | 8.4 ± 3.0 |
Results are shown as mean ± sd reported unless indicated otherwise.
Fig. 1.
Box-and-whisker plots for distribution of 25(OH)D concentrations at times A and B. The bottom and top horizontal lines of the box represent the 25th and 75th percentiles, respectively. The band near the middle of the box represents the median value. The ends of the whiskers represent the 5th and 95th percentiles. Median (25th, 75th percentiles) was 65.9 (45.8, 85.0) for time A and 69.3 (50.9, 92.6) for time B. Wilcoxon signed rank P < 0.0001.
Vitamin D status (i.e. sufficiency vs. insufficiency) did not change between the first and second measurements of 25(OH)D for the majority of the subjects (Table 2); 63.6% remained sufficient (≥50 nmol/liter), 17.5% remained insufficient (<50 nmol/liter), 12.3% had a change in vitamin D status from insufficient to sufficient status, and 6.7% had a change from sufficient to insufficient vitamin D levels. The proportions were similar after excluding the 39 subjects with two samples taken in the same season (63.1, 17.8, 12.0, and 7.0%, respectively). In general, subjects whose vitamin D status remained sufficient were more often daily supplement users (P < 0.01) and less often obese (BMI ≥ 30 kg/m2; P < 0.01). Subjects whose vitamin D status remained insufficient were more likely to be of a non-White race (P < 0.01). Overall, vitamin D status was relatively consistent across the two time points (κ-statistic = 0.52).
Table 2.
Subject characteristics by change in vitamin D status between first and second time of blood draw
| Characteristic | Remained sufficient | Sufficient to insufficient | Insufficient to sufficient | Remained insufficient | P value |
|---|---|---|---|---|---|
| No. of subjects | 342 | 36 | 66 | 94 | |
| Age at blood draw (yr) | |||||
| <60 | 47.1 | 63.9 | 43.9 | 55.3 | 0.12 |
| ≥60 | 52.9 | 36.1 | 56.1 | 44.7 | |
| Gender | |||||
| Female | 62.9 | 41.7 | 53.0 | 64.9 | 0.07 |
| Male | 37.1 | 58.3 | 47.0 | 35.1 | |
| Race | |||||
| White | 68.7 | 50.0 | 60.6 | 38.3 | <0.01 |
| Non-White | 31.3 | 50.0 | 39.4 | 61.7 | |
| Weight (kg) | |||||
| Below median | 56.3 | 24.2 | 42.4 | 41.8 | <0.01 |
| Above median | 43.7 | 75.8 | 57.6 | 58.2 | |
| BMI (kg/m2) | |||||
| <25 | 28.2 | 12.5 | 18.5 | 18.9 | <0.01 |
| 25.0–29.9 | 46.1 | 40.6 | 38.5 | 34.4 | |
| ≥30 | 25.7 | 46.9 | 43.1 | 46.7 | |
| Current smoker | |||||
| No | 89.8 | 91.7 | 83.3 | 85.1 | 0.34 |
| Yes | 10.2 | 8.3 | 16.7 | 14.9 | |
| Latitude tertiles | |||||
| <35° | 25.7 | 33.3 | 27.3 | 27.6 | 0.16 |
| 35–42° | 50.3 | 58.4 | 59.1 | 56.4 | |
| >42° | 24.0 | 8.3 | 13.6 | 16.0 | |
| Daily supplement use | |||||
| No | 37.7 | 61.1 | 56.1 | 68.1 | <0.01 |
| Yes | 62.3 | 38.9 | 43.9 | 31.9 | |
| Ambient UVR exposure | |||||
| <73.7 | 52.9 | 27.8 | 51.5 | 46.8 | 0.03 |
| ≥73.7 | 47.1 | 72.2 | 48.5 | 53.2 |
Results are shown as percentages unless indicated otherwise. P values are reported based on χ 2 test. Insufficient 25(OH)D is less than 50 nmol/liter; sufficient is 50 nmol/liter or higher. Simple κ-coefficient = 0.52 for agreement between vitamin D status at first and second blood draw.
Spearman rank correlation coefficients for the pairwise comparison of the two circulating 25(OH)D measurements stratified by subject characteristics are shown in Table 3. Overall, the correlation between the two 25(OH)D measurements was 0.75 (P < 0.0001). The highest correlation was observed between two measurements taken during months with shorter days (r = 0.88), which corresponds to the high correlations also observed for subjects with lower ambient UVR exposure (r = 0.82) and those residing in areas at higher latitude (r = 0.81). Among subjects whose 25(OH)D concentrations were measured from samples taken in the winter and summer, the Spearman rank correlation coefficient was 0.71. For the 12 subjects whose measures were ascertained in spring and summer, there was a poor correlation of 0.21; however, this finding should be interpreted with caution given the small number of subjects. Correlations did not vary substantially by categories of age, race, BMI, smoking status, or supplement use; however, the correlation between the two 25(OH)D measurements was lower among men (r = 0.66) than women (r = 0.78). The correlations for men and women were not markedly altered when accounting for seasons of blood draw (partial correlations: 0.71 for men and 0.78 for women) (data not shown).
Table 3.
Spearman correlations between vitamin D concentrations by subject characteristics
| Characteristic | n | Correlation coefficient | P value |
|---|---|---|---|
| All subjects | 538 | 0.75 | <0.0001 |
| Age at blood draw (yr) | |||
| <60 | 265 | 0.70 | <0.0001 |
| ≥60 | 273 | 0.78 | <0.0001 |
| Gender | |||
| Female | 326 | 0.78 | <0.0001 |
| Male | 212 | 0.66 | <0.0001 |
| Race | |||
| White | 329 | 0.77 | <0.0001 |
| Non-White | 209 | 0.71 | <0.0001 |
| Weight (kg) | |||
| Below median | 261 | 0.77 | <0.0001 |
| Above median | 261 | 0.71 | <0.0001 |
| BMI (kg/m2) | |||
| <25 | 124 | 0.75 | <0.0001 |
| 25.0–29.9 | 218 | 0.74 | <0.0001 |
| ≥30 | 168 | 0.71 | <0.0001 |
| Current smoker | |||
| No | 462 | 0.75 | <0.0001 |
| Yes | 63 | 0.77 | <0.0001 |
| Latitude tertiles | |||
| <35° | 144 | 0.79 | <0.0001 |
| 35–42° | 285 | 0.70 | <0.0001 |
| >42° | 109 | 0.81 | <0.0001 |
| Daily supplement use | |||
| No | 252 | 0.74 | <0.0001 |
| Yes | 286 | 0.72 | <0.0001 |
| Ambient UVR exposure | |||
| Below median value | 269 | 0.82 | <0.0001 |
| Above median value | 269 | 0.68 | <0.0001 |
| Seasons of blood draws | |||
| Winter-Winter | 12 | 0.86 | 0.0003 |
| Winter-Spring | 35 | 0.81 | <0.0001 |
| Winter-Summer | 287 | 0.71 | <0.0001 |
| Winter-Autumn | 102 | 0.84 | <0.0001 |
| Spring-Spring | 3 | 0.84 | 0.3670 |
| Spring-Summer | 12 | 0.21 | 0.5128 |
| Spring-Autumn | 0 | ||
| Summer-Summer | 1 | ||
| Summer-Autumn | 63 | 0.78 | <0.0001 |
| Autumn-Autumn | 23 | 0.85 | <0.0001 |
| Dark months both times | 53 | 0.88 | <0.0001 |
| Lighter months both times | 1 | ||
| Mixed (dark and light months) | 484 | 0.73 | <0.0001 |
Months with shorter days are considered dark months (November–April); months with longer days are considered lighter months (May–October).
The absolute change in 25(OH)D concentration levels between the two measurements is summarized by participant characteristics in Table 4. White participants had less change in concentration levels than did non-White (15.4 vs. 19.0 nmol/liter). Those living in areas in the lowest and highest tertiles of latitudes had a lower mean change in vitamin D concentrations than did participants living in middle tertile (latitude 35–42°) (mean change = 14.5 and 14.9, respectively, vs. 18.6 nmol/liter). These results did not markedly change after adjustment for age, gender, race, BMI, smoking status, latitude, and supplement use (data not shown).
The within- and between-subject variances adjusted and unadjusted for the predictors age, sex, race, smoking status, BMI, supplement use, latitude, ambient UVR exposure, season, and time are presented in Table 5. The unadjusted and adjusted ICCs are 0.71 and 0.72, which indicates relative consistency between the two measurements. The percent reduction of total random variability after accounting for the predictors is 15.1%. The deattenuation factor of the plasma 25(OH)D levels is 1.39 (i.e. an approximate 40% increase in the estimated regression coefficient), which suggests that using a single measure instead of long-term average 25(OH)D would attenuate the relation by a factor of 0.72. Results were similar when adjusting for weight instead of BMI in the mixed models. Additional results from the mixed-effects linear regression model (Supplemental Table 2) show that 25(OH)D measures obtained in the spring and autumn were not significantly different (P = 0.16), whereas those obtained in winter months (January–March) were lower and those obtained in summer months (July–September) were higher than measures collected in autumn (October–December). Of interest is that circulating 25(OH)D levels collected in winter or summer months were equally different from those obtained in autumn as shown by the β-estimates (±0.06).
Table 5.
Variance components and deattenuation of measurements
| Variance components for unadjusted model | Variance |
|---|---|
| Between-person | 0.18 |
| Within-person | 0.07 |
| Sum of the variance components | 0.25 |
| ICC | 0.72 |
| Deattenuation factor | 1.39 |
| Variance components for adjusted model | |
| Between-person | 0.15 |
| Within-person | 0.06 |
| Sum of the variance components | 0.21 |
| ICC | 0.71 |
| Reduction (%) of total random variation | 15.1 |
Variance components are given for log-transformed 25(OH)D values. The model was adjusted for age, sex, race, BMI, supplement use, smoking status, latitude, ambient UVR exposure, season, and time (i.e. blood draw at time A or time B).
Discussion
Deciding on whether to obtain more than one vitamin D measurement and when blood samples should be collected to reflect average long-term exposure are two important issues for evaluating associations between circulating 25(OH)D and disease risks. We therefore examined the within-subject variability of two measurements of 25(OH)D concentrations in different seasons within a 1-yr period using the DiaSorin LIAISON assay at a single laboratory. We found a relatively high degree of correlation across groups of subject characteristics, including age, race, and BMI, but a lower correlation among men and variability across seasons. Our results from the linear mixed-effects models suggest that a single blood sample obtained in spring or autumn may provide values of 25(OH)D that reflect an average over a 1-yr period. Alternatively, if two samples are drawn, then having one sample in winter (i.e. values lower than typical) and an additional sample in summer (i.e. values higher than typical) would also provide a measure of 25(OH)D closer to the typical 1-yr level on average. However, we found that even though the ICC was relatively high, which may in part be due to these factors not accounting for a large portion of the variation of circulating 25(OH)D among USRT Study participants, there was a resulting attenuation factor of 40%.
Although substantial variation has been reported between vitamin D assay methods, batches, and laboratories (22, 33–38), including assay drift over long periods of time as experienced with the National Health and Nutrition Examination Survey (NHANES) (www.cdc.gov/nchs/data/nhanes/nhanes3/vitaminD_analyticnote.pdf), few studies have examined within-subject variability of 25(OH)D concentrations from blood samples collected over time using the same assay at a single laboratory (24, 25, 39–42). Three of these studies evaluated variability of measures across different years, but within or near the same season (39–42), and an additional study did not indicate whether variation across seasons of blood draw were considered (27). Results from a small study of 29 U.S. adults that examined serum 25(OH)D from nonfasting blood samples three times over a 5-yr period (same month each time), showed an ICC similar to ours of 0.71 (95% confidence interval = 0.63–0.88) and a Spearman rank correlation coefficient of 0.65 when comparing baseline to 1-yr levels of circulating 25(OH)D (40). Similarly, plasma 25(OH)D measured in blood samples that were collected 5 yr apart (same time of year) was examined among 672 older women, and the Spearman rank correlation was 0.61 (41). Among 144 cancer-free U.S. adult males in the Health Professionals Study, a partial correlation of 0.70 was observed between two measurements of plasma 25(OH)D approximately 3 yr apart and in the same season after adjustment for age and race (39). Similarly, a correlation of 0.72 was observed between two measurements of serum 25(OH)D measured approximately 2 yr apart among 352 predominantly White, elderly men (42). Among 71 women in the Nurses' Health Study, the ICC across three measures of 25(OH)D taken at 1, 2, and 3 yr was 0.72 (27). Our findings are consistent with all four studies.
Only two studies, to our knowledge, have specifically examined multiple measures of 25(OH)D concentrations for individual participants across different seasons (24, 25); however, each study was restricted geographically (Japan and Norway) and racially, and one was limited to females (i.e. Japanese women). We observed a higher correlation (r = 0.71) between two measurements taken in summer and winter than those reported among the 122 older Japanese women (r = 0.46) in which the two 25(OH)D measures were obtained in summer (September 1997) and winter (February 1999) (23). Our correlation was also higher than that observed in the Norwegian study (n = 720) between two measurements (i.e. baseline and 14 yr later), which ranged according to season of blood draw from 0.42–0.52. However, the correlation between baseline and 1-yr serum 25(OH)D levels among a subset of the Norwegian adults for whom 1-yr serum vitamin D was measured (n = 94) was similar to the high correlation we observed, r = 0.80 (24), suggesting increased variability between measurements taken more than 1 yr apart.
An important distinction between the present study and the previous five reports is that our study measured 25(OH)D concentrations for most participants in different seasons, included samples from a racially diverse study population that afforded the opportunity to examine White and non-White differences, and was comprised of a large sample of 538 men and women. To our knowledge, this is the largest study of 25(OH)D levels over a 1-yr period among adults in the United States. An additional strength of our study is the detailed information ascertained on personal characteristics and latitude of residential address at the time of blood collection and nationwide distribution of participants. A limitation of the present study is that 25(OH)D concentrations were measured at only two time points, which precluded assessment of within-subject variability in concentrations across all four seasons. Additional longitudinal 25(OH)D measurements would be required to investigate variability across long-term exposures, and additional studies are needed to understand the impact of attenuation due to measurement variability on epidemiologic studies modeling disease risk.
In summary, in a nationwide cohort of U.S. radiologic technologists, we found relatively high agreement in plasma 25(OH)D concentrations taken from the same person in different seasons using the same assay method and laboratory. If confirmed, these findings suggest that although an individual's 25(OH)D level is relatively stable over a 1-yr period, choice of season for blood draw may improve accuracy of measured 25(OH)D to more closely approximate average value for a 1-yr period. Because of the potential differences in variability and agreement in circulating 25(OH)D concentrations across studies, random subsamples with repeat measurements across seasons may be useful to quantify the variability for specific study populations. Our findings may help guide the design and analysis of studies of vitamin D for other populations similar to ours. Additional studies are needed to estimate variability and agreement in plasma 25(OH)D concentrations over longer periods of time and in general populations that include younger adults.
Acknowledgments
We are particularly grateful to the radiologic technologists who participated in this study. We also thank Jerry Reid of the American Registry of Radiologic Technologists for continued support of the project; Diane Kampa of the University of Minnesota for data collection and coordination; Laura Bowen and Li Cheung of Information Management Services, Inc., for biomedical computer assistance; Jackie King of Biorepository Services for plasma sample coordination; and Dr. Ron Horst of Heartland Assays for performing 25(OH(D) assays on the plasma samples.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the U.S. Public Health Service of the Department of Health and Human Services.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- 25(OH)D
- 25-hydroxyvitamin D
- ICC
- intraclass correlation coefficient
- USRT
- U.S. Radiologic Technologist
- UVR
- UV radiation.
References
- 1. Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Grantham N, Ebeling PR, Daly RM. 2011. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care 34:1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giovannucci E. 2009. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol 19:84–88 [DOI] [PubMed] [Google Scholar]
- 3. Holick MF. 2004. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S [DOI] [PubMed] [Google Scholar]
- 4. IARC 2008. Vitamin D and Cancer. In: IARC Working Group Reports. Lyon, France: IARC [Google Scholar]
- 5. Leu M, Giovannucci E. 2011. Vitamin D: epidemiology of cardiovascular risks and events. Best Pract Res Clin Endocrinol Metab 25:633–646 [DOI] [PubMed] [Google Scholar]
- 6. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. 2008. Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford ES, Ajani UA, McGuire LC, Liu S. 2005. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 28:1228–1230 [DOI] [PubMed] [Google Scholar]
- 8. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine 1997. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy of Sciences [Google Scholar]
- 9. Bassuk SS, Manson JE. 2009. Does vitamin D protect against cardiovascular disease? J Cardiovasc Transl Res 2:245–250 [DOI] [PubMed] [Google Scholar]
- 10. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. 2004. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89–90:387–392 [DOI] [PubMed] [Google Scholar]
- 11. Dusso AS, Brown AJ, Slatopolsky E. 2005. Vitamin D. Am J Physiol Renal Physiol 289:F8–F28 [DOI] [PubMed] [Google Scholar]
- 12. Rosen CJ. 2011. Clinical practice. Vitamin D insufficiency. N Engl J Med 364:248–254 [DOI] [PubMed] [Google Scholar]
- 13. Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium 2011. Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Washington, DC: National Academy of Sciences [Google Scholar]
- 14. Krishnan AV, Feldman D. 2011. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 51:311–336 [DOI] [PubMed] [Google Scholar]
- 15. Deeb KK, Trump DL, Johnson CS. 2007. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700 [DOI] [PubMed] [Google Scholar]
- 16. Willett W. 1998. Nutritional epidemiology. 2nd ed New York: Oxford University Press [Google Scholar]
- 17. Gozdzik A, Barta JL, Weir A, Cole DE, Vieth R, Whiting SJ, Parra EJ. 2010. Serum 25-hydroxyvitamin D concentrations fluctuate seasonally in young adults of diverse ancestry living in Toronto. J Nutr 140:2213–2220 [DOI] [PubMed] [Google Scholar]
- 18. Shvetsov YB, Hernandez BY, Wong SH, Wilkens LR, Franke AA, Goodman MT. 2009. Intraindividual variability in serum micronutrients: effects on reliability of estimated parameters. Epidemiology 20:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M. 2010. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer 62:51–57 [DOI] [PubMed] [Google Scholar]
- 20. Nanri A, Foo LH, Nakamura K, Hori A, Poudel-Tandukar K, Matsushita Y, Mizoue T. 2011. Serum 25-hydroxyvitamin d concentrations and season-specific correlates in Japanese adults. J Epidemiol 21:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Boer IH, Levin G, Robinson-Cohen C, Biggs ML, Hoofnagle AN, Siscovick DS, Kestenbaum B. 2012. Serum 25-hydroxyvitamin d concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med 156:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai JK, Lucas RM, Banks E, Ponsonby AL, Ausimmune Investigator G 2012. Variability in vitamin D assays impairs clinical assessment of vitamin D status. Intern Med J 42:43–50 [DOI] [PubMed] [Google Scholar]
- 23. Freedman LS, Schatzkin A, Midthune D, Kipnis V. 2011. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 103:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamura K, Nashimoto M, Yamamoto M. 2000. Summer/winter differences in the serum 25-hydroxyvitamin D3 and parathyroid hormone levels of Japanese women. Int J Biometeorol 44:186–189 [DOI] [PubMed] [Google Scholar]
- 25. Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. 2010. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 171:903–908 [DOI] [PubMed] [Google Scholar]
- 26. Boice JD, Jr, Mandel JS, Doody MM, Yoder RC, McGowan R. 1992. A health survey of radiologic technologists. Cancer 69:586–598 [DOI] [PubMed] [Google Scholar]
- 27. Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, Eliassen AH. 2010. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev 19:938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J, Group IOFCoSANW 2009. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20:1807–1820 [DOI] [PubMed] [Google Scholar]
- 29. Prentice A. 2008. Vitamin D deficiency: a global perspective. Nutr Rev 66:S153–S164 [DOI] [PubMed] [Google Scholar]
- 30. Szklo M, Nieto FJ. 2007. Epidemiology : beyond the basics. 2nd ed Sudbury, MA: Jones and Bartlett [Google Scholar]
- 31. Carroll RJ, Galindo CD. 1998. Measurement error, biases, and the validation of complex models for blood lead levels in chidlren. Environ Health Perspect 106(Suppl 6):1535–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shoben AB, Kestenbaum B, Levin G, Hoofnagle AN, Psaty BM, Siscovick DS, de Boer IH. 2011. Seasonal variation in 25-hydroxyvitamin D concentrations in the cardiovascular health study. Am J Epidemiol 174:1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK. 2004. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89:3152–3157 [DOI] [PubMed] [Google Scholar]
- 34. Glendenning P, Taranto M, Noble JM, Musk AA, Hammond C, Goldswain PR, Fraser WD, Vasikaran SD. 2006. Current assays overestimate 25-hydroxyvitamin D3 and underestimate 25-hydroxyvitamin D2 compared with HPLC: need for assay-specific decision limits and metabolite-specific assays. Ann Clin Biochem 43:23–30 [DOI] [PubMed] [Google Scholar]
- 35. Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. 2008. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem 45:153–159 [DOI] [PubMed] [Google Scholar]
- 36. Binkley N, Krueger D, Gemar D, Drezner MK. 2008. Correlation among 25-hydroxy-vitamin D assays. J Clin Endocrinol Metab 93:1804–1808 [DOI] [PubMed] [Google Scholar]
- 37. Zerwekh JE. 2008. Blood biomarkers of vitamin D status. Am J Clin Nutr 87:1087S–1091S [DOI] [PubMed] [Google Scholar]
- 38. Carter GD. 2011. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets 12:19–28 [DOI] [PubMed] [Google Scholar]
- 39. Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. 2004. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control 15:255–265 [DOI] [PubMed] [Google Scholar]
- 40. Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. 2010. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 19:927–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meng JE, Hovey KM, Wactawski-Wende J, Andrews CA, Lamonte MJ, Horst RL, Genco RJ, Millen AE. 2012. Intraindividual variation in plasma 25-hydroxyvitamin D measures 5 years apart among postmenopausal women. Cancer Epidemiol Biomarkers Prev 21:916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orwoll E, Nielson CM, Marshall LM, Lambert L, Holton KF, Hoffman AR, Barrett-Connor E, Shikany JM, Dam T, Cauley JA. 2009. Vitamin D deficiency in older men. J Clin Endocrinol Metab 94:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]